Paraoxonase-1 Is a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation Through Adipose Stem Cell-Derived Extracellular Vesicles
Abstract
1. Introduction
2. Results
2.1. AHR and Inflammatory Cells in BALF
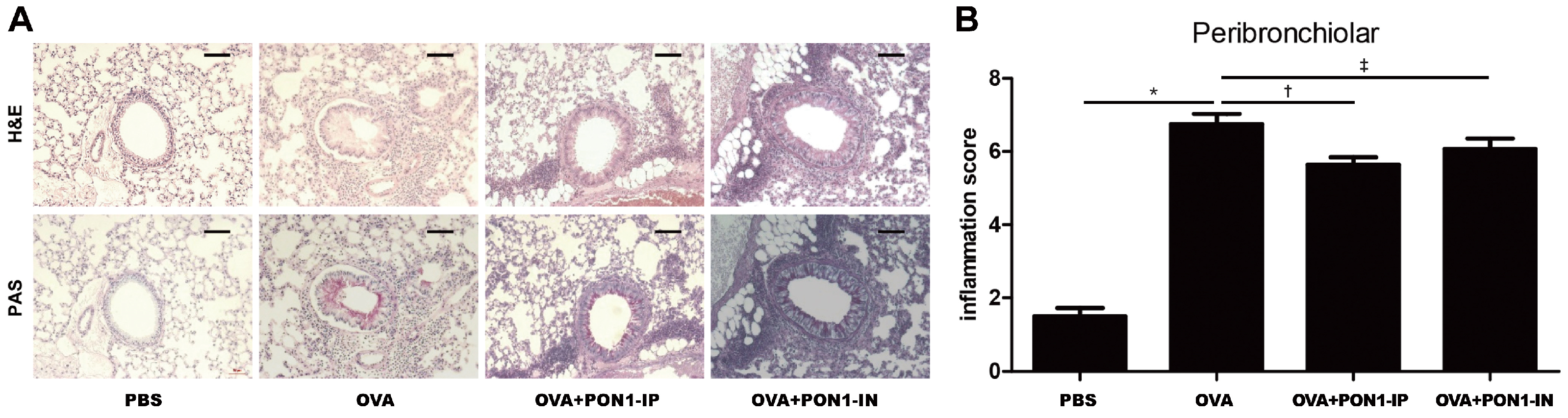
2.2. Lung Histology and Inflammation Score
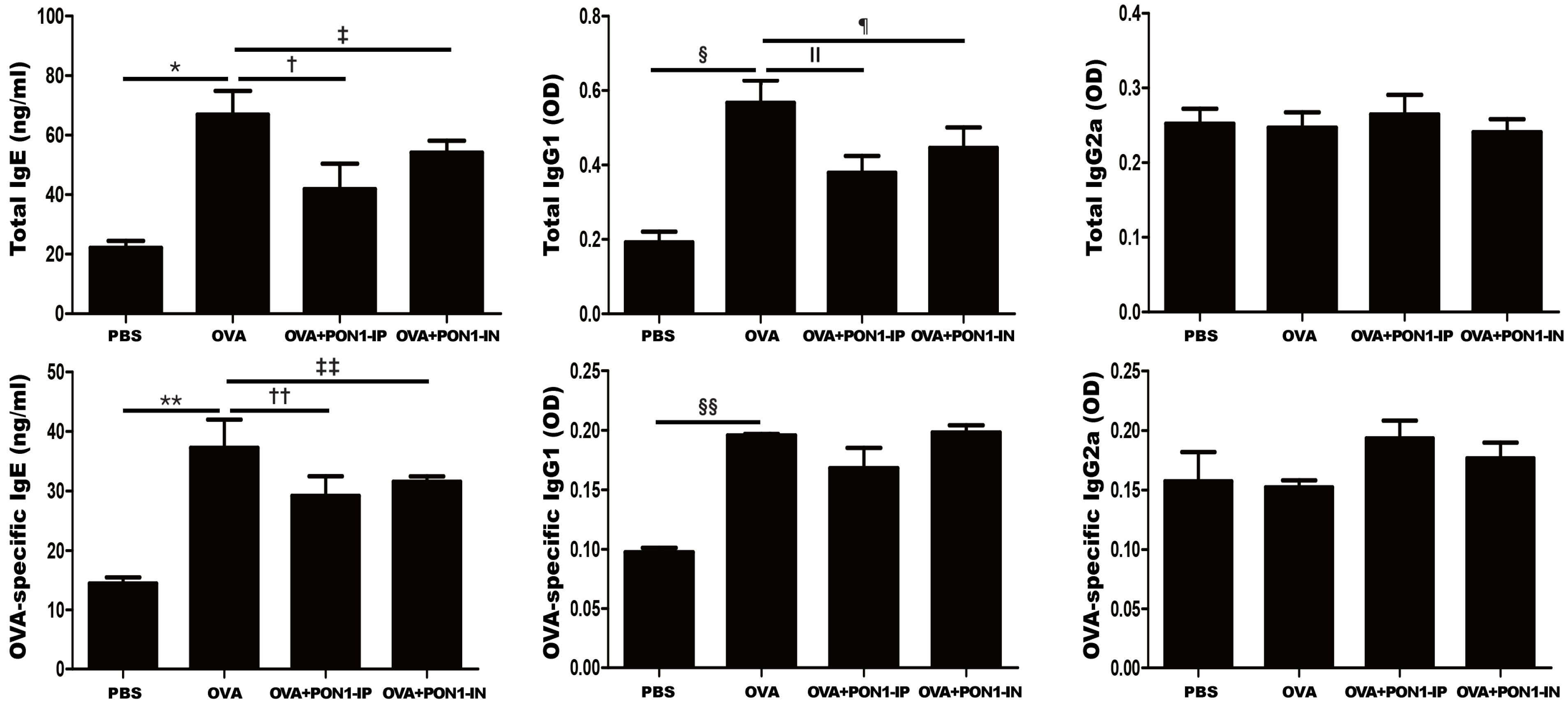
2.3. Serum Total and OVA-Specific IgE, IgG1, and IgG2a Levels
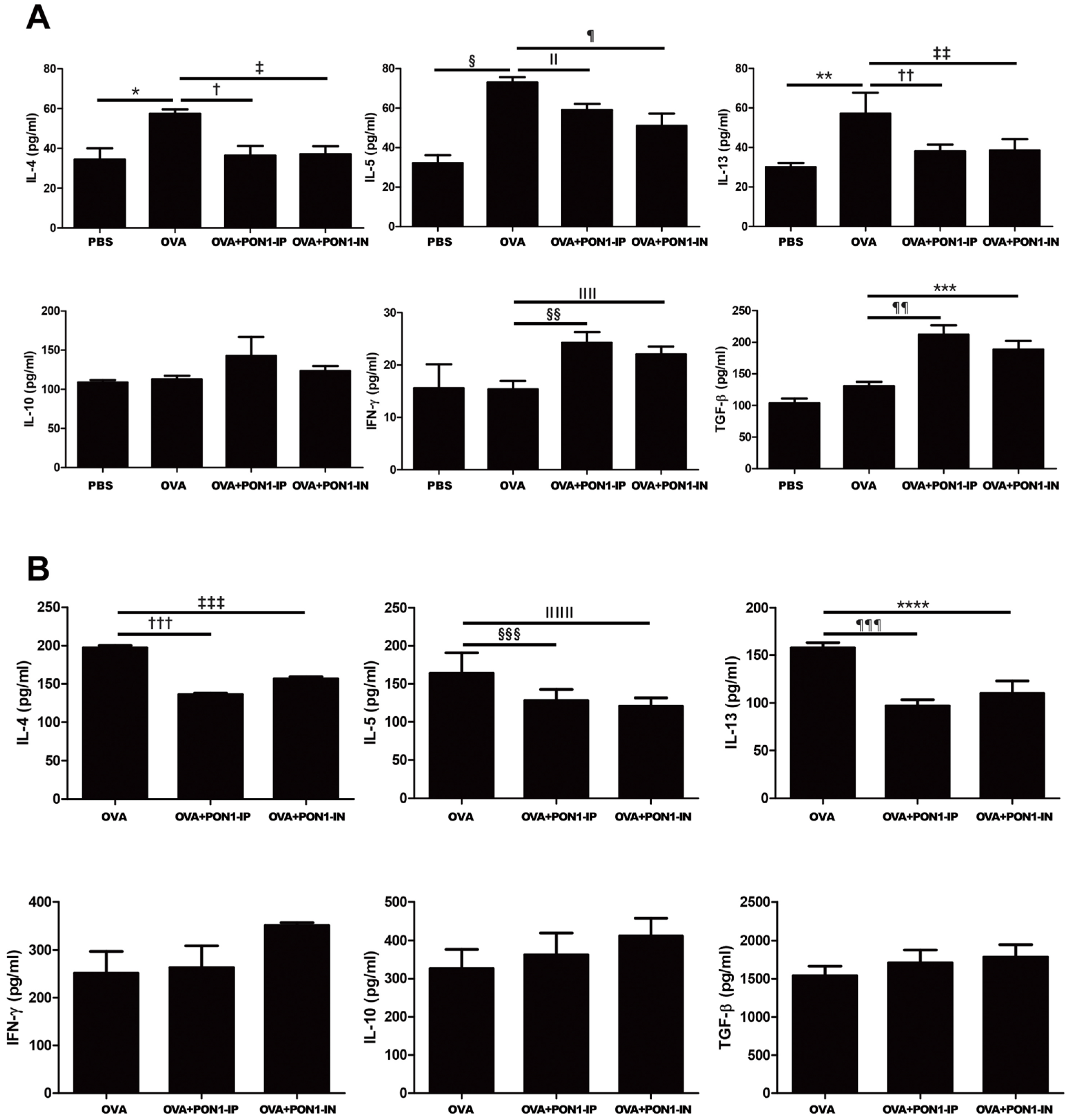
2.4. Expression of Cytokines in the BALF and LLNs
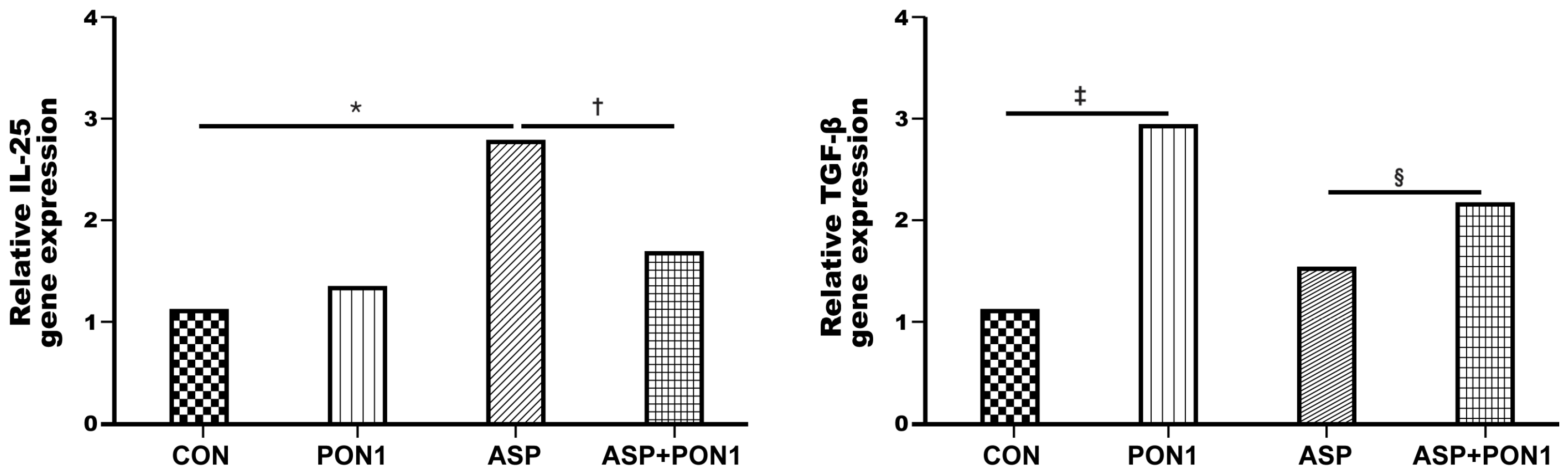
2.5. Expression of IL-25 and TGF-β in MLE-12 Cells
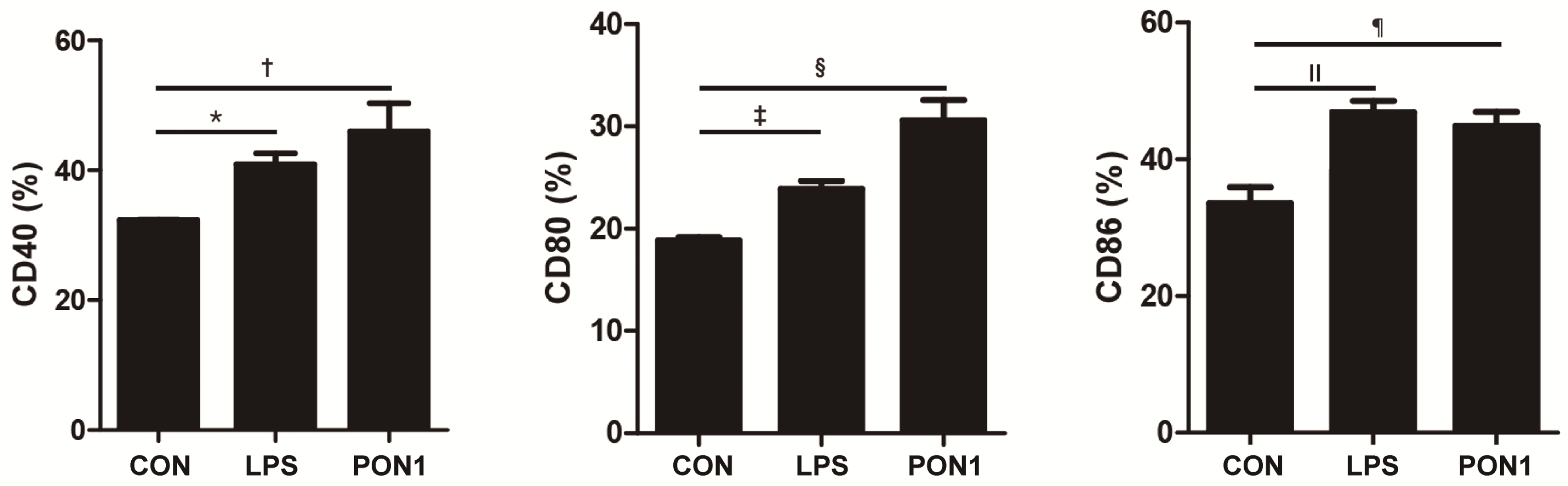
2.6. Activation and Maturation of Dendritic Cells
3. Discussion
4. Materials and Methods
4.1. Animals
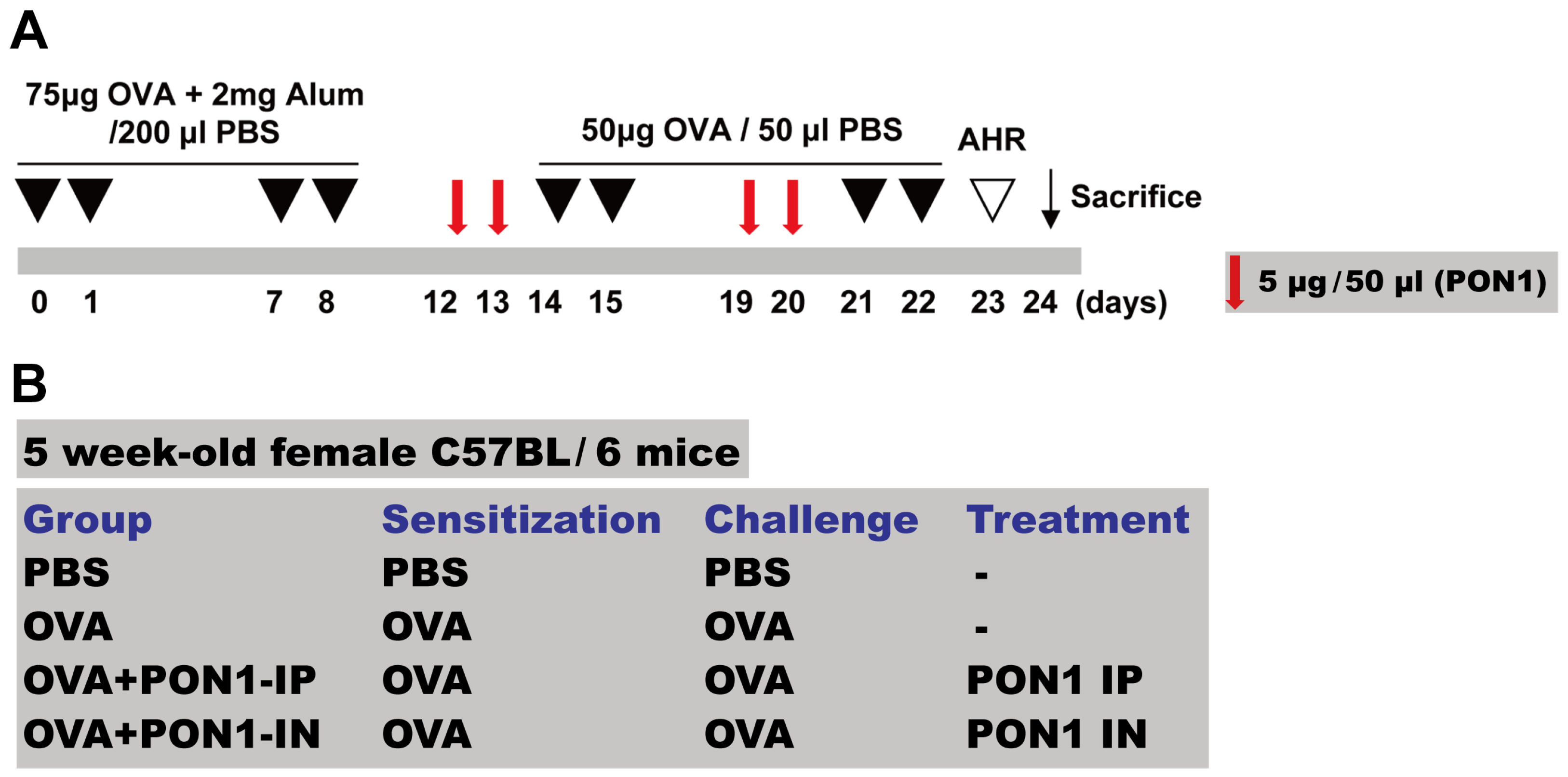
4.2. Mouse Model of Allergic Airway Inflammation
4.3. Intraperitoneal and Intranasal Administration of PON1
4.4. Measurement of AHR to Methacholine
4.5. Differential Cell Counting in Bronchoalveolar Lavage Fluid
4.6. Lung Histology and Inflammation Scoring
4.7. Measurement of Serum Immunoglobulin
4.8. Expression of Cytokines in the BALF and Lung Draining Lymph Nodes
4.9. Treatment of Lung Epithelial Cells and Analysis of Gene Expression
4.10. In Vitro Dendritic Cells Stimulation Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)Len and Allergen). Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Taylor, M.D.; Balic, A.; Finney, C.A.; Lamb, J.R.; Maizels, R.M. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2005, 202, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Z.; Qin, X.J. CD4CD25 regulatory T lymphocytes in allergy and asthma. Allergy 2005, 60, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, Z.; Sivakuru, T.; Roberts, K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J. Immunol. 2004, 172, 3842–3849. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, M.K.; Kang, S.A.; Park, H.Y.; Hong, S.L.; Park, H.K.; Yu, H.S.; Roh, H.J. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediat. Inflamm. 2014, 2014, 436476. [Google Scholar] [CrossRef]
- Kim, R.L.; Bang, J.Y.; Kim, J.; Mo, Y.; Kim, Y.; Lee, C.G.; Elias, J.A.; Kim, H.Y.; Kang, H.R. Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model. Sci. Rep. 2022, 12, 9811. [Google Scholar] [CrossRef]
- Song, J.; Zhu, X.M.; Wei, Q.Y. MSCs reduce airway remodeling in the lungs of asthmatic rats through the Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11199–11211. [Google Scholar]
- Mun, S.J.; Kang, S.A.; Park, H.K.; Yu, H.S.; Cho, K.S.; Roh, H.J. Intranasally administered extracellular vesicles from adipose stem cells have immunomodulatory effects in a mouse model of asthma. Stem Cells Int. 2021, 2021, 6686625. [Google Scholar] [CrossRef]
- Kim, S.D.; Kang, S.A.; Kim, Y.W.; Yu, H.S.; Cho, K.S.; Roh, H.J. Screening and functional pathway analysis of pulmonary genes associated with suppression of allergic airway inflammation by adipose stem cell-derived extracellular vesicles. Stem Cells Int. 2020, 2020, 5684250. [Google Scholar] [CrossRef]
