In Silico Hydrolysis of Lupin (Lupinus angustifolius L.) Conglutins with Plant Proteases Releases Antihypertensive and Antidiabetic Peptides That Are Bioavailable, Non-Toxic, and Gastrointestinal Digestion Stable
Abstract
1. Introduction
2. Results and Discussion
2.1. Biological Activity of Lupin Proteins
2.2. ACE-I and DPP-IV Inhibitory Peptides Released After Simulated Hydrolysis of Lupin Seed Proteins
2.3. ADMET Characteristics and Gastrointestinal Digestion Stability of ACE-I and DPP-IV Inhibitory Peptides
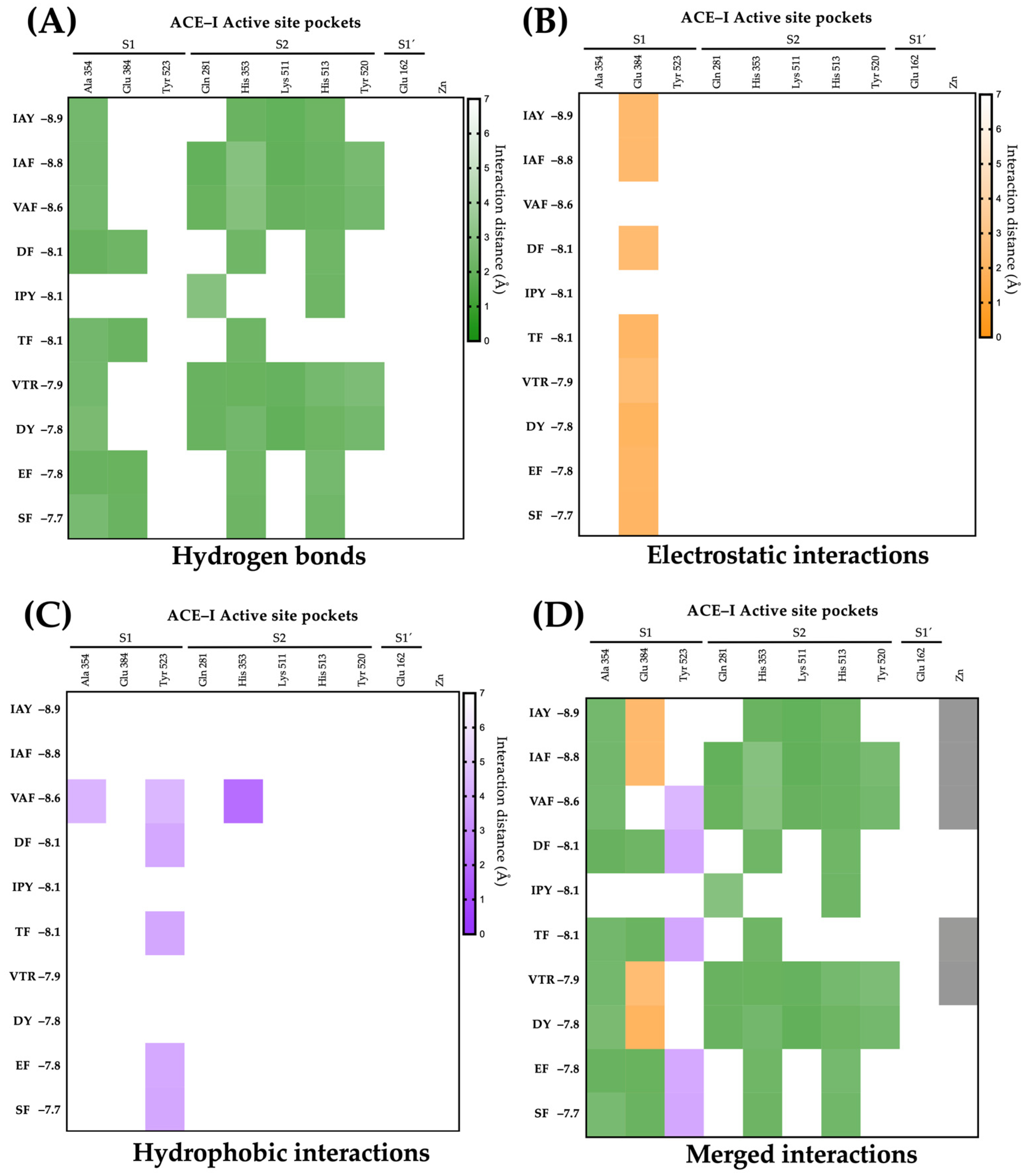
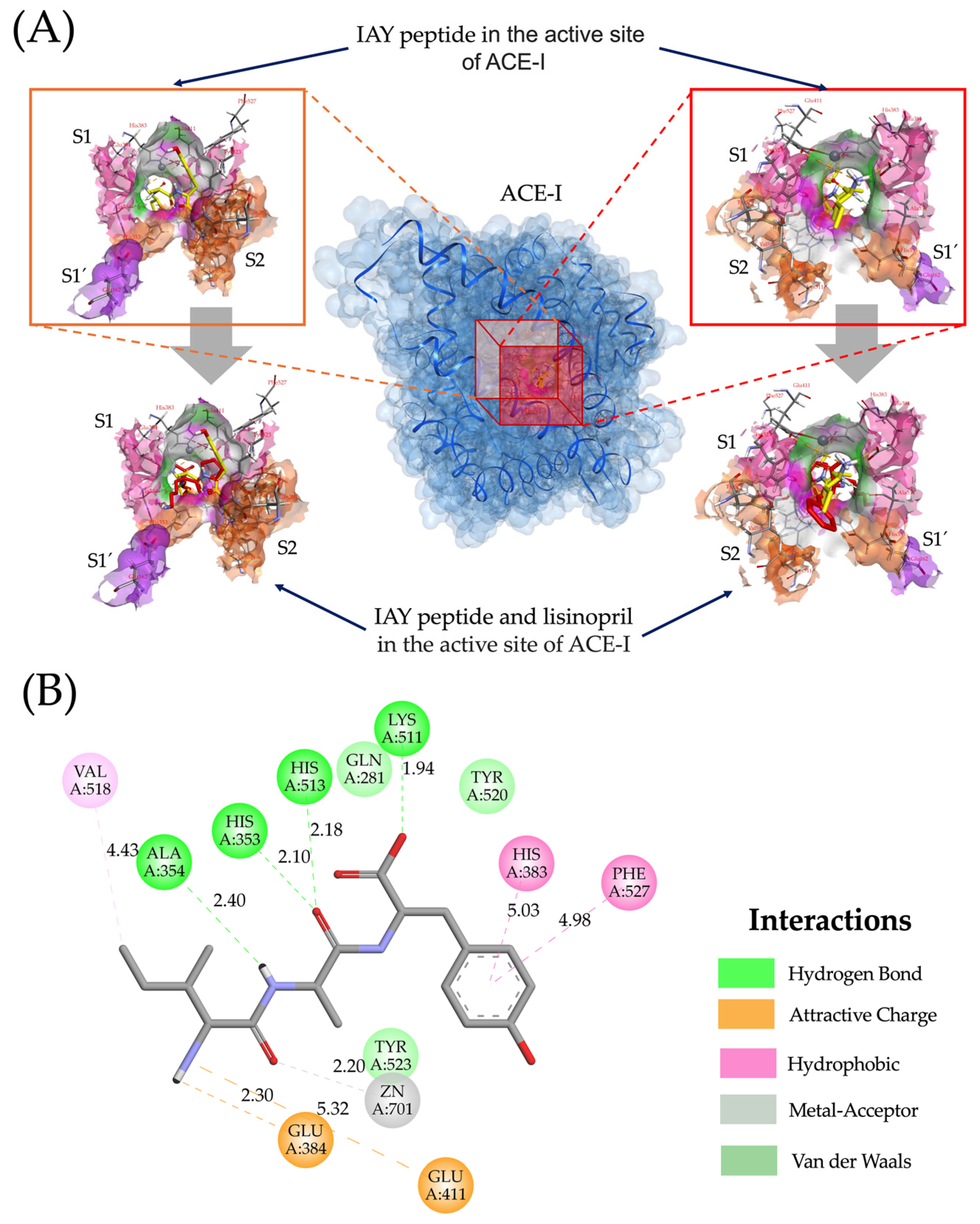
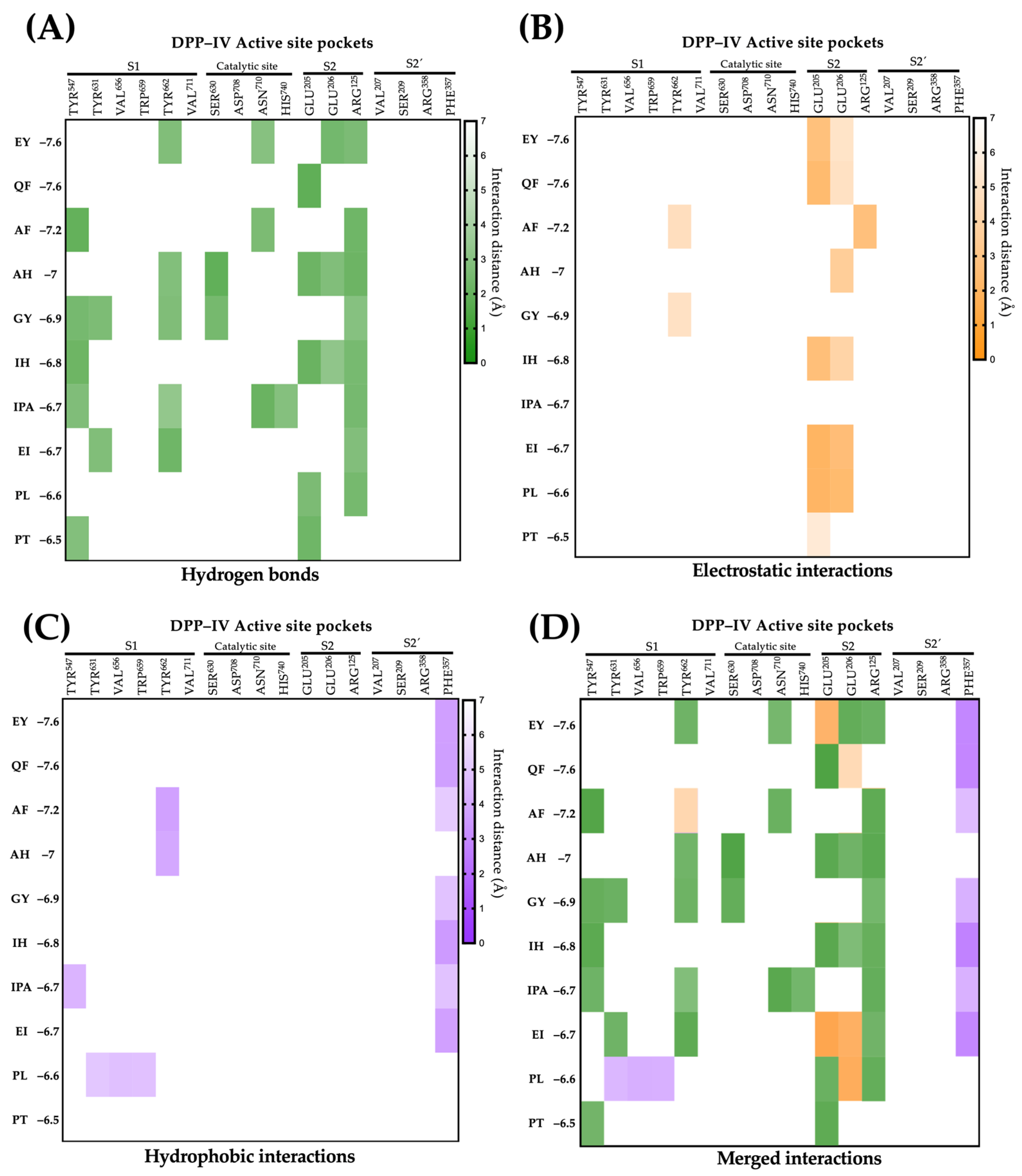
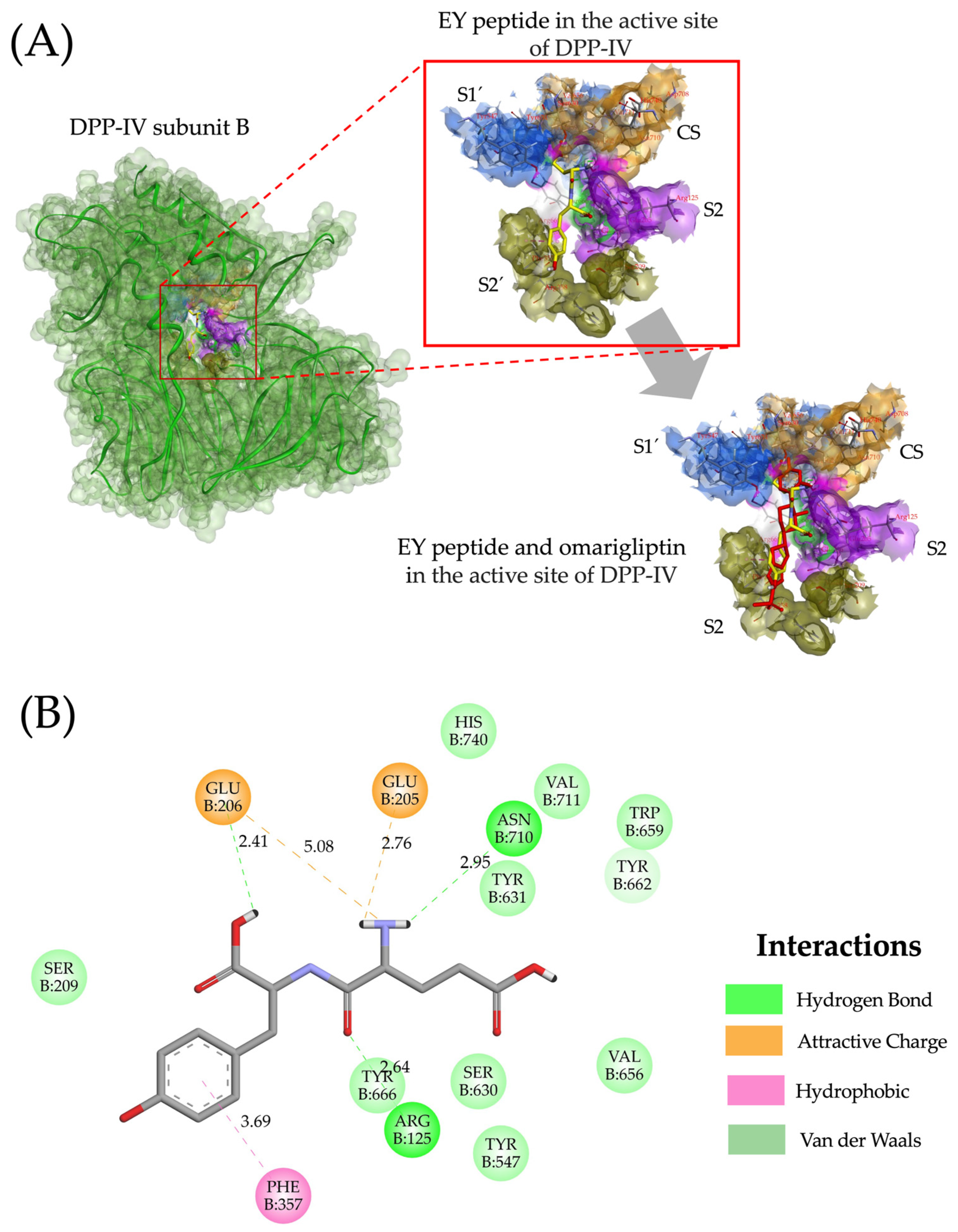
2.4. Molecular Interactions of Lupin Peptides with the Active Sites of ACE-I and DPP-IV
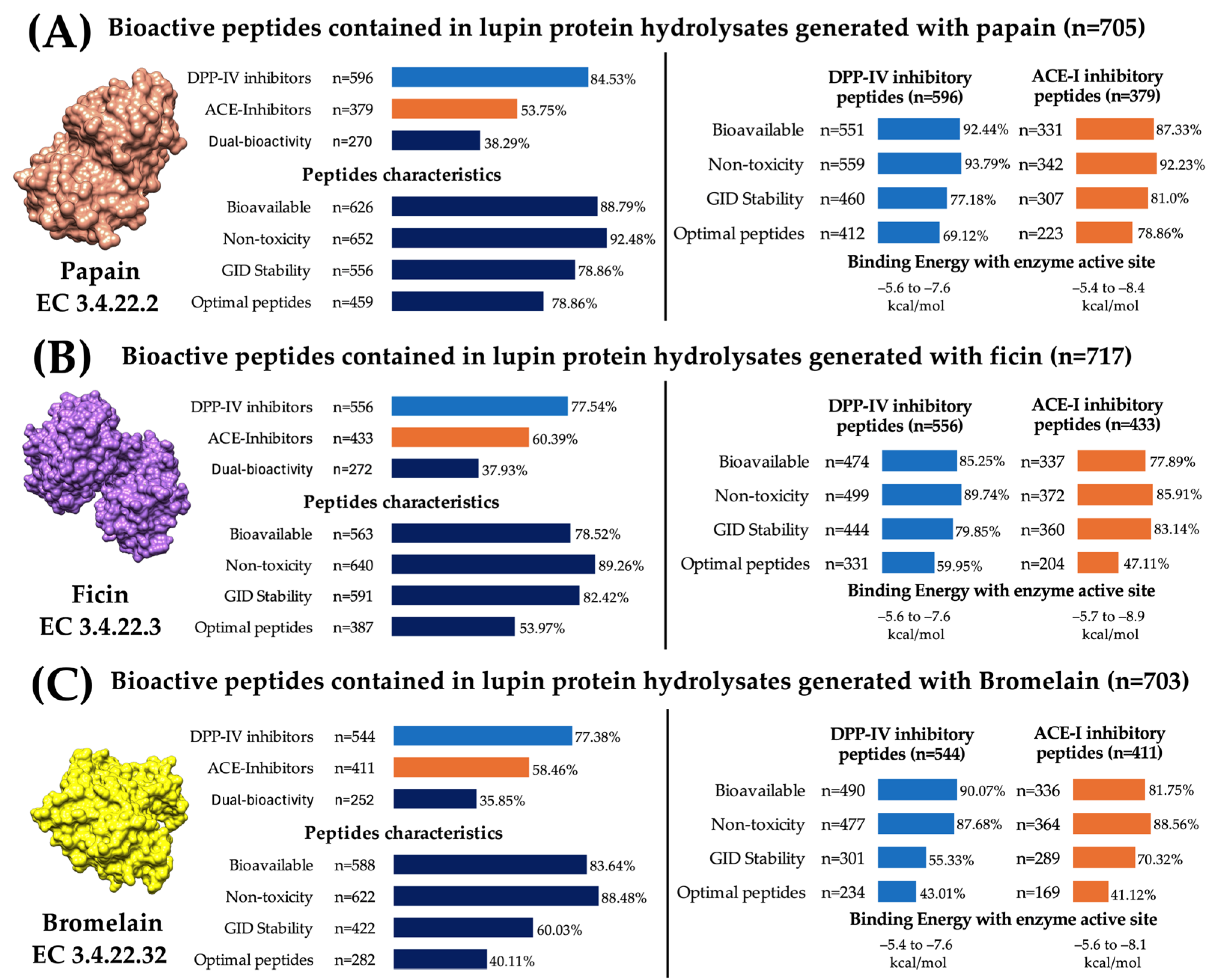
2.5. Plant Proteases Generate Lupine Protein Hydrolysates Containing Bioavailable, Non-Toxic, Digestion-Resistant, and Multi-Bioactive Peptides
3. Materials and Methods
3.1. Protein Sequences and Virtual Screening for Bioactive Peptides
3.2. ADMET Properties and Gastrointestinal Digestion Stability
3.3. Molecular Docking of ACE-I and DPP-IV Inhibitory Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.W.; Esmat, H.A.; Bahee, M.D. Prevalence of Hypertension in Type-2 Diabetes Mellitus. Ann. Med. Surg. 2022, 78, 103758. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2020, 16, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A. A Practical Approach to Hypertension Management in Diabetes. Diabetes Ther. 2017, 8, 981–989. [Google Scholar] [CrossRef]
- Wong, M.K.S. Subchapter 29D—Angiotensin Converting Enzymes. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 263–269. ISBN 978-0-12-801028-0. [Google Scholar]
- Boer, G.A.; Holst, J.J. Incretin Hormones and Type 2 Diabetes—Mechanistic Insights and Therapeutic Approaches. Biology 2020, 9, 473. [Google Scholar] [CrossRef]
- Mirabito Colafella, K.M.; Bovée, D.M.; Danser, A.H.J. The Renin-Angiotensin-Aldosterone System and Its Therapeutic Targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef]
- Sterrett, J.J.; Bragg, S.; Weart, C.W. Type 2 Diabetes Medication Review. Am. J. Med. Sci. 2016, 351, 342–355. [Google Scholar] [CrossRef]
- Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Mora-Melgem, J.A.; Camacho-Cervantes, D.L.; Gracia-Valenzuela, M.H.; Cuevas-Rodríguez, E.O.; Ontiveros, N. Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Foods 2024, 13, 1216. [Google Scholar] [CrossRef]
