Maternal Low-Protein Diet Leads to Mitochondrial Dysfunction and Impaired Energy Metabolism in the Skeletal Muscle of Male Rats
Abstract
1. Introduction
2. Results
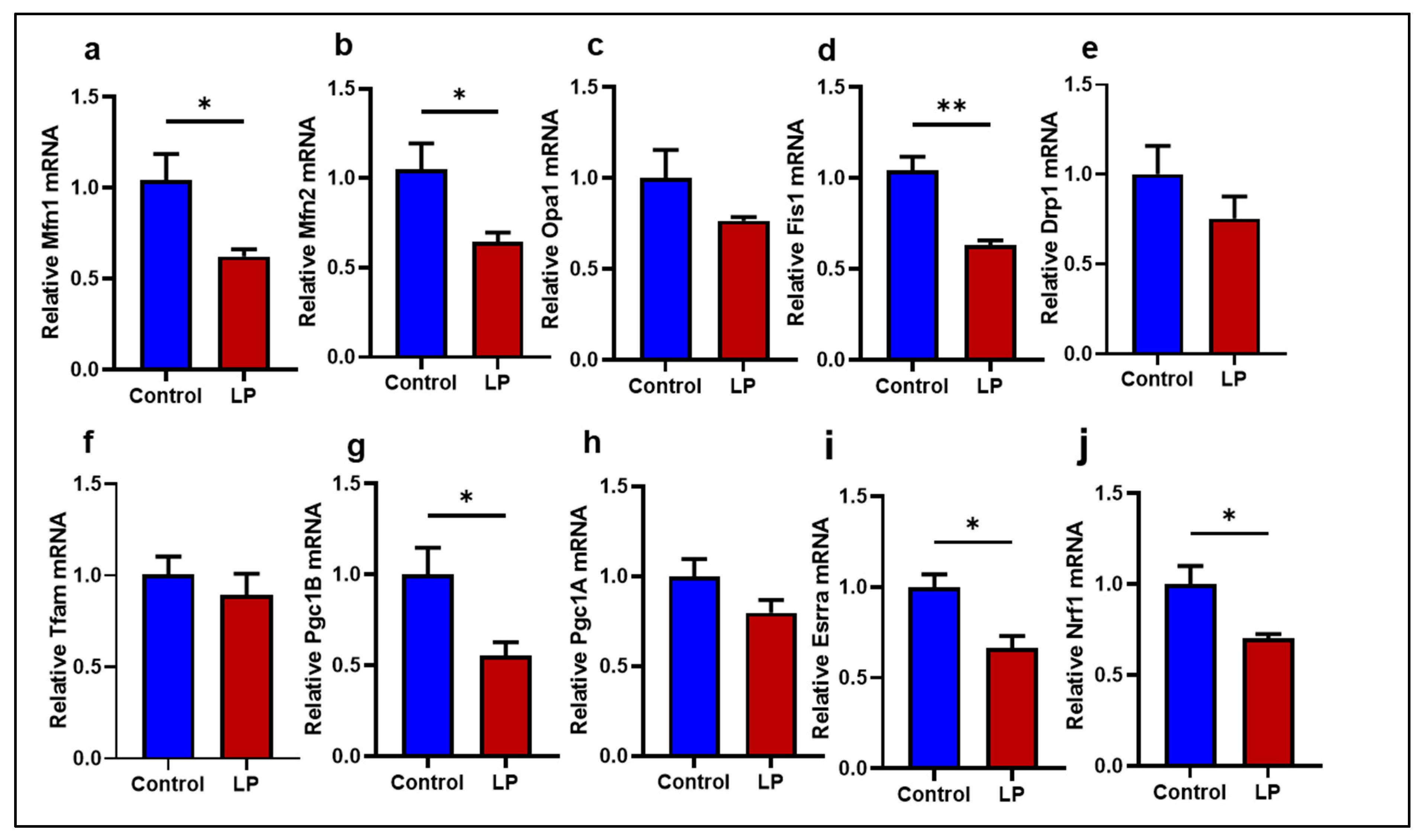
2.1. LP Programming Lowered the Expression of Mitochondrial Dynamics and Biogenesis Genes in the GS Muscle
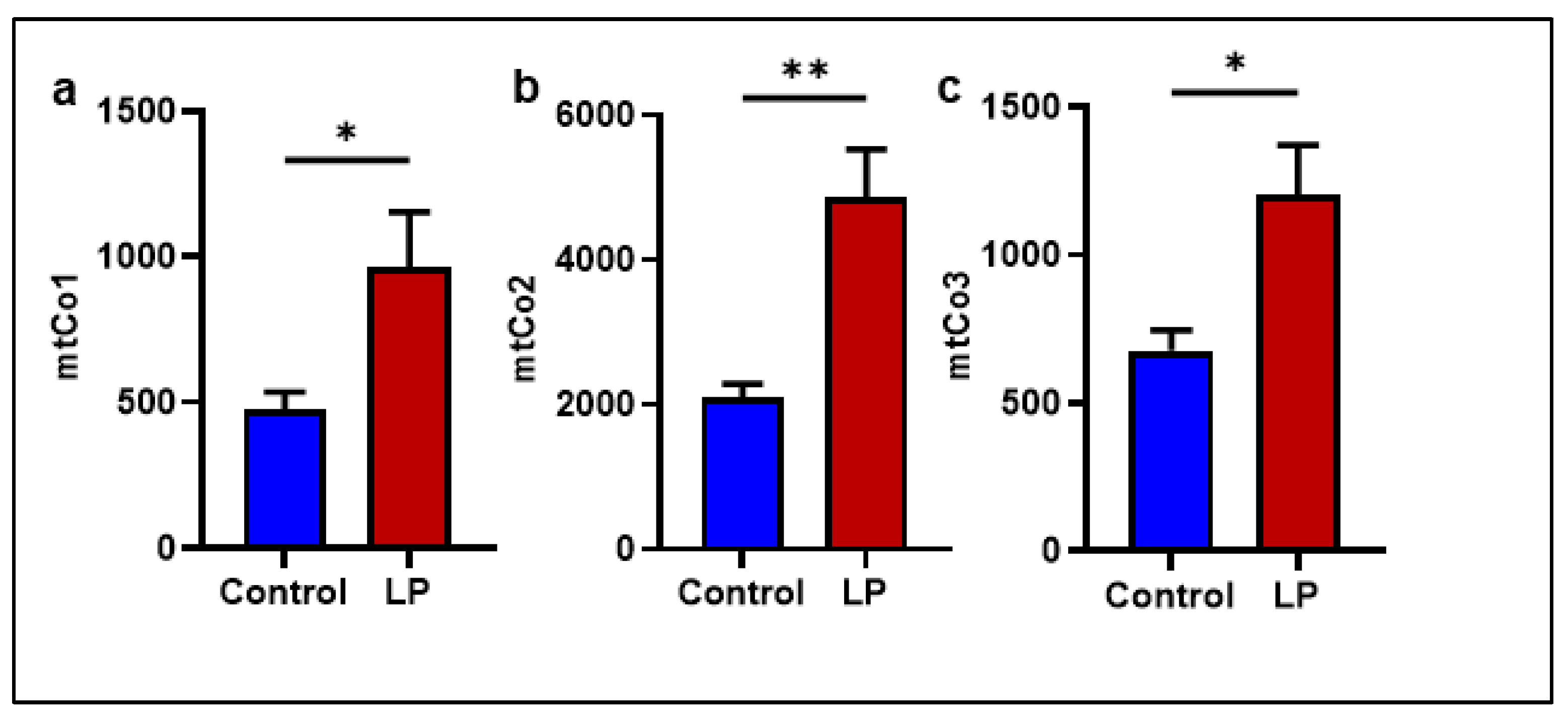
2.2. The LP Programming Increased the mtDNA Copy Number in the GS Muscle
2.3. LP Diet-Induced Fetal Programming Leads to Reduced OCR in the Flexor Digitorum Brevis Muscle
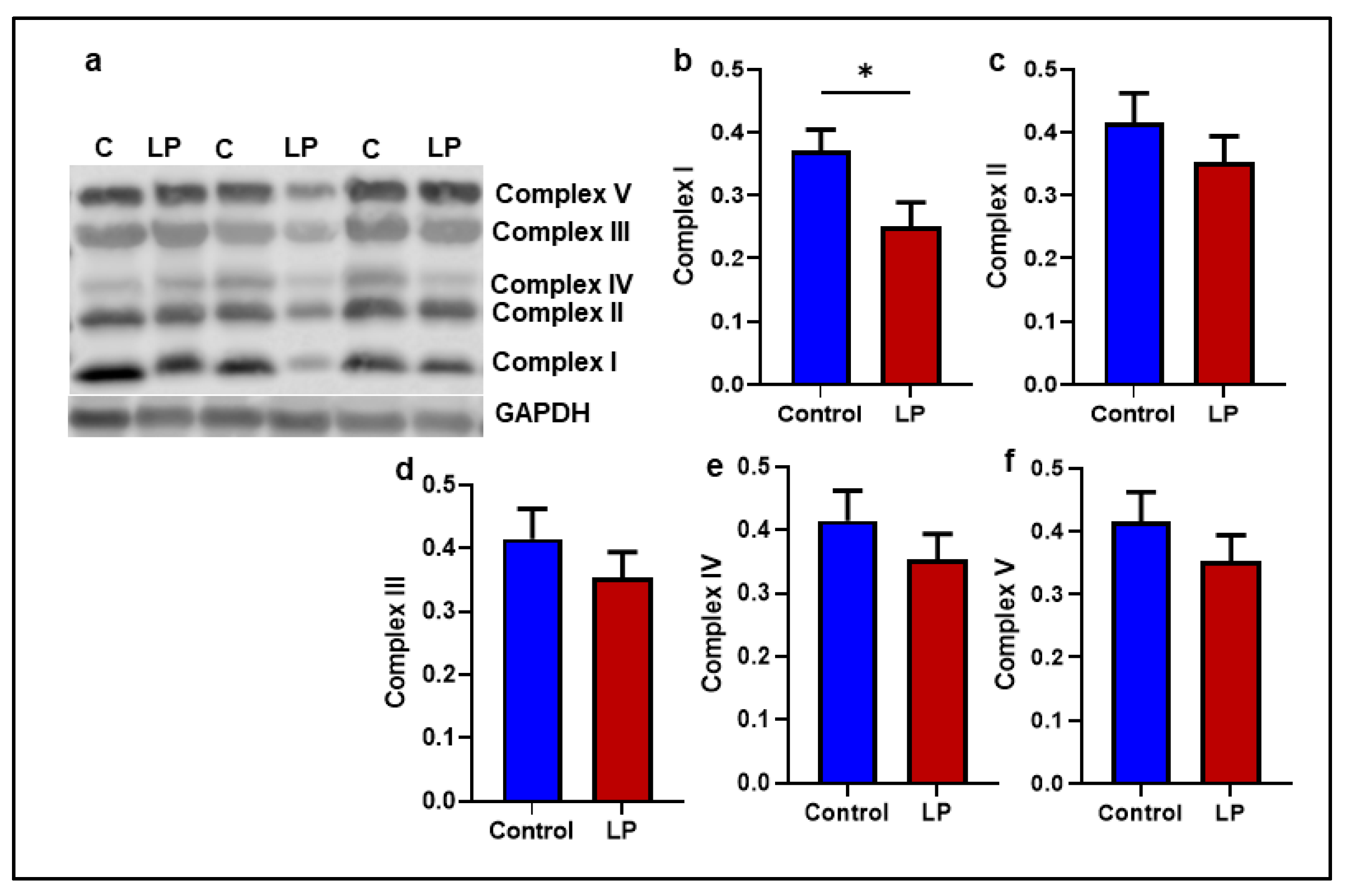
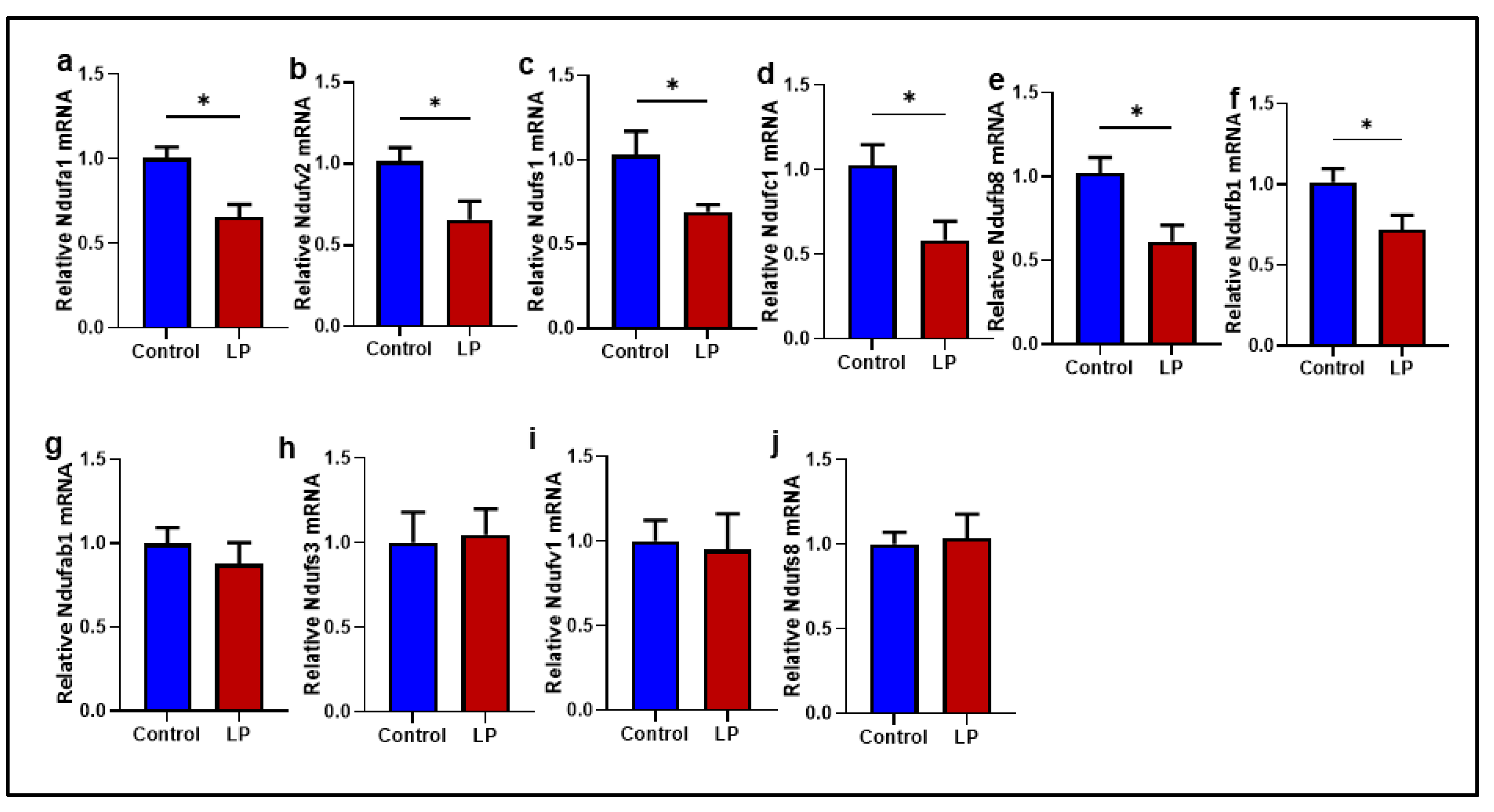
2.4. Mitochondrial Complex I Genes Are Downregulated in LP GS Muscles
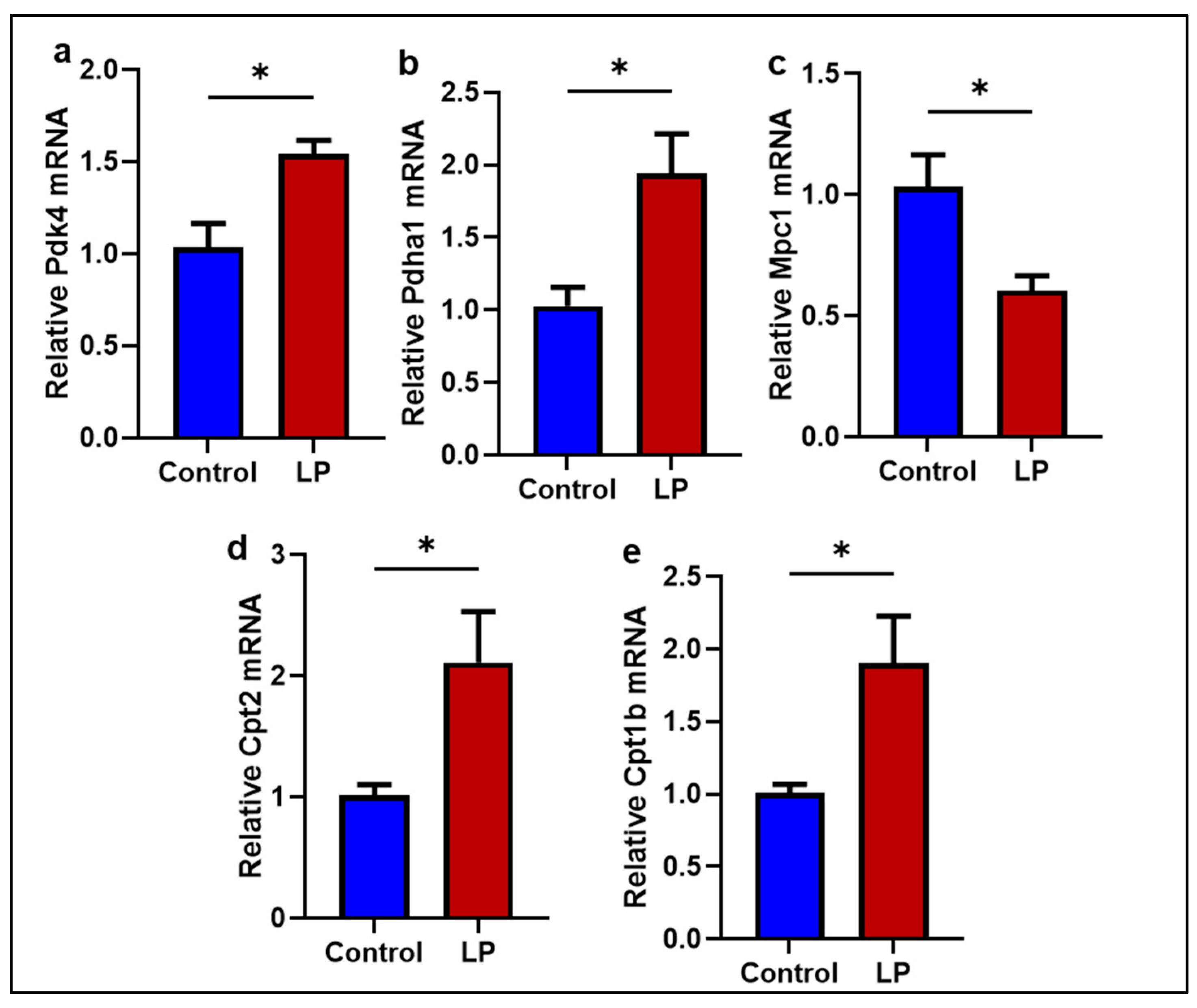
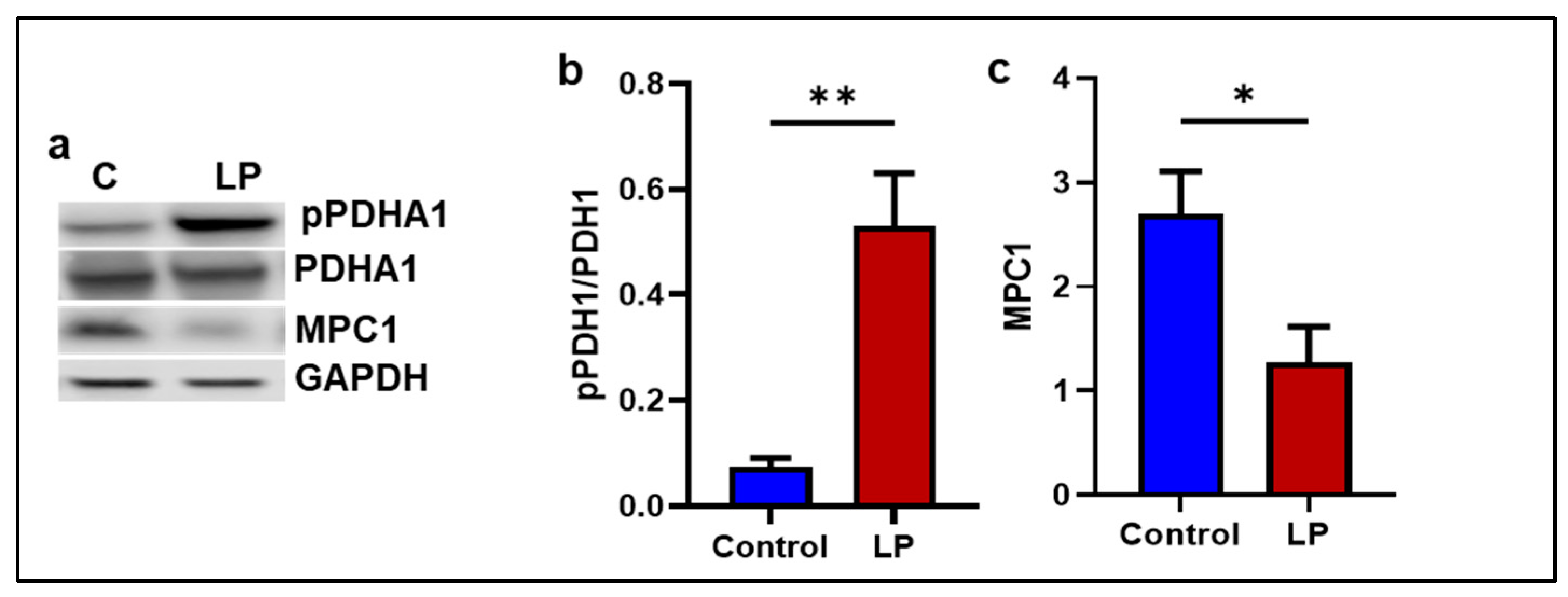
2.5. The LP Diet Altered the Genes Associated with Mitochondrial Substrate Transport and Oxidation in the GS Muscle
2.6. LP Diet Did Not Alter the Mitochondrial Morphology in the GS Muscle of the Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Transmission Electron Microscopy (TEM)
4.3. Mitochondrial DNA Copy Number
4.4. Quantitative Real-Time (qRT)-PCR
4.5. Mitochondrial Oxygen Consumption
4.6. Western Blot
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance. BMJ 1991, 303, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Stroud, D.A.; Surgenor, E.E.; Formosa, L.E.; Reljic, B.; Frazier, A.E.; Dibley, M.G.; Osellame, L.D.; Stait, T.; Beilharz, T.H.; Thorburn, D.R.; et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial disorders of the OXPHOS system. FEBS Lett. 2021, 595, 1062–1106. [Google Scholar] [CrossRef]
- Gao, A.W.; Canto, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Befroy, D.E.; Petersen, K.F.; Dufour, S.; Mason, G.F.; de Graaf, R.A.; Rothman, D.L.; Shulman, G.I. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007, 56, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819888824. [Google Scholar] [CrossRef] [PubMed]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mohlig, M.; Isken, F.; Ristow, M. Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 2419–2421. [Google Scholar] [PubMed]
- Schoenmann, N.; Tannenbaum, N.; Hodgeman, R.M.; Raju, R.P. Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166769. [Google Scholar] [CrossRef]
