The Role of Medium Polarity in the Efficiency of Albumin Binding with Hydrophobic Ligands: Experimental Studies and a Molecular Dynamics Investigation
Abstract
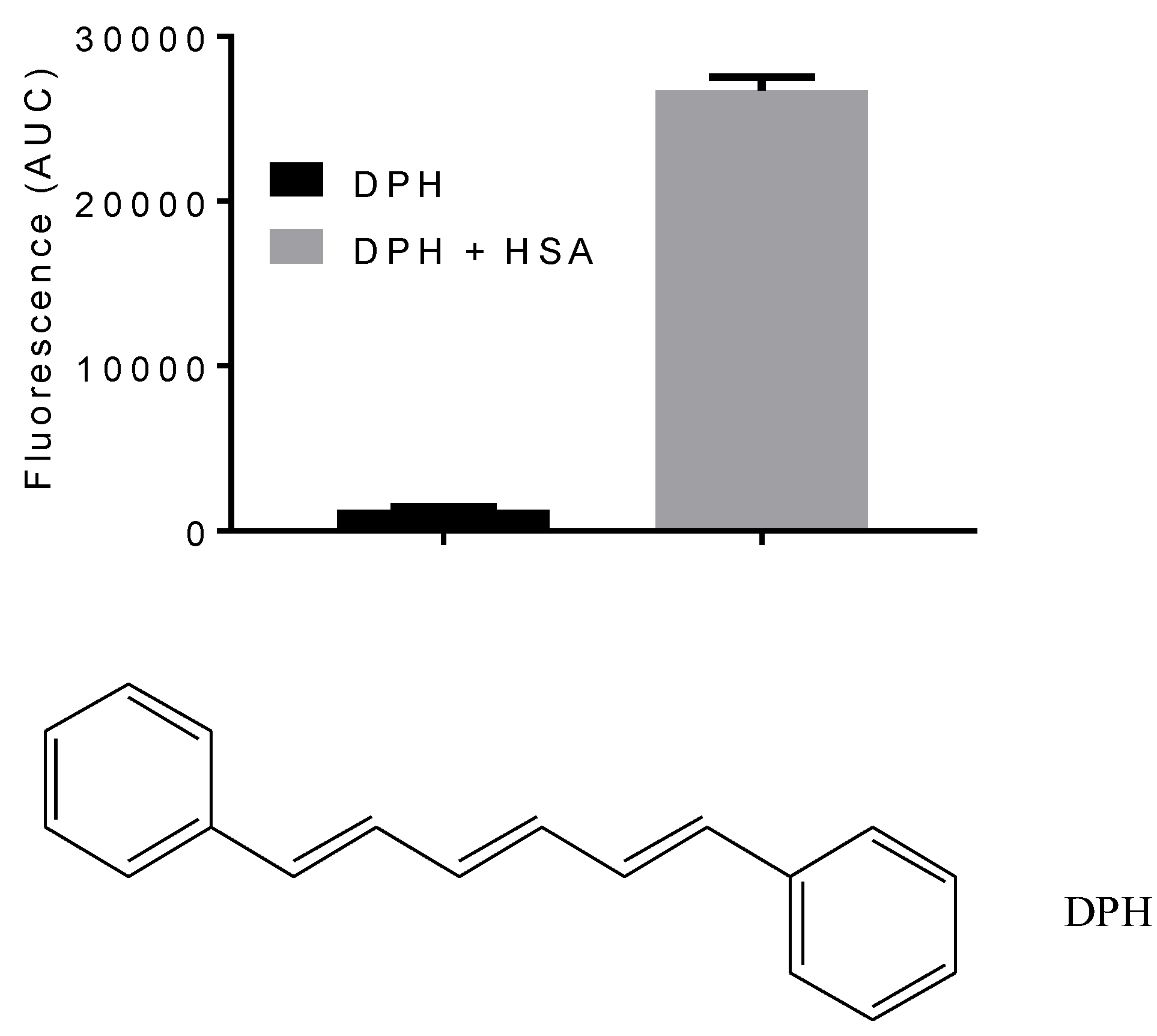
1. Introduction
2. Results and Discussion
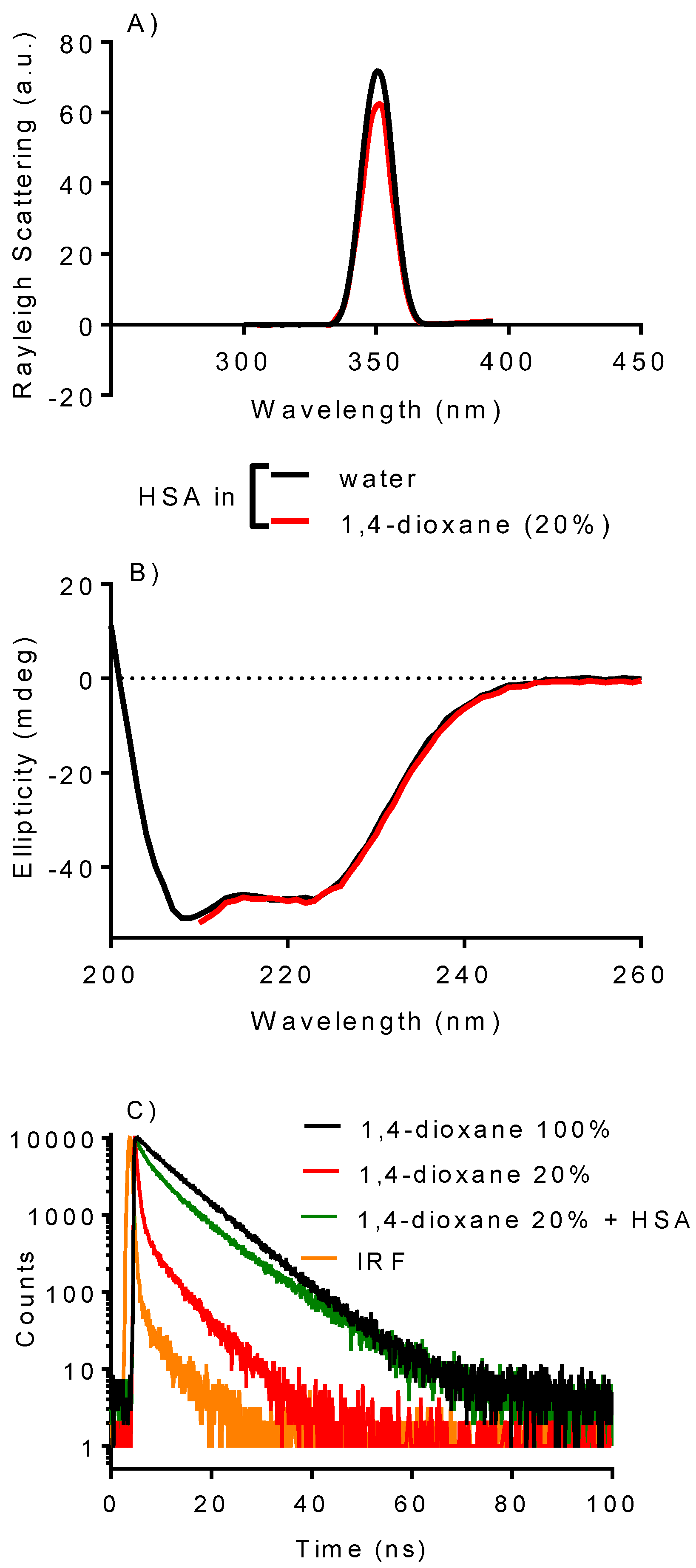
2.1. Integrity of HSA in the Presence of 1,4-Dioxane
- To determine the binding constant of DPH in solvents of different polarities (mixtures of water and 1,4-dioxane);
- To determine the effect of NaCl;
- To evaluate the transfer of DPH from the protein environment to the bulk phase by molecular dynamics.