- Chávez-Ontiveros, J.; Reyes-Moreno, C.; Ramírez-Torres, G.I.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Montoya-Rodríguez, A.; Ontiveros, N.; Cuevas-Rodríguez, E.O. Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods 2022, 11, 2562. [Google Scholar] [CrossRef]
- Mora-Melgem, J.A.; Arámburo-Gálvez, J.G.; Cárdenas-Torres, F.I.; Gonzalez-Santamaria, J.; Ramírez-Torres, G.I.; Arvizu-Flores, A.A.; Figueroa-Salcido, O.G.; Ontiveros, N. Dipeptidyl Peptidase IV Inhibitory Peptides from Chickpea Proteins (Cicer arietinum L.): Pharmacokinetics, Molecular Interactions, and Multi-Bioactivities. Pharmaceuticals 2023, 16, 1109. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and Bioactive Compounds in Mexican Lupin Beans Species: A Mini-Review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Soybean- and Lupin-Derived Peptides Inhibit DPP-IV Activity on In Situ Human Intestinal Caco-2 Cells and Ex Vivo Human Serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients 2023, 15, 1096. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides Derived from Soy and Lupin Protein as Dipeptidyl-Peptidase IV Inhibitors: In Vitro Biochemical Screening and in Silico Molecular Modeling Study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Gan, C.-Y.; Olalere, O.A.; Farahnaky, A.; Gill, H.; Truong, T. Novel Antihypertensive Peptides from Lupin Protein Hydrolysate: An in-silico Identification and Molecular Docking Studies. Food Chem. 2023, 407, 135082. [Google Scholar] [CrossRef]
- Arámburo-Gálvez, J.G.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Ramírez-Torres, G.I.; Flores-Mendoza, L.K.; Gastelum-Acosta, P.E.; Figueroa-Salcido, O.G.; Ontiveros, N. Prediction of ACE-I Inhibitory Peptides Derived from Chickpea (Cicer arietinum L.): In Silico Assessments Using Simulated Enzymatic Hydrolysis, Molecular Docking and ADMET Evaluation. Foods 2022, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Feder, S. Lupin Seeds Storage Protein Composition and Their Interactions with Native Flavonoids. J. Sci. Food Agric. 2019, 99, 4011–4018. [Google Scholar] [CrossRef]
- Foley, R.C.; Jimenez-Lopez, J.C.; Kamphuis, L.G.; Hane, J.K.; Melser, S.; Singh, K.B. Analysis of Conglutin Seed Storage Proteins across Lupin Species Using Transcriptomic, Protein and Comparative Genomic Approaches. BMC Plant Biol. 2015, 15, 106. [Google Scholar] [CrossRef]
