The Therapeutic Effects of SP-8356, a Verbenone Derivative, with Multimodal Cytoprotective Mechanisms in an Ischemic Stroke Rat Model
Abstract
1. Introduction
2. Results
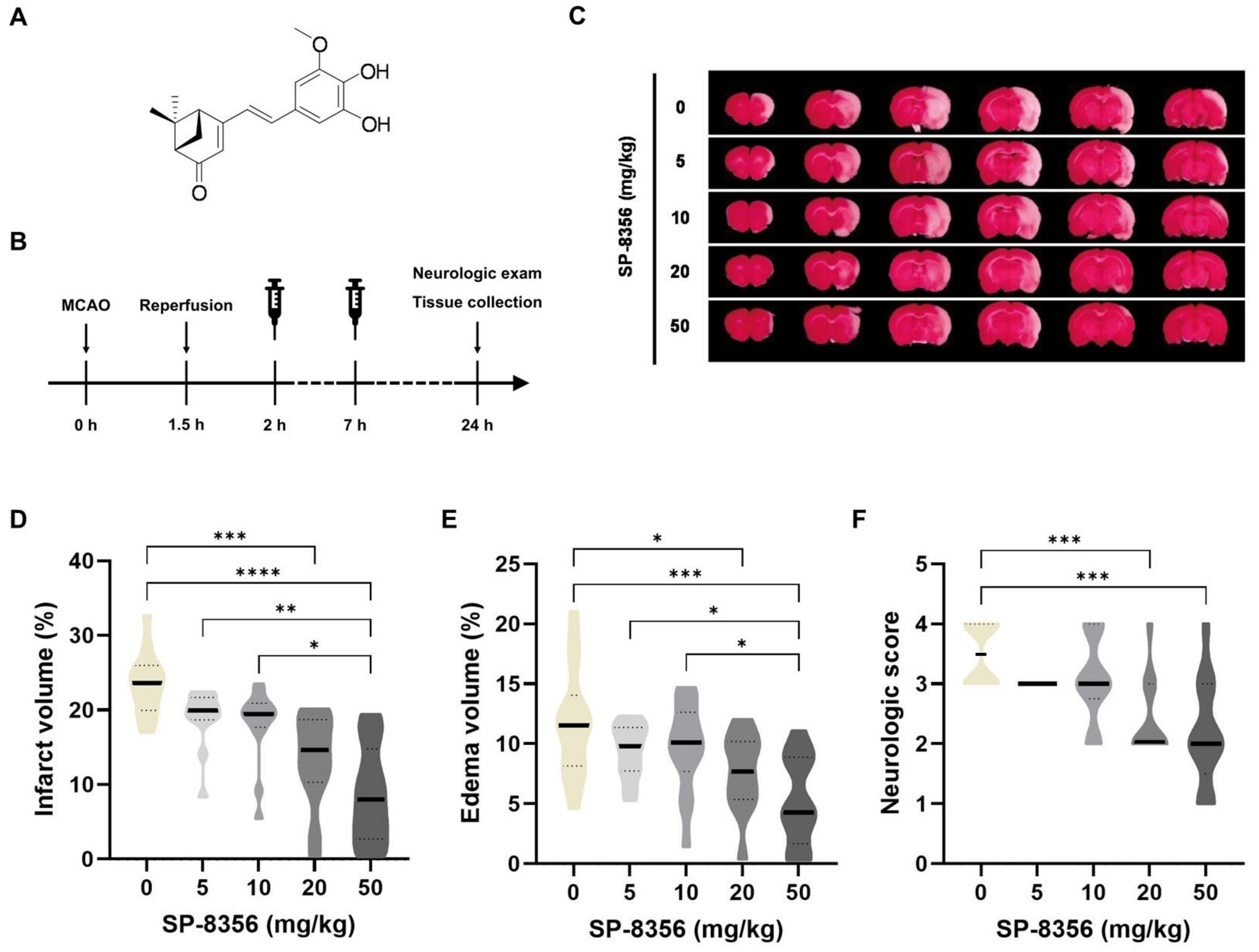
2.1. SP-8356 Shows Cytoprotective Effects Against Acute Ischemia/Reperfusion Brain Injury
2.2. SP-8356 Improves Long-Term Outcomes
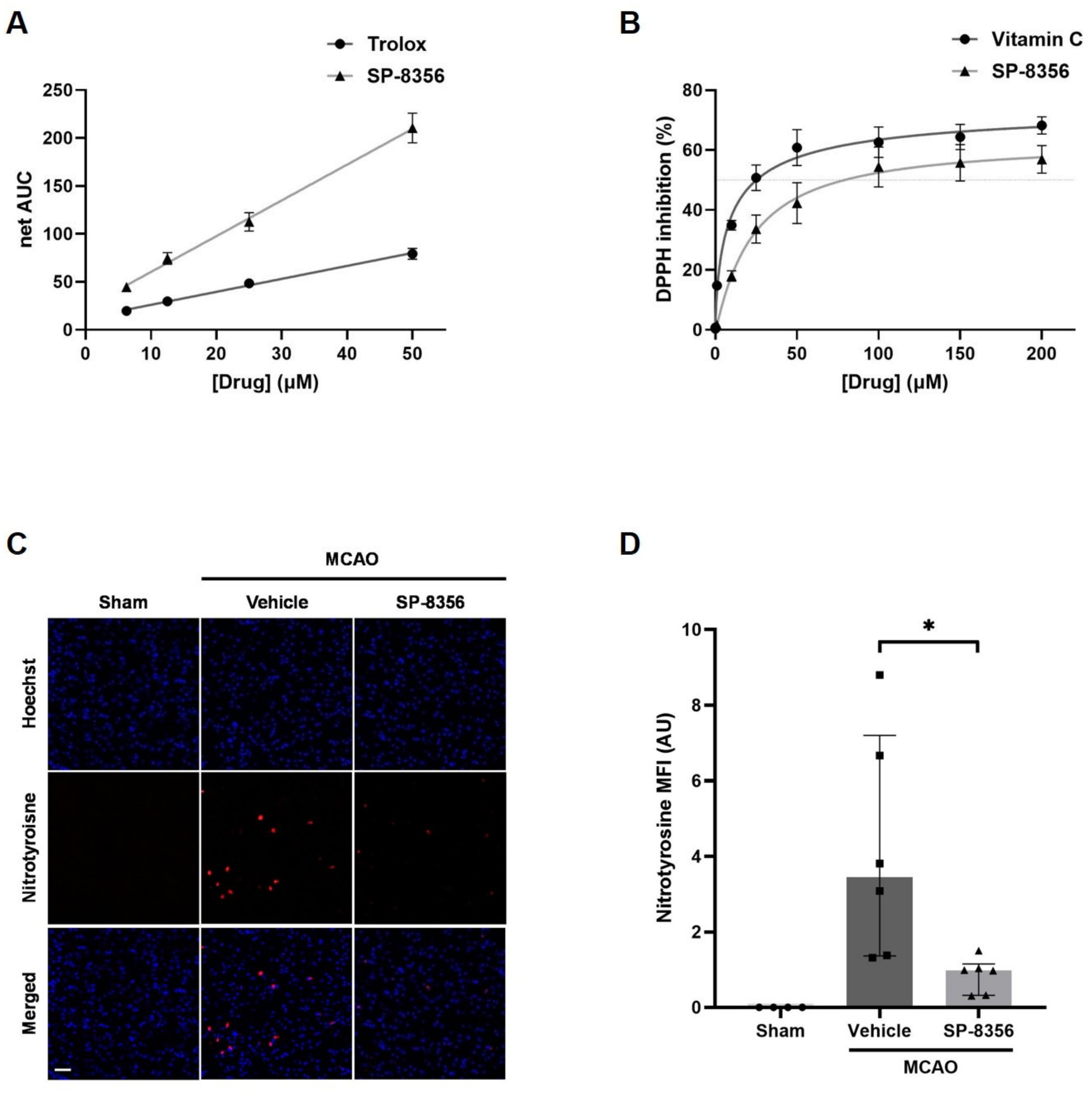
2.3. SP-8356 Alleviates Oxidative/Nitrosative Stress in Ischemia/Reperfusion Injury
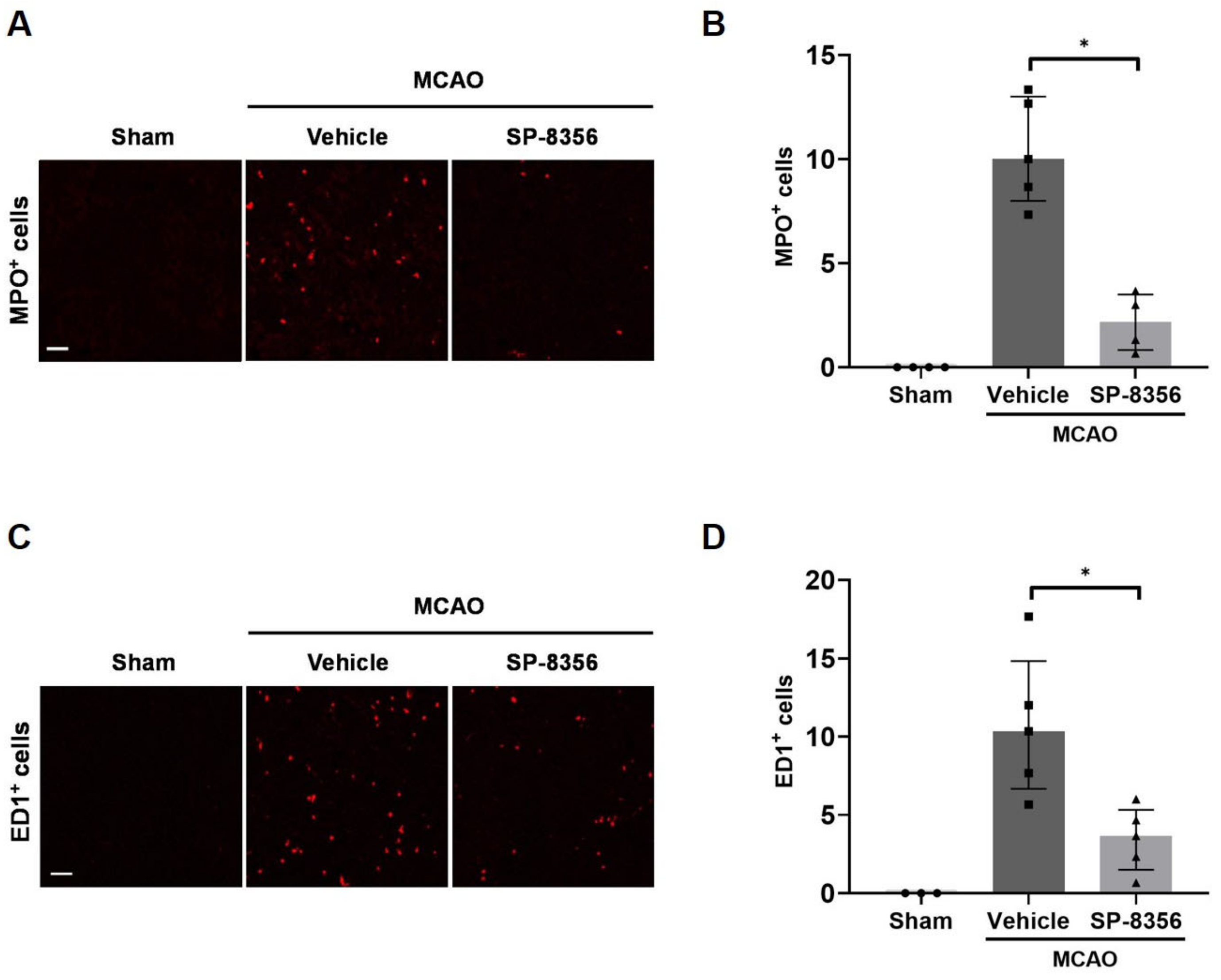
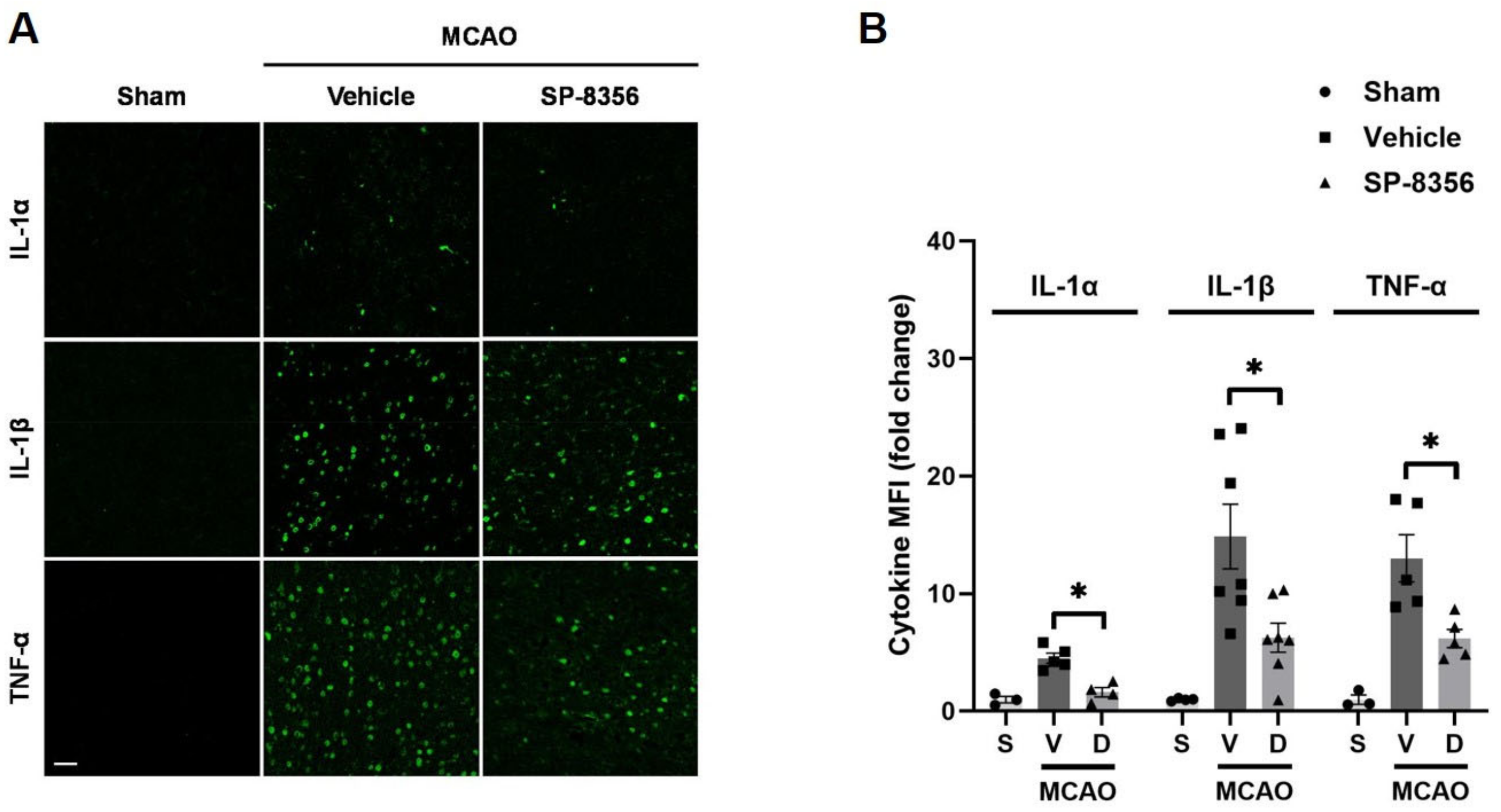
2.4. SP-8356 Reduces Inflammatory Responses in the Ischemic Lesions
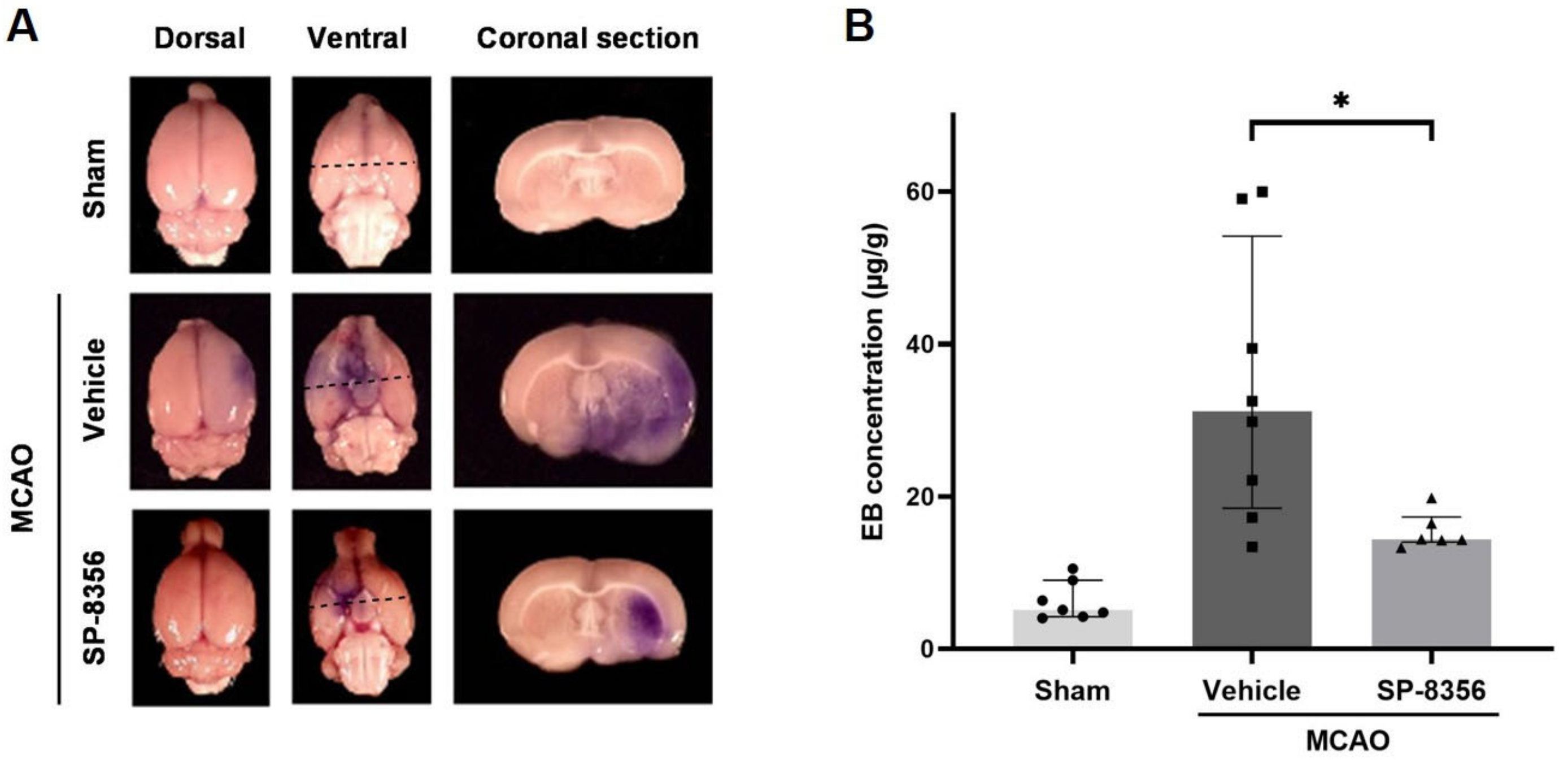
2.5. SP-8356 Prevents BBB Disruption Induced by Ischemia/Reperfusion Injury
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. Transient Intraluminal Middle Cerebral Artery Occlusion (MCAO) Rat Model
4.4. Drug Administration
4.5. Physiological Monitoring
4.6. Measurement of Brain Infarct and Edema Volume
4.7. Assessment of Neurologic Deficits
4.8. Immunofluorescence
4.9. Evans Blue (EB) Leakage
4.10. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.11. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Soldozy, S.; Dalzell, C.; Skaff, A.; Ali, Y.; Norat, P.; Yagmurlu, K.; Park, M.S.; Kalani, M.Y.S. Reperfusion injury in acute ischemic stroke: Tackling the irony of revascularization. Clin. Neurol. Neurosurg. 2023, 225, 107574. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective Strategies for Ischemic Stroke-Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4334. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Norrito, R.L.; Rizzica, S.; Mazzola, M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 6297. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Appleton, J.P.; England, T. The Hazard of Negative (Not Neutral) Trials on Treatment of Acute Stroke: A Review. JAMA Neurol. 2020, 77, 114–124. [Google Scholar] [CrossRef]
- Dhir, N.; Medhi, B.; Prakash, A.; Goyal, M.K.; Modi, M.; Mohindra, S. Pre-clinical to Clinical Translational Failures and Current Status of Clinical Trials in Stroke Therapy: A Brief Review. Curr. Neuropharmacol. 2020, 18, 596–612. [Google Scholar] [CrossRef]
- Lyden, P.D. Cerebroprotection for Acute Ischemic Stroke: Looking Ahead. Stroke 2021, 52, 3033–3044. [Google Scholar] [CrossRef]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke—Renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Oh, Y.K.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorg Med. Chem. Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef]
- Rungqu, P.; Oyedeji, O.; Nkeh-Chungag, B.; Songca, S.; Oluwafemi, O.; Oyedeji, A. Anti-inflammatory activity of the essential oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy from Eastern Cape, South Africa. Asian Pac. J. Trop. Med. 2016, 9, 426–431. [Google Scholar] [CrossRef]
- González-Velasco, H.E.; Pérez-Gutiérrez, M.S.; Alonso-Castro, Á.J.; Zapata-Morales, J.R.; Niño-Moreno, P.D.C.; Campos-Xolalpa, N.; González-Chávez, M.M. Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes parryi A. Gray (Asteraceae) and Verbenone. Molecules 2022, 27, 2612. [Google Scholar] [CrossRef]