- Cho, K.S.; Kang, S.A.; Kim, S.D.; Mun, S.J.; Yu, H.S.; Roh, H.J. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019, 39, 101500. [Google Scholar] [CrossRef]
- Sarioglu, N.; Hismiogullari, A.A.; Erel, F.; Demir, D.; Gencer, N. Paraoxonase 1 phenotype and paraoxonase activity in asthmatic patients. Iran. J. Allergy Asthma Immunol. 2015, 14, 60–66. [Google Scholar] [PubMed]
- Tanimoto, N.; Kumon, Y.; Suehiro, T.; Ohkubo, S.; Ikeda, Y.; Nishiya, K.; Hashimoto, K. Serum paraoxonase activity decreases in rheumatoid arthritis. Life Sci. 2003, 72, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Isik, A.; Koca, S.S.; Ustundag, B.; Celik, H.; Yildirim, A. Paraoxonase and arylesterase levels in rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 342–348. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.W.; Jansen, E.H.; Kruijshoop, M.; Beekhof, P.K.; Blaak, E.; van der Kallen, C.J.; van Greevenbroek, M.M.; Feskens, E.J.M. Paraoxonase 1 phenotype distribution and activity differs in subjects with newly diagnosed Type 2 diabetes (the CODAM Study). Diabet. Med. 2008, 25, 186–193. [Google Scholar] [CrossRef]
- Bahrehmand, F.; Vaisi-Raygani, A.; Rahimi, Z.; Ahmadi, R.; Kiani, A.; Tavilani, H.; Vaisi-Raygani, H.; Pourmotabbed, T. Synergistic effects of BuChE non-UU phenotype and paraoxonase (PON1) 55 M allele on the risk of systemic lupus erythematosus: Influence on lipid and lipoprotein metabolism and oxidative stress, preliminary report. Lupus 2014, 23, 263–272. [Google Scholar] [CrossRef]
