Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality
Abstract
1. Introduction
2. Results
2.1. Comparison of Baseline Clinical and Laboratory Characteristics Between Patients with End-Stage Liver Disease and PREVEND Participants
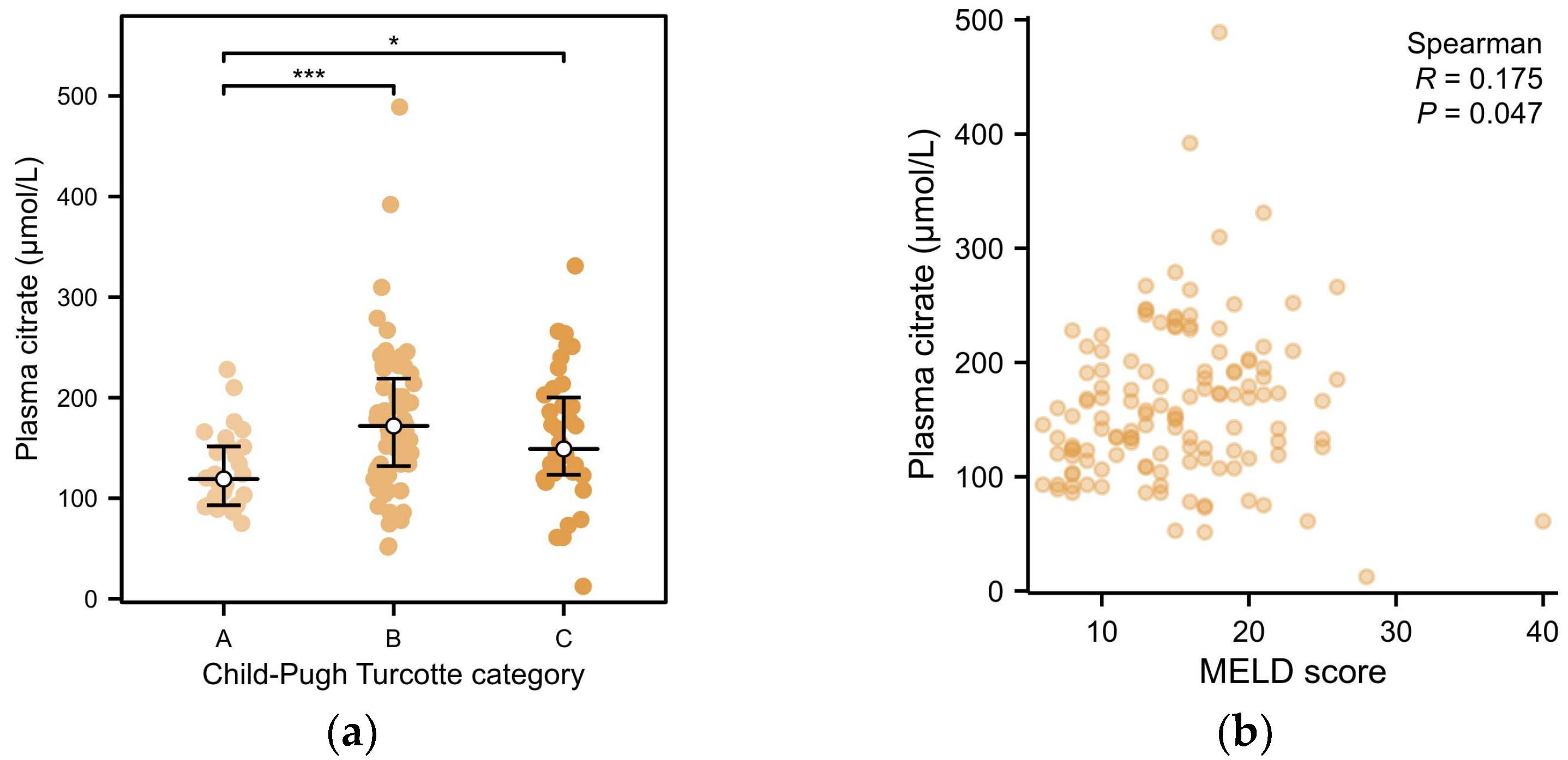
2.2. Associations of Plasma Citrate with Clinical and Laboratory Variables in Patients with End-Stage Liver Disease
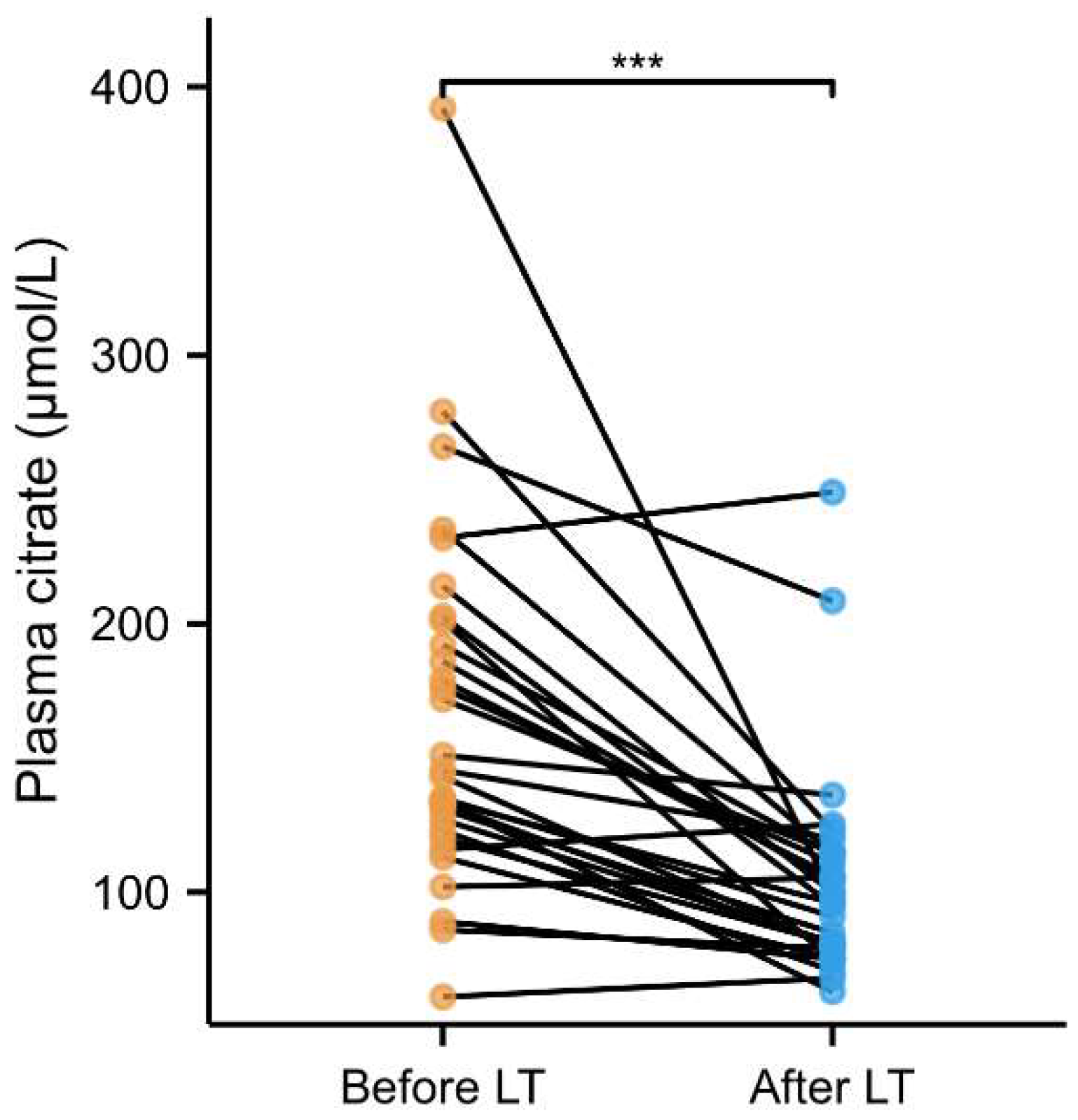
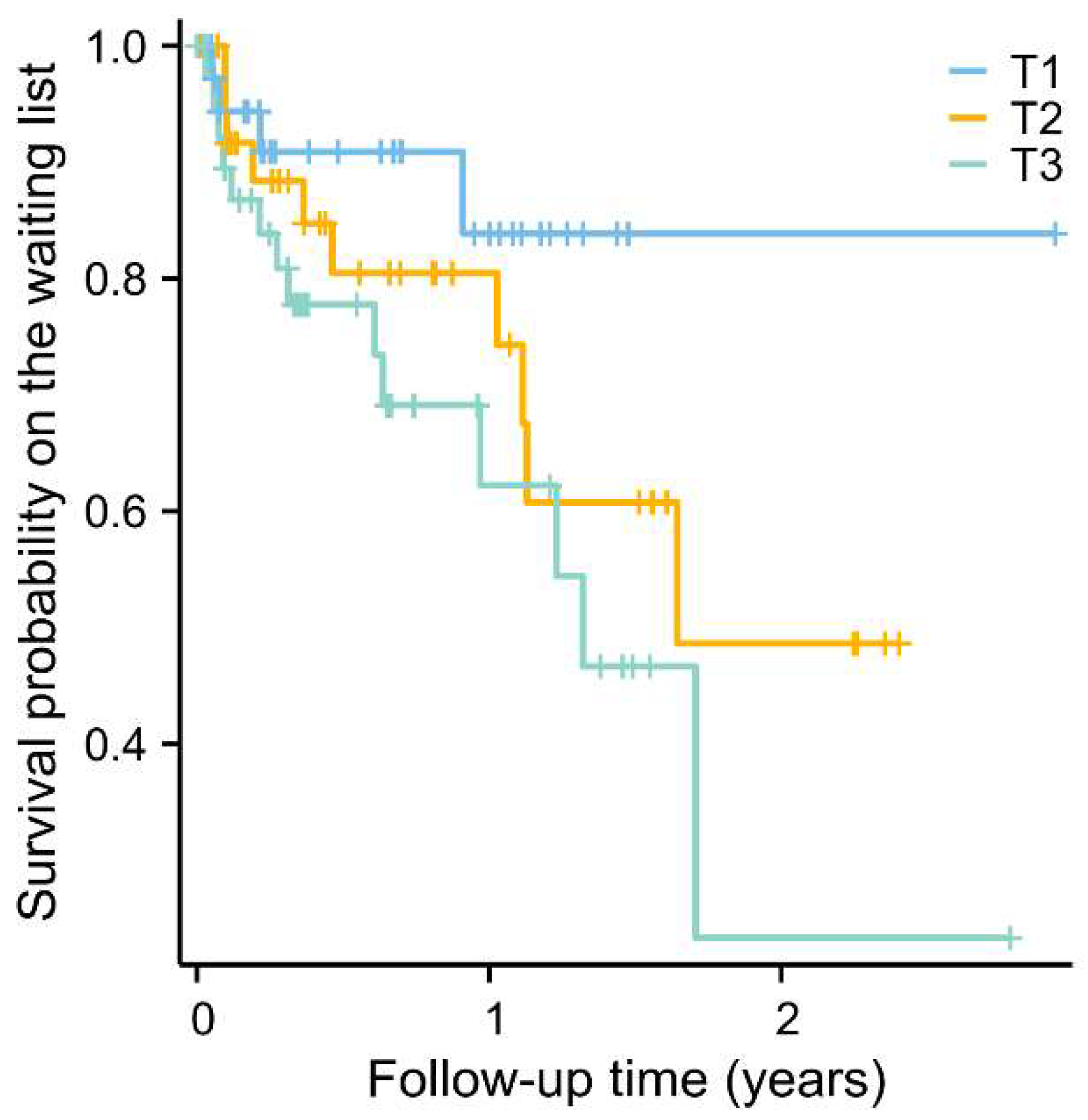
2.3. Longitudinal Analysis of Plasma Citrate with All-Cause Mortality in End-Stage Liver Disease Patients on the Waiting List
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection and Clinical Measurements
4.3. Laboratory Measurements
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, A.; Fortune, B.; Ufere, N.; Brown, R.S.; Rosenblatt, R. National Trends in Location of Death in Patients with End-Stage Liver Disease. Liver Transpl. 2021, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wahid, N.A.; Lee, J.; Kaplan, A.; Fortune, B.E.; Safford, M.M.; Brown, R.S.; Rosenblatt, R. Medicaid Expansion Association with End-Stage Liver Disease Mortality Depends on Leniency of Medicaid Hepatitis C Virus Coverage. Liver Transpl. 2021, 27, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Miro, J.; Laguno, M.; Moreno, A.; Rimola, A.; Hospital Clinic Olt In Hiv Working Group. Management of end stage liver disease (ESLD): What is the current role of orthotopic liver transplantation (OLT)? J. Hepatol. 2006, 44, S140–S145. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75, S49–S66. [Google Scholar] [CrossRef]
- Amjad, W.; Shalaurova, I.; Garcia, E.; Gruppen, E.G.; Dullaart, R.P.F.; DePaoli, A.M.; Jiang, Z.G.; Lai, M.; Connelly, M.A. Circulating Citrate Is Associated with Liver Fibrosis in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2023, 24, 13332. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Connelly, M.A.; Van Goor, H.; Van Dijk, P.R.; Dullaart, R.P.F. Plasma Citrate Levels Are Associated with an Increased Risk of Cardiovascular Mortality in Patients with Type 2 Diabetes (Zodiac-64). J. Clin. Med. 2023, 12, 6670. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.-L.; Mägi, R.; et al. Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W.; Kraus, W.E. Multimarkers of metabolic malnutrition and inflammation and their association with mortality risk in cardiac catheterisation patients: A prospective, longitudinal, observational, cohort study. Lancet Healthy Longev. 2023, 4, e72–e82. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J. Hum. Endocrinol. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Tong, W.; Hannou, S.A.; Wang, Y.; Astapova, I.; Sargsyan, A.; Monn, R.; Thiriveedi, V.; Li, D.; McCann, J.R.; Rawls, J.F.; et al. The intestine is a major contributor to circulating succinate in mice. FASEB J. 2022, 36, e22546. [Google Scholar] [CrossRef]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef]
- Duong, N.; Bajaj, J.S. The impact of the gut microbiome on liver transplantation. Curr. Opin. Organ Transplant. 2021, 26, 587–594. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Stryeck, S.; Gastrager, M.; Degoricija, V.; Trbušić, M.; Potočnjak, I.; Radulović, B.; Pregartner, G.; Berghold, A.; Madl, T.; Frank, S. Serum Concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate are Associated with Increased 3-Month Mortality in Acute Heart Failure Patients. Sci. Rep. 2019, 9, 6743. [Google Scholar] [CrossRef]
- Sanchez-Gimenez, R.; Peiró, Ó.M.; Bonet, G.; Carrasquer, A.; Fragkiadakis, G.A.; Bulló, M.; Papandreou, C.; Bardaji, A. TCA cycle metabolites associated with adverse outcomes after acute coronary syndrome: Mediating effect of renal function. Front. Cardiovasc. Med. 2023, 10, 1157325. [Google Scholar] [CrossRef]