- Garmidolova, A.; Desseva, I.; Mihaylova, D.; Lante, A. Bioactive Peptides from Lupinus Spp. Seed Proteins-State-of-the-Art and Perspectives. Appl. Sci. 2022, 12, 3766. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin γ, a Lupin Seed Protein, Binds Insulin in Vitro and Reduces Plasma Glucose Levels of Hyperglycemic Rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Cerna, E.; Pedreschi, R.; Calsin, M.; Aguilar-Galvez, A.; Campos, D. Multifunctional in Vitro Bioactive Properties: Antioxidant, Antidiabetic, and Antihypertensive of Protein Hydrolyzates from Tarwi (Lupinus mutabilis Sweet) Obtained by Enzymatic Biotransformation. Cereal Chem. 2021, 98, 423–433. [Google Scholar] [CrossRef]
- Lemes, A.C.; De Oliveira Filho, J.G.; Fernandes, S.S.; Gautério, G.V.; Egea, M.B. Bioactive Peptides from Protein-Rich Waste. In Agricultural Waste: Environmental Impact, Useful Metabolites and Energy Production; Ramawat, K.G., Mérillon, J.-M., Arora, J., Eds.; Sustainable Development and Biodiversity; Springer Nature: Singapore, 2023; Volume 31, pp. 139–166. ISBN 978-981-19877-3-1. [Google Scholar]
- Agyei, D.; Danquah, M.K. Industrial-Scale Manufacturing of Pharmaceutical-Grade Bioactive Peptides. Biotechnol. Adv. 2011, 29, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Y. Review and Perspective on Bioactive Peptides: A Roadmap for Research, Development, and Future Opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, A.; Darewicz, M.; Mogut, D.; Minkiewicz, P. Elucidation of the Role of in Silico Methodologies in Approaches to Studying Bioactive Peptides Derived from Foods. J. Funct. Foods 2019, 61, 103486. [Google Scholar] [CrossRef]
- Sharmin, K.N.; Amiza, M.A.; Ahmad, F.; Razali, S.A.; Hashim, F. In Silico Analysis of Gracilaria Changii Proteins for Potential Bioactive Peptides. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012017. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Pliszka, M.; Mogut, D.; Darewicz, M. Characteristics of Biopeptides Released In Silico from Collagens Using Quantitative Parameters. Foods 2020, 9, 965. [Google Scholar] [CrossRef]
- Lafarga, T.; O’Connor, P.; Hayes, M. Identification of Novel Dipeptidyl Peptidase-IV and Angiotensin-I-Converting Enzyme Inhibitory Peptides from Meat Proteins Using in Silico Analysis. Peptides 2014, 59, 53–62. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’ Shea, N.; Gallagher, E.; Lafarga, T. Predicted Release and Analysis of Novel ACE-I, Renin, and DPP-IV Inhibitory Peptides from Common Oat (Avena sativa) Protein Hydrolysates Using in Silico Analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Richel, A.; Hao, Y.; Fan, X.; Everaert, N.; Yang, X.; Ren, G. Novel Dipeptidyl Peptidase-IV and Angiotensin-I-Converting Enzyme Inhibitory Peptides Released from Quinoa Protein by in Silico Proteolysis. Food Sci. Nutr. 2020, 8, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a Source of Peptides with Remarkable Biological Activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibáñez, R.A. Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity. Foods 2022, 11, 2429. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Kailasapathy, K.; Phillips, M. Functional and Potential Therapeutic ACE-Inhibitory Peptides Derived from Bromelain Hydrolysis of Trevally Proteins. J. Funct. Foods 2015, 14, 716–725. [Google Scholar] [CrossRef]
- Osman, A.; El-Hadary, A.; Korish, A.A.; AlNafea, H.M.; Alhakbany, M.A.; Awad, A.A.; Abdel-Hamid, M. Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity. Foods 2021, 10, 468. [Google Scholar] [CrossRef]
- Hanafi, M.A.; Hashim, S.N.; Chay, S.Y.; Ebrahimpour, A.; Zarei, M.; Muhammad, K.; Abdul-Hamid, A.; Saari, N. High Angiotensin-I Converting Enzyme (ACE) Inhibitory Activity of Alcalase-Digested Green Soybean (Glycine max) Hydrolysates. Food Res. Int. 2018, 106, 589–597. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Hanafi, M.A.; Chay, S.Y.; Hussin, F.S.; Auwal, S.M.; Zarei, M.; Sarbini, S.R.; Wan Ibadullah, W.Z.; Karim, R.; Saari, N. Multifunctional Hydrolysates from Kenaf (Hibiscus cannabinus L.) Seed Protein with High Antihypertensive Activity in Vitro and in Vivo. J. Food Meas. Charact. 2021, 15, 652–663. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Gonzalez de Mejia, E. Optimization, Identification, and Comparison of Peptides from Germinated Chickpea (Cicer arietinum) Protein Hydrolysates Using Either Papain or Ficin and Their Relationship with Markers of Type 2 Diabetes. Food Chem. 2022, 374, 131717. [Google Scholar] [CrossRef]
- Ramírez-Torres, G.I.; Ontiveros, N.; López-Teros, V.; Suarez-Jiménez, G.M.; Cabrera-Chávez, F. Food Matrices for the Delivery of Antihypertensive Peptides in Functional Foods. Biotecnia 2018, 20, 165–169. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Lan, X.; Sun, L.; Muhammad, Y.; Wang, Z.; Liu, H.; Sun, J.; Zhou, L.; Feng, X.; Liao, D.; Wang, S. Studies on the Interaction between Angiotensin-Converting Enzyme (ACE) and ACE Inhibitory Peptide from Saurida elongata. J. Agric. Food Chem. 2018, 66, 13414–13422. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, L.; Han, X.; Cheng, D. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides from Protease Hydrolysates of Qula Casein: Quantitative Structure-Activity Relationship Modeling and Molecular Docking Study. J. Funct. Foods 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Gao, J.; Gong, H.; Mao, X. Dipeptidyl Peptidase-IV Inhibitory Activity and Related Molecular Mechanism of Bovine α-Lactalbumin-Derived Peptides. Molecules 2020, 25, 3009. [Google Scholar] [CrossRef]
- Valenzuela Zamudio, F.; Hidalgo-Figueroa, S.N.; Ortíz Andrade, R.R.; Hernández Álvarez, A.J.; Segura Campos, M.R. Identification of Antidiabetic Peptides Derived from in Silico Hydrolysis of Three Ancient Grains: Amaranth, Quinoa and Chia. Food Chem. 2022, 394, 133479. [Google Scholar] [CrossRef]
- Gupta, A.; Jacobson, G.A.; Burgess, J.R.; Jelinek, H.F.; Nichols, D.S.; Narkowicz, C.K.; Al-Aubaidy, H.A. Citrus Bioflavonoids Dipeptidyl Peptidase-4 Inhibition Compared with Gliptin Antidiabetic Medications. Biochem. Biophys. Res. Commun. 2018, 503, 21–25. [Google Scholar] [CrossRef]
- Sneha, P.; Doss, C.G.P. Gliptins in Managing Diabetes—Reviewing Computational Strategy. Life Sci. 2016, 166, 108–120. [Google Scholar] [CrossRef]
- Pina, A.S.; Roque, A.C.A. Studies on the Molecular Recognition between Bioactive Peptides and Angiotensin-Converting Enzyme. J. Mol. Recognit. 2009, 22, 162–168. [Google Scholar] [CrossRef]
- Fu, Y.; Alashi, A.M.; Young, J.F.; Therkildsen, M.; Aluko, R.E. Enzyme Inhibition Kinetics and Molecular Interactions of Patatin Peptides with Angiotensin I-Converting Enzyme and Renin. Int. J. Biol. Macromol. 2017, 101, 207–213. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina Sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef]
- Bünning, P.; Riordan, J.F. The Functional Role of Zinc in Angiotensin Converting Enzyme: Implications for the Enzyme Mechanism. J. Inorg. Biochem. 1985, 24, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, H.; Bian, X.; Li, J.; Li, J.; Zhang, H. Insight into the Binding of ACE-Inhibitory Peptides to Angiotensin-Converting Enzyme: A Molecular Simulation. Mol. Simul. 2019, 45, 215–222. [Google Scholar] [CrossRef]
- Ma, M.; Feng, Y.; Miao, Y.; Shen, Q.; Tang, S.; Dong, J.; Zhang, J.Z.H.; Zhang, L. Revealing the Sequence Characteristics and Molecular Mechanisms of ACE Inhibitory Peptides by Comprehensive Characterization of 160,000 Tetrapeptides. Foods 2023, 12, 1573. [Google Scholar] [CrossRef] [PubMed]