- Asefi, M.; Vaisi-Raygani, A.; Bahrehmand, F.; Kiani, A.; Rahimi, Z.; Nomani, H.; Ebrahimi, A.; Tavilani, H.; Pourmotabbed, T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br. J. Dermatol. 2012, 167, 1279–1286. [Google Scholar] [CrossRef]
- Tolgyesi, G.; Molnar, V.; Semsei, A.F.; Kiszel, P.; Ungvari, I.; Pocza, P.; Wiener, Z.; Komlosi, Z.I.; Kunos, L.; Galffy, G.; et al. Gene expression profiling of experimental asthma reveals a possible role of paraoxonase-1 in the disease. Int. Immunol. 2009, 21, 967–975. [Google Scholar] [CrossRef][Green Version]
- Emin, O.; Hasan, A.; Rusen, D.M. Plasma paraoxonase, oxidative status level, and their relationship with asthma control test in children with asthma. Allergol. Immunopathol. 2015, 43, 346–352. [Google Scholar] [CrossRef]
- Chen, W.Q.; Xie, Z.Z.; Wang, X.; Zhao, J.H.; Hu, Q.; Chen, Y.H.; Gao, W.Y.; Liu, Y. Influences of PON1 on airway inflammation and remodeling in bronchial asthma. J. Cell. Biochem. 2018, 119, 793–805. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, I.K.; Yoon, H.K.; Kwon, S.S.; Rhee, C.K.; Lee, S.Y. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-β/smad signaling pathway in chronic asthma model. Allergy Asthma Immunol. Res. 2017, 9, 25–34. [Google Scholar] [CrossRef]