- Vanderperre, B.; Bender, T.; Kunji, E.R.; Martinou, J.-C. Mitochondrial pyruvate import and its effects on homeostasis. Curr. Opin. Cell Biol. 2015, 33, 35–41. [Google Scholar] [CrossRef]
- Zheng, H.; Li, Q.; Li, S.; Li, Z.; Brotto, M.; Weiss, D.; Prosdocimo, D.; Xu, C.; Reddy, A.; Puchowicz, M.; et al. Loss of Ptpmt1 limits mitochondrial utilization of carbohydrates and leads to muscle atrophy and heart failure in tissue-specific knockout mice. Elife 2023, 12, RP86944. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Akyol, A.; Cetin, A.K.; Gulec, A.; Dasgin, H.; Ayaz, A.; Onbasilar, I. Maternal low-quality protein diet exerts sex-specific effects on plasma amino acid profile and alters hepatic expression of methyltransferases in adult rat offspring. J. Dev. Orig. Health Dis. 2018, 9, 409–416. [Google Scholar] [CrossRef]
- Almeida, D.L.; Simoes, F.S.; Saavedra, L.P.J.; Moraes, A.M.P.; Matiusso, C.C.I.; Malta, A.; Palma-Rigo, K.; Mathias, P.C.d.F. Maternal low-protein diet during lactation combined with early overfeeding impair male offspring’s long-term glucose homeostasis. Endocrine 2019, 63, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, J.; Chen, H.; Pan, Y.-X. A maternal low-protein diet during gestation induces hepatic autophagy-related gene expression in a sex-specific manner in Sprague-Dawley rats. Br. J. Nutr. 2022, 128, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Gazzard, S.E.; McEwen, L.A.C.; Bertram, J.F.; Hutt, K.J. Maternal low-protein diet programmes low ovarian reserve in offspring. Reproduction 2018, 156, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Wang, T.; Yu, M.; Xu, J. Maternal Low-Protein Diet Modulates Glucose Metabolism and Hepatic MicroRNAs Expression in the Early Life of Offspring dagger. Nutrients 2017, 9, 205. [Google Scholar] [CrossRef]
- Serpente, P.; Zhang, Y.; Islimye, E.; Hart-Johnson, S.; Gould, A.P. Quantification of fetal organ sparing in maternal low-protein dietary models. Wellcome Open Res. 2021, 6, 218. [Google Scholar] [CrossRef]
- Blesson, C.S.; Schutt, A.K.; Balakrishnan, M.P.; Pautler, R.G.; Pedersen, S.E.; Sarkar, P.; Gonzales, D.; Zhu, G.; Marini, J.C.; Chacko, S.K.; et al. Novel lean type 2 diabetic rat model using gestational low-protein programming. Am. J. Obstet. Gynecol. 2016, 214, 540.e1–540.e7. [Google Scholar] [CrossRef]