- Natesh, R.; Schwager, S.L.U.; Evans, H.R.; Sturrock, E.D.; Acharya, K.R. Structural Details on the Binding of Antihypertensive Drugs Captopril and Enalaprilat to Human Testicular Angiotensin I-Converting Enzyme. Biochemistry 2004, 43, 8718–8724. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. Dipeptidyl Peptidase IV and Its Inhibitors: Therapeutics for Type 2 Diabetes and What Else? J. Med. Chem. 2014, 57, 2197–2212. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Dietary Proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef]
- Mathur, V.; Alam, O.; Siddiqui, N.; Jha, M.; Manaithiya, A.; Bawa, S.; Sharma, N.; Alshehri, S.; Alam, P.; Shakeel, F. Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents. Molecules 2023, 28, 5860. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Dellafiora, L.; Paolella, S.; Galaverna, G.; Cozzini, P.; FitzGerald, R.J. In Silico Approaches Applied to the Study of Peptide Analogs of Ile-Pro-Ile in Relation to Their Dipeptidyl Peptidase IV Inhibitory Properties. Front. Endocrinol. 2018, 9, 329. [Google Scholar] [CrossRef]
- Gonzatti, M.B.; Júnior, J.E.M.; Rocha, A.J.; de Oliveira, J.S.; Evangelista, A.J.d.J.; Fonseca, F.M.P.; Ceccatto, V.M.; de Oliveira, A.C.; da Cruz Freire, J.E. Mechanism of Molecular Interaction of Sitagliptin with Human DPP4 Enzyme—New Insights. Adv. Med. Sci. 2023, 68, 402–408. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. In Silico Approaches to Predict the Potential of Milk Protein-Derived Peptides as Dipeptidyl Peptidase IV (DPP-IV) Inhibitors. Peptides 2014, 57, 43–51. [Google Scholar] [CrossRef]
- Lolok, N.; Ramadhan, D.S.F.; Sumiwi, S.A.; Sahidin, I.; Levita, J. Molecular Docking of β-Sitosterol and Stigmasterol Isolated from Morinda Citrifolia with α-Amylase, α-Glucosidase, Dipeptidylpeptidase-IV, and Peroxisome Proliferator-Activated Receptor-γ. Rasayan J. Chem. 2022, 15, 20. [Google Scholar] [CrossRef]
- You, H.; Wu, T.; Wang, W.; Li, Y.; Liu, X.; Ding, L. Preparation and Identification of Dipeptidyl Peptidase IV Inhibitory Peptides from Quinoa Protein. Food Res. Int. 2022, 156, 111176. [Google Scholar] [CrossRef] [PubMed]
- Matkawala, F.; Nighojkar, S.; Nighojkar, A. Next-Generation Nutraceuticals: Bioactive Peptides from Plant Proteases. BioTechnologia 2022, 103, 397–408. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
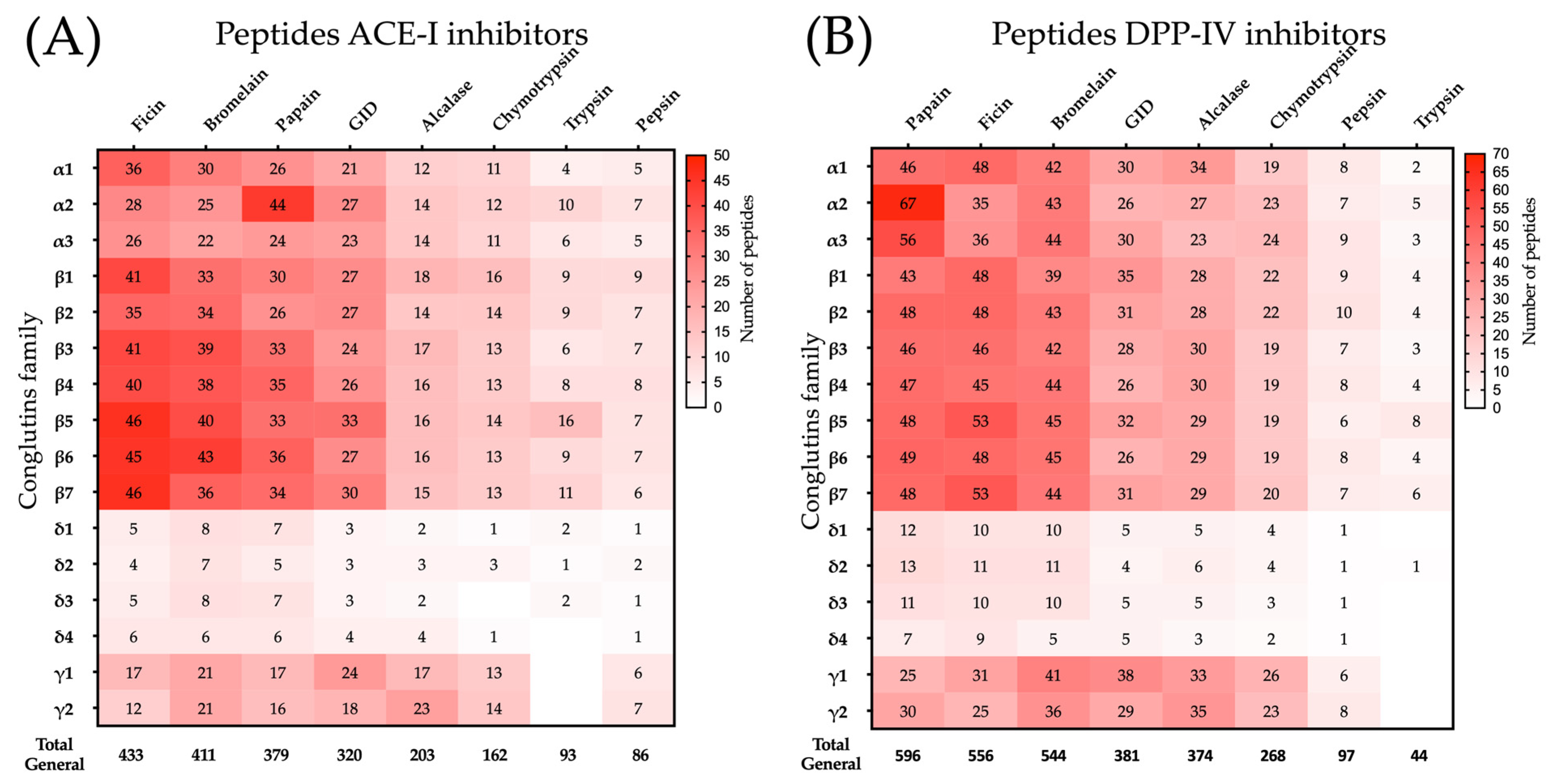