- Cheng, H.; Guan, X.; Chen, D.; Ma, W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms 2019, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H.; Brightling, C.E.; Pavord, I.D.; Wardlaw, A.J. Management of asthma in adults: Current therapy and future directions. Postgrad. Med. J. 2003, 79, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Armentaro, G.; Calabrese, C.; Sciacqua, A.; Gallelli, L.; Vatrella, A. Biological therapy of severe asthma with dupilumab, a dual receptor antagonist of interleukin 4 and 13. Vaccines 2022, 10, 974. [Google Scholar] [CrossRef]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhuansun, Y.X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef]
- Yu, H.S.; Park, M.K.; Kang, S.A.; Cho, K.S.; Mun, S.J.; Roh, H.J. Culture supernatant of adipose stem cells can ameliorate allergic airway inflammation via recruitment of CD4+CD25+Foxp3 T cells. Stem Cell Res. Ther. 2017, 8, 8. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, K.S. Immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in allergic airway disease. Life 2022, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Moroni, C.; Savino, S.; Liuzzi, A.; Balzola, F.; Bicchiega, V. Paraoxonase activity in high-density lipoproteins: A comparison between healthy and obese females. J. Clin. Endocrinol. Metab. 2005, 90, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Fulop, T.; Berrougui, H. Role of paraoxonase1 in the regulation of high-density lipoprotein functionality and in cardiovascular protection. Antioxid. Redox Signal. 