- Blesson, C.S.; Chinnathambi, V.; Kumar, S.; Yallampalli, C. Gestational Protein Restriction Impairs Glucose Disposal in the Gastrocnemius Muscles of Female Rats. Endocrinology 2017, 158, 756–767. [Google Scholar] [CrossRef][Green Version]
- Blesson, C.S.; Sathishkumar, K.; Chinnathambi, V.; Yallampalli, C. Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology 2014, 155, 3036–3046. [Google Scholar] [CrossRef]
- Vidyadharan, V.A.; Betancourt, A.; Smith, C.; Yallampalli, C.; Blesson, C.S. Prenatal Low-Protein Diet Affects Mitochondrial Structure and Function in the Skeletal Muscle of Adult Female Offspring. Nutrients 2022, 14, 1158. [Google Scholar] [CrossRef]
- Vipin, V.A.; Blesson, C.S.; Yallampalli, C. Maternal low protein diet and fetal programming of lean type 2 diabetes. World J. Diabetes 2022, 13, 185–202. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [PubMed]
- Houzelle, A.; Jörgensen, J.A.; Schaart, G.; Daemen, S.; van Polanen, N.; Fealy, C.E.; Hesselink, M.K.C.; Schrauwen, P.; Hoeks, J. Human skeletal muscle mitochondrial dynamics in relation to oxidative capacity and insulin sensitivity. Diabetologia 2021, 64, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef]
- Fealy, C.E.; Mulya, A.; Axelrod, C.L.; Kirwan, J.P. Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl. Res. 2018, 202, 69–82. [Google Scholar] [CrossRef]
- Hernandez-Alvarez, M.I.; Thabit, H.; Burns, N.; Shah, S.; Brema, I.; Hatunic, M.; Finucane, F.; Liesa, M.; Chiellini, C.; Naon, D.; et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 2010, 33, 645–651. [Google Scholar] [CrossRef]
- Hoeks, J.; Schrauwen, P. Muscle mitochondria and insulin resistance: A human perspective. Trends Endocrinol. Metab. 2012, 23, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Song, G.; Wang, C.; Ma, H.; Ren, L.; Nie, Q.; Zhang, X.; Gan, K. Overexpression of mitofusin 2 improves translocation of glucose transporter 4 in skeletal muscle of highfat dietfed rats through AMPactivated protein kinase signaling. Mol. Med. Rep. 2013, 8, 205–210. [Google Scholar] [CrossRef]