- Schneider, J.; Han, W.H.; Matthew, R.; Sauvé, Y.; Lemieux, H. Age and sex as confounding factors in the relationship between cardiac mitochondrial function and type 2 diabetes in the Nile Grass rat. PLoS ONE 2020, 15, e0228710. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Chella Krishnan, K.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949.e4. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Perreault, L.; Macias, E.; Newsom, S.A.; Harrison, K.; Bui, H.H.; Milligan, P.; Roth, K.D.; Nemkov, T.; D’Alessandro, A.; et al. Sex Differences in Insulin Sensitivity are Related to Muscle Tissue Acylcarnitine But Not Subcellular Lipid Distribution. Obesity 2021, 29, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Gomes-Neto, A.W.; Van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Kunutsor, S.K.; Kieneker, L.M.; Van Der Vegt, B.; Connelly, M.A.; De Bock, G.H.; Gansevoort, R.T.; Bakker, S.J.L.; Dullaart, R.P.F. GlycA, a novel pro-inflammatory glycoprotein biomarker is associated with mortality: Results from the PREVEND study and meta-analysis. J. Intern. Med. 2019, 286, 596–609. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F.; on behalf of the PREVEND study group. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein: (Apo)lipoproteins and cardiovascular risk. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 2005, 60, 646–649. [Google Scholar] [CrossRef]
- Bedi, S.; Garcia, E.; Jeyarajah, E.; Shalaurova, I.; Perez-Matos, M.; Jiang, Z.; Dullaart, R.; Matyus, S.; Kirk, W.; Otvos, J.; et al. Characterization of LP-Z Lipoprotein Particles and Quantification in Subjects with Liver Disease Using a Newly Developed NMR-Based Assay. J. Clin. Med. 2020, 9, 2915. [Google Scholar] [CrossRef]

| ESLD Patients (n = 129) | Before Propensity Score Matching | After Propensity Score Matching | |||
|---|---|---|---|---|---|
| PREVEND (n = 4837) | p-Value | PREVEND (n = 129) | p-Value | ||
| Age (years) | 58 ± 10 | 54 ± 12 | <0.001 | 55 ± 13 | 0.10 |