- Pahk, K.; Noh, H.; Joung, C.; Jang, M.; Song, H.Y.; Kim, K.W.; Han, K.; Hwang, J.I.; Kim, S.; Kim, W.K. A novel CD147 inhibitor, SP-8356, reduces neointimal hyperplasia and arterial stiffness in a rat model of partial carotid artery ligation. J. Transl. Med. 2019, 17, 274. [Google Scholar] [CrossRef]
- Pahk, K.; Joung, C.; Song, H.Y.; Kim, S.; Kim, W.-K. SP-8356, a Novel Inhibitor of CD147-Cyclophilin A Interactions, Reduces Plaque Progression and Stabilizes Vulnerable Plaques in apoE-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Mander, S.; Kim, D.H.; Thi Nguyen, H.; Yong, H.J.; Pahk, K.; Kim, E.Y.; Lee, K.; Seong, J.Y.; Kim, W.K.; Hwang, J.I. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci. Rep. 2019, 9, 6595. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yong, H.J.; Mander, S.; Nguyen, H.T.; Nguyen, L.P.; Park, H.K.; Cha, H.K.; Kim, W.K.; Hwang, J.I. SP-8356, a (1S)-(-)-Verbenone Derivative, Inhibits the Growth and Motility of Liver Cancer Cells by Regulating NF-κB and ERK Signaling. Biomol. Ther. 2021, 29, 331–341. [Google Scholar] [CrossRef]
- Nguyen, T.U.; Hurh, S.; In, S.; Nguyen, L.P.; Cho, M.; Mykhailova, K.; Kim, H.R.; Ham, B.J.; Choi, Y.; Kim, W.K.; et al. SP-8356 inhibits acute lung injury by suppressing inflammatory cytokine production and immune cell infiltration. Int. Immunopharmacol. 2024, 131, 111847. [Google Scholar] [CrossRef] [PubMed]
- Joung, C.; Noh, H.; Jung, J.; Song, H.Y.; Bae, H.; Pahk, K.; Kim, W.K. A Novel CD147 Inhibitor, SP-8356, Attenuates Pathological Fibrosis in Alkali-Burned Rat Cornea. Int. J. Mol. Sci. 2020, 21, 2990. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Hong, D.; Chen, F.; Ji, X.; Cao, G. White matter injury in ischemic stroke. Prog. Neurobiol. 2016, 141, 45–60. [Google Scholar] [CrossRef]