- Yu, R.; Jin, S.; Lendahl, U.; Nistér, M.; Zhao, J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 2019, 38, e99748. [Google Scholar] [CrossRef]
- Noone, J.; O’Gorman, D.J.; Kenny, H.C. OPA1 regulation of mitochondrial dynamics in skeletal and cardiac muscle. Trends Endocrinol. Metab. 2022, 33, 710–721. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ide, T.; Fujino, T.; Arai, S.; Saku, K.; Kakino, T.; Tyynismaa, H.; Yamasaki, T.; Yamada, K.-I.; Kang, D.; et al. Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS ONE 2015, 10, e0119687. [Google Scholar] [CrossRef]
- Otten, A.B.C.; Kamps, R.; Lindsey, P.; Gerards, M.; Pendeville-Samain, H.; Muller, M.; van Tienen, F.H.J.; Smeets, H.J.M. Tfam Knockdown Results in Reduction of mtDNA Copy Number, OXPHOS Deficiency and Abnormalities in Zebrafish Embryos. Front. Cell Dev. Biol. 2020, 8, 381. [Google Scholar] [CrossRef]
- Knorre, D.A. Intracellular quality control of mitochondrial DNA: Evidence and limitations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190176. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lu, C.Y.; Chi, C.W.; Wei, Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348 Pt 2, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Van Huynh, T.; Rethi, L.; Rethi, L.; Chen, C.H.; Chen, Y.J.; Kao, Y.H. The Complex Interplay between Imbalanced Mitochondrial Dynamics and Metabolic Disorders in Type 2 Diabetes. Cells 2023, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 2019, 21, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Banh, R.S.; Iorio, C.; Marcotte, R.; Xu, Y.; Cojocari, D.; Rahman, A.A.; Pawling, J.; Zhang, W.; Sinha, A.; Rose, C.M.; et al. PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia. Nat. Cell Biol. 2016, 18, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Phielix, E.; Schrauwen-Hinderling, V.B.; Mensink, M.; Lenaers, E.; Meex, R.; Hoeks, J.; Kooi, M.E.; Moonen-Kornips, E.; Sels, J.-P.; Hesselink, M.K.; et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 2008, 57, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Toledo, F.G.; Menshikova, E.V.; Ritov, V.B.; Azuma, K.; Radikova, Z.; DeLany, J.; Kelley, D.E. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 2007, 56, 2142–2147. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Blesson, C.S.; Schutt, A.; Chacko, S.; Marini, J.C.; Mathew, P.R.; Tanchico, D.; Balakrishnan, M.; Yallampalli, C. Sex Dependent Dysregulation of Hepatic Glucose Production in Lean Type 2 Diabetic Rats. Front. Endocrinol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Distelmaier, F.; Koopman, W.J.; Heuvel, L.P.v.D.; Rodenburg, R.J.; Mayatepek, E.; Willems, P.H.; Smeitink, J.A. Mitochondrial complex I deficiency: From organelle dysfunction to clinical disease. Brain 2009, 132 Pt 4, 833–842. [Google Scholar] [CrossRef]
- Moran, M.; Rivera, H.; Sánchez-Aragó, M.; Blázquez, A.; Merinero, B.; Ugalde, C.; Arenas, J.; Cuezva, J.; Martín, M. Mitochondrial bioenergetics and dynamics interplay in complex I-deficient fibroblasts. Biochim. Biophys. Acta 2010, 1802, 443–453. [Google Scholar] [CrossRef]
- Yamada, T.; Ivarsson, N.; Hernández, A.; Fahlström, A.; Cheng, A.J.; Zhang, S.; Bruton, J.D.; Ulfhake, B.; Westerblad, H. Impaired mitochondrial respiration and decreased fatigue resistance followed by severe muscle weakness in skeletal muscle of mitochondrial DNA mutator mice. J. Physiol. 2012, 590, 6187–6197. [Google Scholar] [CrossRef]
- Choksi, K.B.; Nuss, J.E.; Boylston, W.H.; Rabek, J.P.; Papaconstantinou, J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 2007, 43, 1423–1438. [Google Scholar] [CrossRef]
- Choksi, K.B.; Nuss, J.E.; DeFord, J.H.; Papaconstantinou, J. Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 2008, 45, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Ritov, V.B.; Menshikova, E.V.; Azuma, K.; Wood, R.; Toledo, F.G.S.; Goodpaster, B.H.; Ruderman, N.B.; Kelley, D.E. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E49–E58. [Google Scholar] [CrossRef]
- Vidyadharan, V.A.; Blesson, C.S.; Tanchico, D.; Betancourt, A.; Smith, C.; Yallampalli, C. Low Protein Programming Causes Increased Mitochondrial Fusion and Decreased Oxygen Consumption in the Hepatocytes of Female Rats. Nutrients 2023, 15, 1568. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, O.H.; Olsen, H.L.; Frandsen, L.; Nielsen, P.; Nielsen, F.C.; Grunnet, N.; Quistorff, B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J. Biomed. Sci. 2010, 17 (Suppl. 