| Sex | <0.001 | 0.06 | |||
| Male, n (%) | 84 (65.1) | 2388 (49.4) | 69 (53.5) | ||
| Female, n (%) | 45 (34.9) | 2449 (50.6) | 60 (46.5) | ||
| BMI (kg/m2) | 28.3 ± 4.8 | 26.7 ± 4.4 | <0.001 | 27.5 ± 4.9 | 0.22 |
| SBP (mmHg) | 120 ± 18 | 126 ± 19 | 0.002 | 128 ± 21 | 0.002 |
| DBP (mmHg) | 67 ± 11 | 73 ± 9 | <0.001 | 74 ± 10 | <0.001 |
| Current smoking, n (%) | 16 (12.4) | 1321 (27.3) | <0.001 | 27 (20.9) | 0.07 |
| Alcohol consumption (g/day) | <0.001 | <0.001 | |||
| 0/rarely, n (%) | 124 (96.1) | 1697 (35.1) | 46 (35.7) | ||
| 0.1–10, n (%) | 5 (3.9) | 1218 (25.2) | 33 (25.6) | ||
| 10–30, n (%) | 0 (0) | 993 (20.5) | 26 (20.2) | ||
| ≥30, n (%) | 0 (0) | 929 (19.2) | 24 (18.6) | ||
| Diabetes, n (%) | 36 (27.9) | 294 (6.1) | <0.001 | 33 (25.6) | 0.67 |
| History of cardiovascular disease, n (%) | 6 (4.7) | 301 (6.2) | 0.46 | 15 (11.6) | 0.04 |
| Blood glucose-lowering drugs, n (%) | 35 (27.1) | 178 (3.7) | <0.001 | 18 (14) | 0.009 |
| Lipid-lowering drugs, n (%) | 19 (14.7) | 458 (9.5) | 0.045 | 13 (10.1) | 0.26 |
| Antihypertensives, n (%) | 80 (62) | 854 (17.7) | <0.001 | 13 (10.1) | <0.001 |
| Etiology, n (%) | |||||
| Storage diseases | 4 (3.1) | ||||
| Autoimmune hepatitis | 10 (7.8) | ||||
| Cholestatic liver diseases | 33 (25.6) | ||||
| Viral | 12 (9.3) | ||||
| ALD | 28 (21.7) | ||||
| MASLD | 33 (25.6) | ||||
| Others | 9 (7) | ||||
| Child–Turcotte–Pugh classification | |||||
| A, n (%) | 28 (21.7) | ||||
| B, n (%) | 63 (48.8) | ||||
| C, n (%) | 38 (29.5) | ||||
| MELD score | 15 (10, 19) | ||||
| Total cholesterol (mmol/L) | 3.2 (2.5, 4.1) | 5.3 (4.7, 6.1) | <0.001 | 5.3 (4.7, 6.0) | <0.001 |
| Fasting glucose (mmol/L) | 6.2 (5.0, 8.2) | 4.8 (4.4, 5.3) | <0.001 | 5.0 (4.5, 6.1) | <0.001 |
| Serum creatinine (µmol/L) | 73.2 (55.6, 95.7) | 83.2 (73.9, 92.4) | <0.001 | 86.2 (76.0, 95.5) | <0.001 |
| eGFR (mL/min/1.73 m2) | 99.5 (75.5, 109.5) | 93.7 (81.6, 104.3) | 0.16 | 91.7 (78.8, 101.4) | 0.09 |