- Rogers, D.C.; Campbell, C.A.; Stretton, J.L.; Mackay, K.B. Correlation Between Motor Impairment and Infarct Volume After Permanent and Transient Middle Cerebral Artery Occlusion in the Rat. Stroke 1997, 28, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Cipak Gasparovic, A.; Zarkovic, N.; Zarkovic, K.; Semen, K.; Kaminskyy, D.; Yelisyeyeva, O.; Bottari, S.P. Biomarkers of oxidative and nitro-oxidative stress: Conventional and novel approaches. Br. J. Pharmacol. 2017, 174, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Laaker, C.; Hsu, M.; Cismaru, P.; Sandor, M.; Fabry, Z. Molecular Mechanisms of Neuroimmune Crosstalk in the Pathogenesis of Stroke. Int. J. Mol. Sci. 2021, 22, 9486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, K.; Wang, Y.; Shi, F.D. Neurovascular Inflammation and Complications of Thrombolysis Therapy in Stroke. Stroke 2023, 54, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Mohammed Ali, A.-M.; Ziad, M. Antioxidant Activity: The Presence and Impact of Hydroxyl Groups in Small Molecules of Natural and Synthetic Origin. In Antioxidants; Viduranga, W., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef]
- Chamorro, A.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Martí-Fábregas, J.; Gállego, J.; Krupinski, J.; Gomis, M.; Cánovas, D.; et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014, 13, 453–460. [Google Scholar] [CrossRef]