1), S38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antoun, G.; McMurray, F.; Thrush, A.B.; Patten, D.A.; Peixoto, A.C.; Slack, R.S.; McPherson, R.; Dent, R.; Harper, M.-E. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 2015, 58, 2861–2866. [Google Scholar] [CrossRef]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Biophys. Acta 2000, 1486, 1–17. [Google Scholar] [CrossRef]
- Ngo, J.; Choi, D.W.; Stanley, I.A.; Stiles, L.; Molina, A.J.; Chen, P.H.; Lako, A.; Sung, I.C.H.; Goswami, R.; Kim, M.-Y.; et al. Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 2023, 42, e111901. [Google Scholar] [CrossRef]
- Yiew, N.K.H.; Finck, B.N. The mitochondrial pyruvate carrier at the crossroads of intermediary metabolism. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E33–E52. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.; Chanda, D.; Thoudam, T.; Kang, H.-J.; Harris, R.A.; Lee, I.-K. The Role of Pyruvate Metabolism in Mitochondrial Quality Control and Inflammation. Mol. Cells 2023, 46, 259–267. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Kuang, F.; Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Xie, Y.; Tang, D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021, 34, 108767. [Google Scholar] [CrossRef]
- Sugden, M.C.; Holness, M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E855–E862. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Buzzigoli, G.; Bonadonna, R.; Brandi, L.S.; Oleggini, M.; Boni, C.; Geloni, M.; Ferrannini, E. Operation of Randle’s cycle in patients with NIDDM. Diabetes 1990, 39, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef]
- Chappell, N.R.; Zhou, B.; Schutt, A.K.; E Gibbons, W.; Blesson, C.S. Prenatal androgen induced lean PCOS impairs mitochondria and mRNA profiles in oocytes. Endocr. Connect. 2020, 9, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.A.; Jackson, K.C.; Khairallah, R.J.; Ward, C.W.; Spangenburg, E.E. Measuring mitochondrial respiration in intact single muscle fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R712–R719. [Google Scholar] [CrossRef]
- Blesson, C.S.; Schutt, A.K.; Vipin, V.A.; Tanchico, D.T.; Mathew, P.R.; Balakrishnan, M.; Betancourt, A.; Yallampalli, C. In utero low-protein-diet-programmed type 2 diabetes in adult offspring is mediated by sex hormones in ratsdagger. Biol. Reprod. 2020, 103, 1110–1120. [Google Scholar] [CrossRef]