| Total bilirubin (µmol/L) | 42.0 (23.2, 98.5) | 7.0 (5.0, 9.0) | <0.001 | 6.0 (5.0, 9.0) | <0.001 |
| ALT (U/L) | 40.0 (28.0, 60.0) | 17.0 (13.0, 24.0) | <0.001 | 18.0 (13.0, 27.0) | <0.001 |
| AST (U/L) | 54.0 (44.0, 84.0) | 22.0 (19.0, 26.0) | <0.001 | 23.0 (20.0, 27.0) | <0.001 |
| GGT (U/L) | 95.0 (48.5, 150.5) | 24.0 (16.0, 38.0) | <0.001 | 28.0 (19.0, 41.0) | <0.001 |
| ALP (U/L) | 141.0 (98.5, 213.5) | 66.0 (55.0, 79.0) | <0.001 | 70.0 (56.0, 84.0) | <0.001 |
| Hemoglobin (mmol/L) | 6.8 (5.8, 7.8) | 8.5 (8.0, 9.0) | <0.001 | 8.6 (7.9, 8.9) | <0.001 |
| Plasma citrate (µmol/L) | 153.0 (118.0, 195.0) | 106.2 (91.1, 122.8) | <0.001 | 106.2 (93.2, 124.9) | <0.001 |
| T1 <126 μmol/L (n = 43) | T2 126–179 μmol/L (n = 44) | T3 >179 μmol/L (n = 42) | p-Value | |
|---|---|---|---|---|
| Age (years) | 55 (48, 63) | 61 (57, 67) | 60 (55, 64) | 0.012 |
| Sex | 0.41 | |||
| Male, n (%) | 27 (62.8) | 32 (72.7) | 25 (59.5) | |
| Female, n (%) | 16 (37.2) | 12 (27.3) | 17 (40.5) | |
| BMI (kg/m2) | 26.4 (23.3, 29.6) | 27.8 (25.5, 31.5) | 28.8 (25.0, 32.1) | 0.076 |
| SBP (mmHg) | 120 (108, 135) | 121 (108, 129) | 112 (106, 125) | 0.34 |
| DBP (mmHg) | 69 (61, 75) | 64.0 (59, 77) | 65 (59, 70) | 0.85 |
| Current smoking, n (%) | 4 (9.3) | 5 (11.4) | 7 (16.7) | 0.57 |
| Alcohol consumption (g/day) | 0.063 | |||
| 0/rarely, n (%) | 39 (90.7) | 44 (100) | 41 (97.6) | |
| 0.1–10, n (%) | 4 (9.3) | 0 (0) | 1 (2.4) | |
| Diabetes, n (%) | 9 (20.9) | 13 (29.5) | 14 (33.3) | 0.42 |
| History of cardiovascular disease, n (%) | 1 (2.3) | 4 (9.1) | 1 (2.4) | 0.35 |
| Blood glucose-lowering drugs, n (%) | 9 (20.9) | 12 (27.3) | 14 (33.3) | 0.44 |
| Lipid-lowering drugs, n (%) | 5 (11.6) | 9 (20.5) | 5 (11.9) | 0.42 |
| Antihypertensives, n (%) | 19 (44.2) | 30 (68.2) | 31 (73.8) | 0.011 |
| Etiology, n (%) | ||||
| Storage diseases | 0 (0) | 2 (4.5) | 2 (4.8) | 0.48 |