- Llull, L.; Laredo, C.; Renú, A.; Pérez, B.; Vila, E.; Obach, V.; Urra, X.; Planas, A.; Amaro, S.; Chamorro, Á. Uric Acid Therapy Improves Clinical Outcome in Women With Acute Ischemic Stroke. Stroke 2015, 46, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Amaro, S.; Llull, L.; Renú, A.; Laredo, C.; Perez, B.; Vila, E.; Torres, F.; Planas, A.M.; Chamorro, Á. Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Ann. Neurol. 2015, 77, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Amaro, S.; Castellanos, M.; Gomis, M.; Urra, X.; Blasco, J.; Arenillas, J.F.; Román, L.S.; Muñoz, R.; Macho, J.; et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int. J. Stroke 2017, 12, 377–382. [Google Scholar] [CrossRef]
- Bae, H. SP-8356, a (1S)-(-)-Verbenone Derivative, Attenuates Adhesion Molecule Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Inhibiting NF-κB Signaling. Graduate School, Korea University: Seoul, Republic of Korea, 2020. [Google Scholar]
- Asgari, R.; Vaisi-Raygani, A.; Aleagha, M.S.E.; Mohammadi, P.; Bakhtiari, M.; Arghiani, N. CD147 and MMPs as key factors in physiological and pathological processes. Biomed. Pharmacother. 2023, 157, 113983. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhou, X.; Yang, Z.-H.; Si, X.-K.; Sun, X. Stroke-induced damage on the blood–brain barrier. Front. Neurol. 2023, 14, 1248970. [Google Scholar] [CrossRef]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Wysocka, A.; Szczygielski, J.; Kopańska, M.; Oertel, J.M.; Głowniak, A. Matrix Metalloproteinases in Cardioembolic Stroke: From Background to Complications. Int. J. Mol. Sci. 2023, 24, 3628. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, P.; Tang, X.; Chang, N.; Shi, H.; Guo, L.; Wang, B.; Yang, P.; Zhu, T.; Zhao, X. The mechanisms of minocycline in alleviating ischemic stroke damage and cerebral ischemia-reperfusion injury. Eur. J. Pharmacol. 2023, 955, 175903. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Y.; Li, H.; Zheng, W.; Xia, B.; Zhang, X.; Yong, V.W.; Xue, M. Efficacy of Minocycline in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis of Rodent and Clinical Studies. Front. Neurol. 2018, 9, 1103. [Google Scholar] [CrossRef]
- Naderi, Y.; Panahi, Y.; Barreto, G.E.; Sahebkar, A. Neuroprotective effects of minocycline on focal cerebral ischemia injury: A systematic review. Neural Regen. Res. 2020, 15, 773–782. [Google Scholar]
- Gwag, B.J.; Lee, Y.A.; Ko, S.Y.; Lee, M.J.; Im, D.S.; Yun, B.S.; Lim, H.R.; Park, S.M.; Byun, H.Y.; Son, S.J.; et al. Marked Prevention of Ischemic Brain Injury by Neu2000, an NMDA Antagonist and Antioxidant Derived from Aspirin and Sulfasalazine. J. Cereb. Blood Flow. Metab. 2007, 27, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Lee, J.S.; Lee, Y.-B.; Shin, D.H.; Shin, D.-I.; Hwang, Y.-H.; Ahn, S.H.; Kim, J.G.; Sohn, S.-I.; Kwon, S.U.; et al. Nelonemdaz for Patients With Acute Ischemic Stroke Undergoing Endovascular Reperfusion Therapy: A Randomized Phase II Trial. Stroke 2022, 53, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, J.S.; Gwag, B.J.; Choi, D.W.; An, C.S.; Kang, H.G.; Song, T.J.; Ahn, S.H.; Kim, C.H.; Shin, D.I.; et al. The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz (RODIN) Trial: Protocol for a Double-Blinded Clinical Trial of