| Gene | Accession NO: | Primers F = Forward; R = Reverse | Amplicon Size (bp) |
|---|---|---|---|
| mtCox1 | MW209726.1 | F: 5′-ATCGCAATTCCTACAGGCGT-3′ R: 5′-TGTTAGGCCCCCTACTGTGA-3′ | 129 |
| mtCox2 | MW209726.1 | F: 5′-CAAGACGCCACATCACCTATC-3′ R: 5′-TTGGGCGTCTATTGTGCTTG-3′ | 150 |
| mtCox3 | MW209726.1 | F: 5′-GGAACATACCAAGGCCACCA-3′ R: 5′-TCGTGGGTAGGAACTAGGCT-3′ | 140 |
| Esrra | NM_001008511.2 | F: 5′-AAAGTCCTGGCCCATTTCTATG-3′ R: 5′-CCCTTGCCTCAGTCCATCAT-3′ | 101 |
| Drp1 | NM_053655.3 | F: 5′-CTGGTCCACGTTTCACCAGA-3′ R: 5′-CCCATTCTTCTGCTTCAACTCC-3′ | 73 |
| Tfam | NM_031326.2 | F: 5′-TCGCCTGTCAGCCTTATCTG-3′ R: 5′-TTACATCTGGGTGTTTAGCTT-3′ | 133 |
| Cyclophilin A | XM_006250801.5 | F: 5′-TATCTGCACTGCCAAGACTGAGTG-3′ R: 5′-CTTCTTGCTGGTCTTGCCATTCC-3′ | 127 |
| Fis1 | NM_001105919. | F: 5′-GTGCCTGGTTCGAAGCAAATA-3′ R: 5′-CATATTCCCGCTGCTCCTCTT-3′ | 101 |
| Mfn1 | NM_138976.2 | F: 5′-ATCTTCGGCCAGTTACTGGAGTT-3′ R: 5′-AGATCATCCTCGGTTGCTATCC-3′ | 101 |
| Mfn2 | NM_001429969.1 | F: 5′-CCTTGAAGACACCCACAGGAATA-3′ R: 5′-CGCTGATTCCCCTGACCTT-3′ | 101 |
| Nrf1 | NM_001100708.1 | F: 5′-CTCTGCATCTCACCCTCCAAAC-3′ R: 5′-TCTTCCAGGATCATGCTCTTGTAC-3′ | 101 |
| OPA1 | NM_133585.3 | F: 5′-AAAAGCCCTTCCCAGTTCAGA-3′ R: 5′-TACCCGCAGTGAAGAAATCCTT-3′ | 101 |
| Pgc1a | NM_031347.1 | F: 5′-CTACAATGAATGCAGCGGTCTT-3′ R: 5′-TGCTCCATGAATTCTCGGTCTT-3′ | 101 |
| Pgc1b | NM_176075.3 | F: 5′-TCGGTGAAGGTCGTGTGGTATAC-3′ R: 5′-GCACTCGACTATCTCACCAAACA-3′ | 101 |
| Beta actin | V01217.1 | F: 5′-CCACCATGTACCCAGGCATT-3′ R: 5′-GCTGACCACACCCCACTATG-3′ | 119 |
| Tuba1a | XM_063263380.1 | F: 5′-ATGGTCTTGTCGCTTGGCAT-3′ R: 5′-CCCCTTTCCACAGCGTGAGT-3′ | 135 |
| Ndufa1 | NM_001108813.2 | F: 5′-GGGGGCAAGGAAAAGAGAGT-3′ R: 5′-CAGAGATGCGTCTATCGCGT-3′ | 73 |
| Ndufv2 | NM_031064.2 | F: 5′-AGCCAGTTGGGAAGTACCAC-3′ R: 5′-CCCAGCTTTCTCTGAAGGGT-3′ | 97 |
| Ndufs1 | NM_001005550.1 | F: 5′-CCAAGTGTGTCAAAGCCGTC-3′ R: 5′-TGTCCGTAGCAAAACAGGGT-3′ | 96 |
| Ndufc1 | NM_001399603.1 | F: 5′-GTACTGCGCTCGTTTTCGC-3′ R: 5′-GTTTGGCATTGACTGGCTCC-3′ | 100 |
| Ndufb8 | NM_001106360.3 | F: 5′-AGGCGGTGATCCTTCCAAAG-3′ R: 5′-GAGTCCCATTCAGAGGGCAC-3′ | 91 |