| Autoimmune hepatitis | 3 (7) | 3 (6.8) | 4 (9.5) | 0.83 |
| Cholestatic liver diseases | 16 (37.2) | 9 (20.5) | 8 (19) | 0.10 |
| Viral | 6 (14) | 2 (4.5) | 4 (9.5) | 0.31 |
| ALD | 4 (9.3) | 15 (34.1) | 9 (21.4) | 0.02 |
| MASLD | 9 (20.9) | 11 (25) | 13 (31) | 0.57 |
| Others | 5 (11.6) | 2 (4.5) | 2 (4.8) | 0.47 |
| Child–Turcotte–Pugh classification | 0.003 | |||
| A, n (%) | 17 (39.5) | 9 (20.5) | 2 (4.8) | |
| B, n (%) | 14 (32.6) | 23 (52.3) | 26 (61.9) | |
| C, n (%) | 12 (27.9) | 12 (27.3) | 14 (33.3) | |
| MELD score | 14 (9, 18) | 14 (11, 18) | 16 (13, 19) | 0.06 |
| Total cholesterol (mmol/L) | 3.5 (2.8, 4.7) | 2.9 (2.4, 3.7) | 3.1 (2.6, 3.7) | 0.032 |
| Albumin (g/L) | 35.0 (29.8, 41.2) | 30.0 (27.0, 35.0) | 29.5 (27.0, 33.2) | 0.05 |
| Fasting glucose (mmol/L) | 7.3 (4.6, 8.9) | 6.2 (5.0, 7.1) | 6.3 (5.3, 8.0) | 0.59 |
| HbA1c (%) | 5.4 (4.8, 6.3) | 5.0 (4.3, 5.6) | 4.7 (4.5, 5.5) | 0.32 |
| Serum creatinine (µmol/L) | 69.6 (50.5, 82.6) | 70.2 (55.8, 85.7) | 85.8 (64.6, 104.6) | 0.041 |
| eGFR (mL/min/1.73 m2) | 102.8 (88.5, 119.3) | 102.2 (81.2, 109.4) | 84.0 (68.1, 100.6) | 0.003 |
| Total bilirubin (µmol/L) | 49.0 (10.8, 168.8) | 32.5 (23.8, 75.0) | 53.5 (28.0, 81.8) | 0.35 |
| ALT (U/L) | 47.0 (32.0, 74.0) | 37.5 (28.5, 58.8) | 38.0 (27.5, 45.8) | 0.25 |
| AST (U/L) | 58.0 (37.0, 122.0) | 51.0 (43.2, 65.8) | 54.0 (44.8, 84.2) | 0.55 |
| GGT (U/L) | 101.0 (60.0, 255.0) | 101.5 (57.5, 149.8) | 71.0 (35.5, 135.5) | 0.33 |
| ALP (U/L) | 129.0 (80.0, 220.0) | 141.5 (119.2, 185.5) | 144.5 (86.8, 221.2) | 0.83 |
| Hemoglobin (mmol/L) | 6.4 (5.6, 7.9) | 6.9 (6.2, 8.1) | 6.7 (6.0, 7.2) | 0.60 |
| Thrombocytes (*109/L) | 138.5 (91.0, 202.8) | 99.0 (72.0, 137.0) | 109.0 (85.5, 132.2) | 0.069 |
| Leucocytes (*109/L) | 5.3 (4.0, 7.7) | 4.2 (3.5, 6.8) | 5.2 (3.6, 7.6) | 0.59 |
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Std. β (95% CI) | p-Value | Std. β (95% CI) | p-Value | Std. β (95% CI) | p-Value | Std. β (95% CI) | p-Value | |