| Bioactivity | Lupin Conglutin Families | Total General | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1 | α2 | α3 | β1 | β2 | β3 | β4 | β5 | β6 | β7 | δ1 | δ2 | δ3 | δ4 | γ1 | γ2 | ||
| DPP-IV inhibitor | 148 | 147 | 158 | 155 | 151 | 150 | 151 | 149 | 149 | 149 | 55 | 109 | 55 | 40 | 149 | 147 | 2062 |
| ACE-I inhibitor | 132 | 116 | 112 | 122 | 119 | 116 | 117 | 117 | 117 | 118 | 37 | 72 | 37 | 33 | 92 | 101 | 1558 |
| DPP III inhibitor | 21 | 23 | 19 | 24 | 23 | 21 | 21 | 23 | 22 | 22 | 7 | 11 | 7 | 2 | 21 | 20 | 287 |
| Antioxidative | 26 | 20 | 23 | 21 | 17 | 20 | 19 | 19 | 19 | 17 | 5 | 11 | 6 | 3 | 21 | 21 | 268 |
| Stimulating | 11 | 12 | 9 | 12 | 12 | 11 | 11 | 11 | 11 | 11 | 7 | 14 | 7 | 5 | 10 | 8 | 162 |
| Renin inhibitor | 12 | 8 | 8 | 11 | 11 | 9 | 9 | 9 | 10 | 10 | 1 | 2 | 1 | 6 | 7 | 114 | |
| Alpha-glucosidase inhibitor | 3 | 5 | 6 | 5 | 4 | 4 | 4 | 6 | 4 | 5 | 1 | 1 | 1 | 5 | 54 | ||
| Regulating | 3 | 4 | 2 | 3 | 4 | 4 | 4 | 5 | 4 | 4 | 1 | 2 | 1 | 3 | 3 | 47 | |
| Antithrombotic | 5 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 4 | 2 | 2 | 2 | 40 | |
| Anti-inflammatory | 2 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | 2 | 36 | |||
| Neuropeptide | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 33 | |||
| CaMPDE inhibitor | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | 31 | ||||
| Activating ubiquitin-mediated proteolysis | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 3 | 3 | 28 |
| Anti-amnestic | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 3 | 2 | 28 | |
| Hypocholesterolemic | 2 | 3 | 1 | 3 | 1 | 1 | 1 | 12 | |||||||||
| Hypolipidemic | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | |||||
| Immunomodulating | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ||||||
| HMG-CoA reductase inhibitor | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||||||||
| Hypotensive | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||||||||||
| Anticancer | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||
| Bacterial permease ligand | 1 | 1 | 1 | 1 | 4 | ||||||||||||
| Immunostimulating | 1 | 1 | 1 | 3 | |||||||||||||
| Antibacterial | 1 | 1 | 2 | ||||||||||||||
| Embryotoxic | 1 | 1 | 2 | ||||||||||||||
| Opioid | 1 | 1 | |||||||||||||||
| Total general | 376 | 358 | 356 | 379 | 362 | 355 | 353 | 359 | 353 | 356 | 118 | 231 | 121 | 88 | 317 | 331 | 4813 |
| Parameters | General (n = 201) | DPP-IV (n = 151) | ACE-I (n = 110) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| HIA | 157 | 78.10 | 123 | 81.46 | 83 | 75.45 |