Nelonemdaz in Patients with Hyperacute Ischemic Stroke and Endovascular Thrombectomy. J. Stroke 2023, 25, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.J.; Lee, S.H.; Shin, K.Y.; Lee, C.K.; Cho, I.H.; Kim, H.S.; Suh, Y.H. SP-8203 reduces oxidative stress via SOD activity and behavioral deficit in cerebral ischemia. Pharmacol. Biochem. Behav. 2011, 98, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.J.; Lee, J.M.; Lee, K.S.; Hong, H.S.; Lee, C.K.; Cho, I.H.; Kim, H.S.; Suh, Y.H. SP-8203 shows neuroprotective effects and improves cognitive impairment in ischemic brain injury through NMDA receptor. Pharmacol. Biochem. Behav. 2011, 100, 73–80. [Google Scholar] [CrossRef]
- Song, H.Y.; Chung, J.I.; Jalin, A.M.A.; Ju, C.; Pahk, K.; Joung, C.; Lee, S.; Jin, S.; Kim, B.S.; Lee, K.S.; et al. The Quinazoline Otaplimastat (SP-8203) Reduces the Hemorrhagic Transformation and Mortality Aggravated after Delayed rtPA-Induced Thrombolysis in Cerebral Ischemia. Int. J. Mol. Sci. 2022, 23, 1403. [Google Scholar] [CrossRef]
- Ruan, J.; Yao, Y. Behavioral tests in rodent models of stroke. Brain Hemorrhages 2020, 1, 171–184. [Google Scholar] [CrossRef]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle Cerebral Artery Occlusion in the Rat by Intraluminal Suture. Stroke 1996, 27, 1616–1623. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Models Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed; Elsevier Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Golanov, E.V.; Reis, D.J. Contribution of cerebral edema to the neuronal salvage elicited by stimulation of cerebellar fastigial nucleus after occlusion of the middle cerebral artery in rat. J. Cereb. Blood Flow Metab. 1995, 15, 172–174. [Google Scholar] [CrossRef]