| Age | 0.263 (0.092, 0.434) | 0.003 | 0.111 (−0.087, 0.320) | 0.26 | 0.164 (−0.032, 0.377) | 0.097 | 0.160 (−0.043, 0.381) | 0.12 |
| Sex | 0.102 (−0.069, 0.273) | 0.241 | 0.012 (−0.175, 0.199) | 0.90 | 0.015 (−0.181, 0.184) | 0.99 | 0.000 (−0.184, 0.184) | 1.00 |
| Anti- hypertensives | 0.169 (−0.012, 0.358) | 0.066 | 0.141 (−0.038, 0.325) | 0.12 | 0.139 (−0.043, 0.326) | 0.13 | ||
| eGFR | −0.306 (−0.553, −0.130) | 0.002 | −0.244 (−0.486, −0.059) | 0.013 | −0.250 (−0.506, −0.052) | 0.016 | ||
| CTP classification | ||||||||
| A | ref | ref | ||||||
| B | 0.327 (0.093, 0.575) | 0.007 | 0.335 (0.077, 0.608) | 0.012 | ||||
| C | 0.219 (−0.018, 0.457) | 0.070 | 0.233 (−0.065, 0.534) | 0.12 | ||||
| MELD score | −0.020 (−0.275, 0.233) | 0.87 | ||||||
| Per 1 Ln SD Increment | T1 | T2 | T3 | ||
|---|---|---|---|---|---|
| HR [95%CI] | p-Value | HR [95%CI] | HR [95%CI] | ||
| All | |||||
| Model 1 | 1.65 [1.03–2.63] | 0.037 | Reference | 2.00 [0.62–6.41] p = 0.219 | 3.13 [1.03–9.54] p = 0.044 |
| Model 2 | 1.59 [0.97–2.61] | 0.065 | Reference | 1.61 [0.48–5.41] p = 0.442 | 2.79 [0.91–8.54] p = 0.072 |
| Model 3 | 1.49 [0.89–2.48] | 0.13 | Reference | 1.60 [0.47–5.42] p = 0.455 | 2.58 [0.83–7.96] p = 0.100 |
| Model 4 | 1.60 [0.93–2.75] | 0.088 | Reference | 1.95 [0.55–6.93] p = 0.303 | 3.09 [0.94–10.12] p = 0.063 |
| Males (n = 84, 17 deaths) | |||||
| Model 1 | 2.04 [1.08–3.85] | 0.027 | Reference | 5.23 [0.64–42.64] p = 0.122 | 8.19 [1.02–65.67] p = 0.048 |
| Model 2 | 2.11 [1.06–4.22] | 0.034 | Reference | 4.02 [0.47–34.15] p = 0.202 | 7.44 [0.93–59.82] p = 0.059 |
| Model 3 | 1.94 [0.88–4.26] | 0.10 | Reference | 3.63 [0.41–32.08] p = 0.246 | 6.45 [0.75–55.35] p = 0.089 |
| Model 4 | 1.96 [0.86–4.49] | 0.11 | Reference | 3.51 [0.38–32.09] p = 0.266 | 6.02 [0.67–54.37] p = 0.11 |
| Females (n = 45, 12 deaths) | |||||