| F20% | 176 | 87.56 | 138 | 91.39 | 95 | 86.36 |
| F30% | 164 | 81.59 | 128 | 84.77 | 90 | 81.82 |
| VD | 201 | 100.00 | 151 | 100.00 | 110 | 100.00 |
| T ½ | 156 | 77.61 | 111 | 73.51 | 95 | 86.36 |
| ROAT | 184 | 91.56 | 139 | 92.05 | 100 | 90.91 |
| LIPINSKI | 191 | 95.02 | 104 | 68.87 | 104 | 94.55 |
| GDS | 179 | 89.05 | 97 | 64.23 | 82 | 74.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arámburo-Gálvez, J.G.; Tinoco-Narez-Gil, R.; Mora-Melgem, J.A.; Sánchez-Cárdenas, C.A.; Gracia-Valenzuela, M.H.; Flores-Mendoza, L.K.; Figueroa-Salcido, O.G.; Ontiveros, N. In Silico Hydrolysis of Lupin (Lupinus angustifolius L.) Conglutins with Plant Proteases Releases Antihypertensive and Antidiabetic Peptides That Are Bioavailable, Non-Toxic, and Gastrointestinal Digestion Stable. Int. J. Mol. Sci. 2024, 25, 12866. https://doi.org/10.3390/ijms252312866
Arámburo-Gálvez JG, Tinoco-Narez-Gil R, Mora-Melgem JA, Sánchez-Cárdenas CA, Gracia-Valenzuela MH, Flores-Mendoza LK, Figueroa-Salcido OG, Ontiveros N. In Silico Hydrolysis of Lupin (Lupinus angustifolius L.) Conglutins with Plant Proteases Releases Antihypertensive and Antidiabetic Peptides That Are Bioavailable, Non-Toxic, and Gastrointestinal Digestion Stable. International Journal of Molecular Sciences. 2024; 25(23):12866. https://doi.org/10.3390/ijms252312866
Chicago/Turabian StyleArámburo-Gálvez, Jesús Gilberto, Raúl Tinoco-Narez-Gil, José Antonio Mora-Melgem, Cesar Antonio Sánchez-Cárdenas, Martina Hilda Gracia-Valenzuela, Lilian Karem Flores-Mendoza, Oscar Gerardo Figueroa-Salcido, and Noé Ontiveros. 2024. "In Silico Hydrolysis of Lupin (Lupinus angustifolius L.) Conglutins with Plant Proteases Releases Antihypertensive and Antidiabetic Peptides That Are Bioavailable, Non-Toxic, and Gastrointestinal Digestion Stable" International Journal of Molecular Sciences 25, no. 23: 12866. https://doi.org/10.3390/ijms252312866
APA StyleArámburo-Gálvez, J. G., Tinoco-Narez-Gil, R., Mora-Melgem, J. A., Sánchez-Cárdenas, C. A., Gracia-Valenzuela, M. H., Flores-Mendoza, L. K., Figueroa-Salcido, O. G., & Ontiveros, N. (2024). In Silico Hydrolysis of Lupin (Lupinus angustifolius L.) Conglutins with Plant Proteases Releases Antihypertensive and Antidiabetic Peptides That Are Bioavailable, Non-Toxic, and Gastrointestinal Digestion Stable. International Journal of Molecular Sciences, 25(23), 12866. https://doi.org/10.3390/ijms252312866