| NdufAb1 | NM_001106294.1 | F: 5′-GGCTGCTGACTGGAACTTACT-3′ R: 5′-TTTGGGGCCAAATCTTCAGC-3′ | 100 |
| Ndufb1 | NM_001402546.1 | F: 5′-CCTATGGGATTCGCCTTTGGA-3′ R: 5′-TTATTCCGGAAGGCAGTGAGC-3′ | 71 |
| Ndufs3 | NM_001106489.1 | F: 5′-ATTTCCACTTCCGGTCCGTG-3′ R: 5′-CATGTTCCTTAGGGTGCCGA-3′ | 83 |
| Ndufv1 | NM_001006972.1 | F: 5′-ACCTCATTTGGCTCGCTGAA-3′ R: 5′-CCTTCAGCCTCCAGTCATGG-3′ | 76 |
| Ndufs8 | NM_001106322.2 | F: 5′-GAGCCGCTGCACTTCAAGAT-3′ R: 5′-GGCCATTAAGATGTCCTGTGC-3′ | 91 |
| Pdk4 | NM_053551.2 | F: 5′-AGCAGTAGTCGAAGATGCCT-3′ R: 5′-CACGATGTGGATTGGTTGGC-3′ | 124 |
| Pdha1 | NM_001004072.2 | F: 5′-GCAGCCAGCACGGATTACTA-3′ R: 5′-TCAGGATAGGCCCCTTACCA-3′ | 136 |
| Mpc1 | NM_133561.2 | F: 5′-CGCAAAGCAGCGGACTATGT-3′ R: 5′-GGGCCCCAGAAGTGCGTA-3′ | 71 |
| Cpt2 | NM_001429335.1 | F: 5′-CTAAGAGATGCTCCGAGGCG-3′ R: 5′-GGTCAGCTGGCCATGGTATT-3′ | 104 |
| Cpt1b | NM_013200.2 | F: 5′-CGAGTTCAGAAACGAACGCC-3′ R: 5′-TGGTGTGTCTCCTGGTCTCA-3′ | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidyadharan, V.A.; Betancourt, A.; Smith, C.; Blesson, C.S.; Yallampalli, C. Maternal Low-Protein Diet Leads to Mitochondrial Dysfunction and Impaired Energy Metabolism in the Skeletal Muscle of Male Rats. Int. J. Mol. Sci. 2024, 25, 12860. https://doi.org/10.3390/ijms252312860
Vidyadharan VA, Betancourt A, Smith C, Blesson CS, Yallampalli C. Maternal Low-Protein Diet Leads to Mitochondrial Dysfunction and Impaired Energy Metabolism in the Skeletal Muscle of Male Rats. International Journal of Molecular Sciences. 2024; 25(23):12860. https://doi.org/10.3390/ijms252312860
Chicago/Turabian StyleVidyadharan, Vipin A., Ancizar Betancourt, Craig Smith, Chellakkan S. Blesson, and Chandra Yallampalli. 2024. "Maternal Low-Protein Diet Leads to Mitochondrial Dysfunction and Impaired Energy Metabolism in the Skeletal Muscle of Male Rats" International Journal of Molecular Sciences 25, no. 23: 12860. https://doi.org/10.3390/ijms252312860
APA StyleVidyadharan, V. A., Betancourt, A., Smith, C., Blesson, C. S., & Yallampalli, C. (2024). Maternal Low-Protein Diet Leads to Mitochondrial Dysfunction and Impaired Energy Metabolism in the Skeletal Muscle of Male Rats. International Journal of Molecular Sciences, 25(23), 12860. https://doi.org/10.3390/ijms252312860