| Model 1 | 1.23 [0.62–2.45] | 0.56 | Reference | 0.85 [0.16–4.53] p = 0.851 | 1.44 [0.34–6.02] p = 0.621 |
| Model 2 | 1.19 [0.58–2.43] | 0.63 | Reference | 0.70 [0.12–4.08] p = 0.694 | 1.31 [0.31–5.56] p = 0.71 |
| Model 3 | 1.17 [0.57–2.40] | 0.67 | Reference | 0.73 [0.12–4.57] p = 0.736 | 1.324 [0.31–5.66] p = 0.70 |
| Model 4 | 1.99 [0.78–5.04] | 0.15 | Reference | 2.62 [0.34–20.18] p = 0.35 | 5.46 [0.66–44.92] p = 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chvatal-Medina, M.; Trillos-Almanza, M.C.; Bourgonje, A.R.; Connelly, M.A.; Moshage, H.; Bakker, S.J.L.; de Meijer, V.E.; Blokzijl, H.; Dullaart, R.P.F., on behalf of the TransplantLines Investigators. Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. Int. J. Mol. Sci. 2024, 25, 12806. https://doi.org/10.3390/ijms252312806
Li Y, Chvatal-Medina M, Trillos-Almanza MC, Bourgonje AR, Connelly MA, Moshage H, Bakker SJL, de Meijer VE, Blokzijl H, Dullaart RPF on behalf of the TransplantLines Investigators. Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. International Journal of Molecular Sciences. 2024; 25(23):12806. https://doi.org/10.3390/ijms252312806
Chicago/Turabian StyleLi, Yakun, Mateo Chvatal-Medina, Maria Camila Trillos-Almanza, Arno R. Bourgonje, Margery A. Connelly, Han Moshage, Stephan J. L. Bakker, Vincent E. de Meijer, Hans Blokzijl, and Robin P. F. Dullaart on behalf of the TransplantLines Investigators. 2024. "Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality" International Journal of Molecular Sciences 25, no. 23: 12806. https://doi.org/10.3390/ijms252312806
APA StyleLi, Y., Chvatal-Medina, M., Trillos-Almanza, M. C., Bourgonje, A. R., Connelly, M. A., Moshage, H., Bakker, S. J. L., de Meijer, V. E., Blokzijl, H., & Dullaart, R. P. F., on behalf of the TransplantLines Investigators. (2024). Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. International Journal of Molecular Sciences, 25(23), 12806. https://doi.org/10.3390/ijms252312806