- Uyama, O.; Okamura, N.; Yanase, M.; Narita, M.; Kawabata, K.; Sugita, M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J. Cereb. Blood Flow Metab. 1988, 8, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.Y.; Jin, S.; Lee, S.; Jalin, A.M.A.; Roh, K.-H.; Kim, W.-K. The Therapeutic Effects of SP-8356, a Verbenone Derivative, with Multimodal Cytoprotective Mechanisms in an Ischemic Stroke Rat Model. Int. J. Mol. Sci. 2024, 25, 12769. https://doi.org/10.3390/ijms252312769
Song HY, Jin S, Lee S, Jalin AMA, Roh K-H, Kim W-K. The Therapeutic Effects of SP-8356, a Verbenone Derivative, with Multimodal Cytoprotective Mechanisms in an Ischemic Stroke Rat Model. International Journal of Molecular Sciences. 2024; 25(23):12769. https://doi.org/10.3390/ijms252312769
Chicago/Turabian StyleSong, Hwa Young, Sejong Jin, Sekwang Lee, Angela Melinda Anthony Jalin, Kyung-Hye Roh, and Won-Ki Kim. 2024. "The Therapeutic Effects of SP-8356, a Verbenone Derivative, with Multimodal Cytoprotective Mechanisms in an Ischemic Stroke Rat Model" International Journal of Molecular Sciences 25, no. 23: 12769. https://doi.org/10.3390/ijms252312769
APA StyleSong, H. Y., Jin, S., Lee, S., Jalin, A. M. A., Roh, K.-H., & Kim, W.-K. (2024). The Therapeutic Effects of SP-8356, a Verbenone Derivative, with Multimodal Cytoprotective Mechanisms in an Ischemic Stroke Rat Model. International Journal of Molecular Sciences, 25(23), 12769. https://doi.org/10.3390/ijms252312769