2021, 34, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, H.; Kuczkowski, A.; Ruehl, M.; Lamkemeyer, T.; Brodesser, S.; Horke, S.; Dryer, S.; Schermer, B.; Benzing, T.; Brinkkoetter, P.T. Breaking the chain at the membrane: Paraoxonase 2 counteracts lipid peroxidation at the plasma membrane. FASEB J. 2014, 28, 1769–1779. [Google Scholar] [CrossRef]
- Priyanka, K.; Singh, S.; Gill, K. Paraoxonase 3: Structure and its role in pathophysiology of coronary artery disease. Biomolecules 2019, 9, 817. [Google Scholar] [CrossRef]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef]
- Ekmekci, O.B.; Donma, O.; Ekmekci, H.; Yildirim, N.; Uysal, O.; Sardogan, E.; Demirel, H.; Demir, T. Plasma paraoxonase activities, lipoprotein oxidation, and trace element interaction in asthmatic patients. Biol. Trace Elem. Res. 2006, 111, 41–52. [Google Scholar] [CrossRef]
- Cheraghi, M.; Shahsavari, G.; Maleki, A.; Ahmadvand, H. Paraoxonase 1 activity, lipid profile, and atherogenic indexes status in coronary heart disease. Rep. Biochem. Mol. Biol. 2017, 6, 1–7. [Google Scholar]
- Elkiran, E.T.; Mar, N.; Aygen, B.; Gursu, F.; Karaoglu, A.; Koca, S. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 2007, 7, 48. [Google Scholar] [CrossRef]
- Lavie, L.; Vishnevsky, A.; Lavie, P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004, 27, 123–128. [Google Scholar] [PubMed]
- Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and chronic obstructive pulmonary disease: A meta-analysis. Antioxidants 2021, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Gornicka, G.; Beltowski, J.; Wojcicka, G.; Jamroz, A. Serum paraoxonase activity, total antioxidant potential and lipid peroxidation products in children with bronchial asthma exacerbation. Wiad. Lek. 2002, 55, 257–263. [Google Scholar] [PubMed]
- Bassu, S.; Mangoni, A.A.; Argiolas, D.; Carru, C.; Pirina, P.; Fois, A.G.; Zinellu, A. A systematic review and meta-analysis of paraoxonase-1 activity in asthma. Clin. Exp. Med. 2023, 23, 1067–1074. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Park, H.; Wang, Y.H.; Wang, Y.H.; Chang, S.H.; Corry, D.B.; Liu, Y.J.; Zhu, Z.; Dong, C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007, 204, 1509–1517. [Google Scholar] [CrossRef]
- Cella, M.; Sallusto, F.; Lanzavecchia, A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997, 9, 10–16. [Google Scholar] [CrossRef]
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; van der Strate, B.W.; Kerstjens, H.A.M.; Timens, W.; Hylkema, M.N. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy 2005, 35, 1496–1503. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, J.H.; Park, M.K.; Park, H.K.; Yu, H.S.; Roh, H.J. Prostaglandin E2 and Transforming Growth Factor-β Play a Critical Role in Suppression of Allergic Airway Inflammation by Adipose-Derived Stem Cells. PLoS ONE 2015, 10, e0131813. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Wu, K.; Azhati, B.; Rexiati, M. Culture and identification of mouse bone marrow-derived dendritic cells and their capability to induce T lymphocyte proliferation. Med. Sci. Monit. 2016, 22, 244–250. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.H.; Kang, S.A.; Park, J.-H.; Kim, S.-D.; Yu, H.S.; Mun, S.J.; Cho, K.-S. Paraoxonase-1 Is a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation Through Adipose Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 12756. https://doi.org/10.3390/ijms252312756
Jung JH, Kang SA, Park J-H, Kim S-D, Yu HS, Mun SJ, Cho K-S. Paraoxonase-1 Is a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation Through Adipose Stem Cell-Derived Extracellular Vesicles. International Journal of Molecular Sciences. 2024; 25(23):12756. https://doi.org/10.3390/ijms252312756
Chicago/Turabian StyleJung, Jae Hoon, Shin Ae Kang, Ji-Hwan Park, Sung-Dong Kim, Hak Sun Yu, Sue Jean Mun, and Kyu-Sup Cho. 2024. "Paraoxonase-1 Is a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation Through Adipose Stem Cell-Derived Extracellular Vesicles" International Journal of Molecular Sciences 25, no. 23: 12756. https://doi.org/10.3390/ijms252312756
APA StyleJung, J. H., Kang, S. A., Park, J.-H., Kim, S.-D., Yu, H. S., Mun, S. J., & Cho, K.-S. (2024). Paraoxonase-1 Is a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation Through Adipose Stem Cell-Derived Extracellular Vesicles. International Journal of Molecular Sciences, 25(23), 12756. https://doi.org/10.3390/ijms252312756