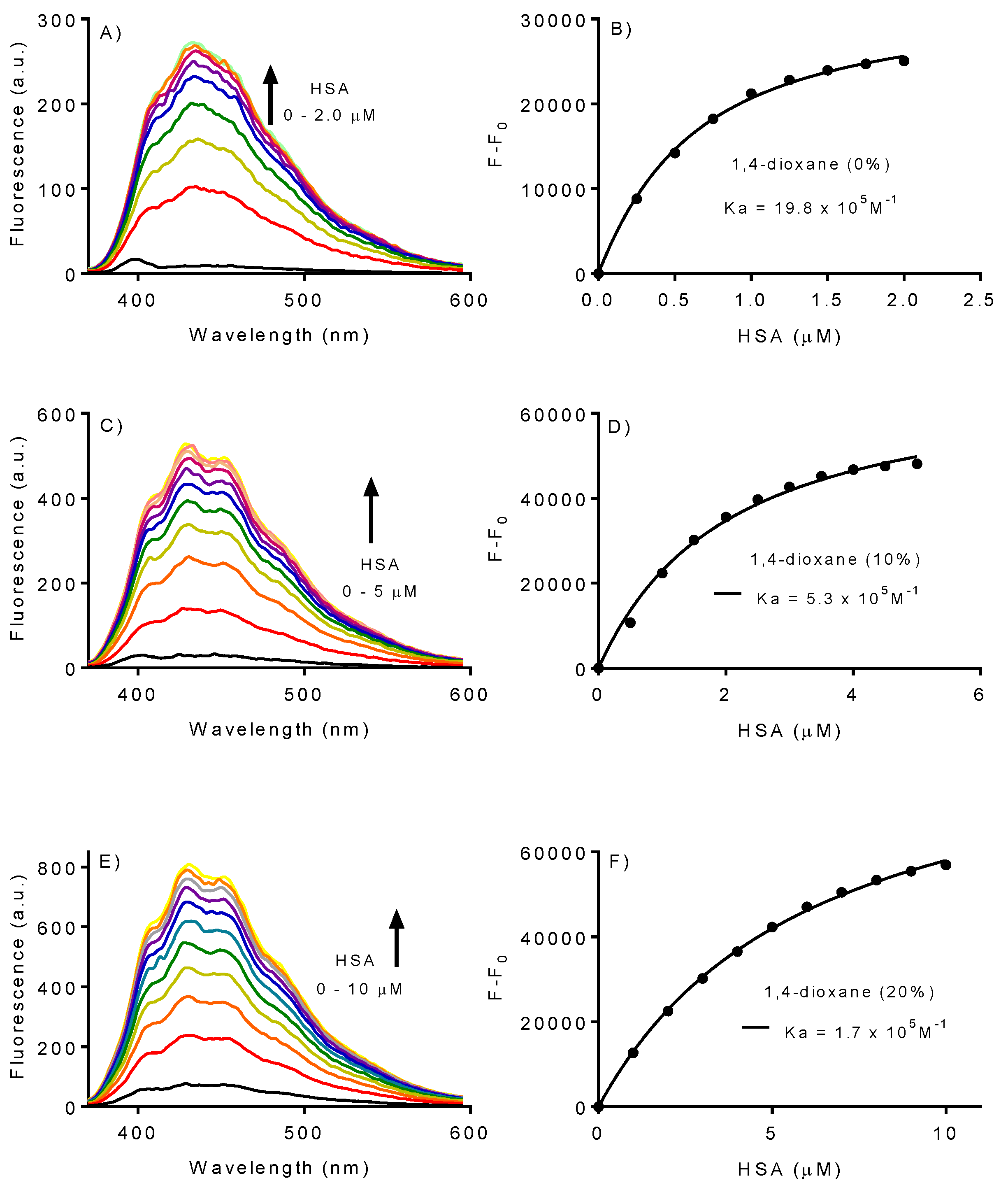
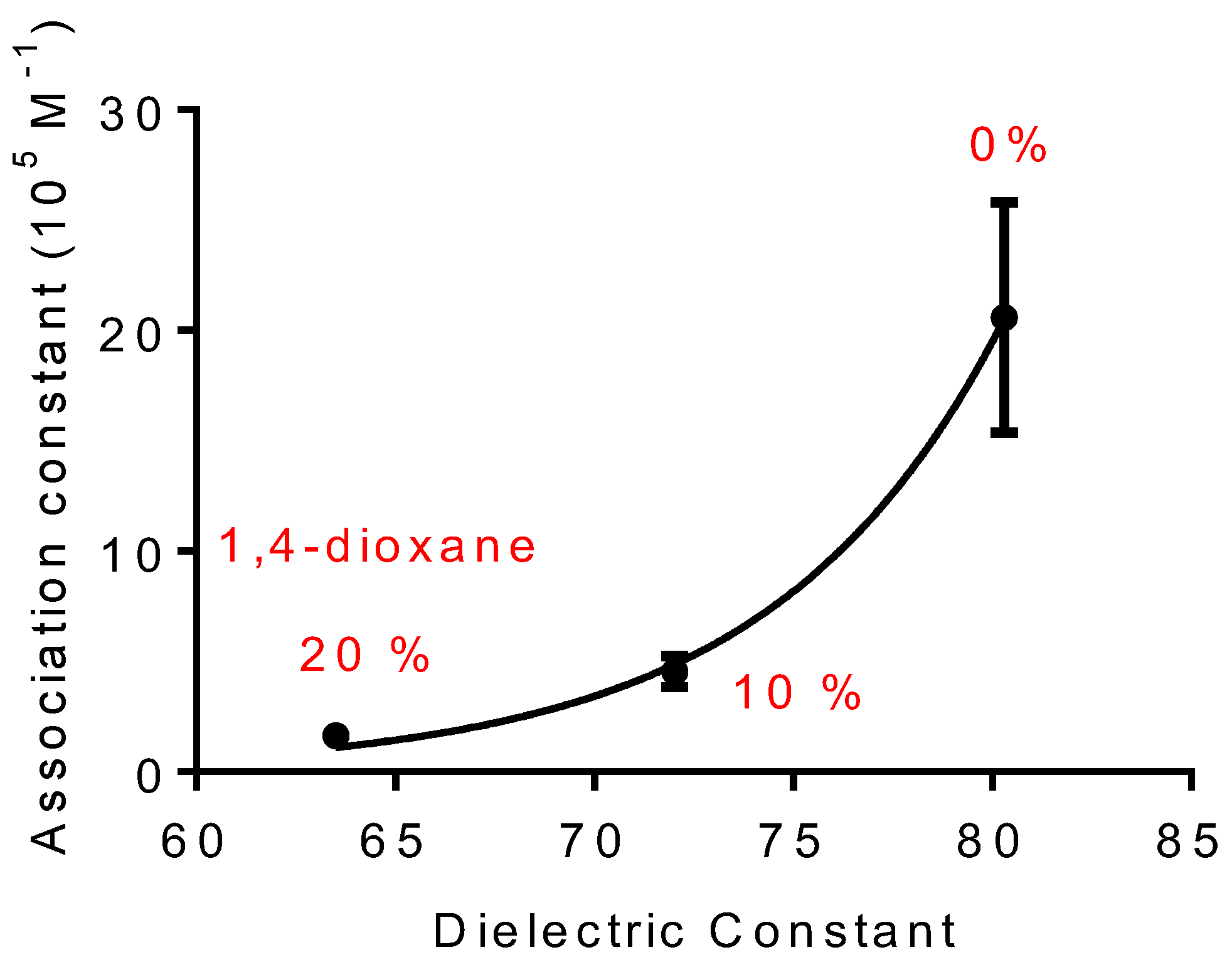
2.2. Effect of 1,4-Dioxane on the Association Constant of DPH
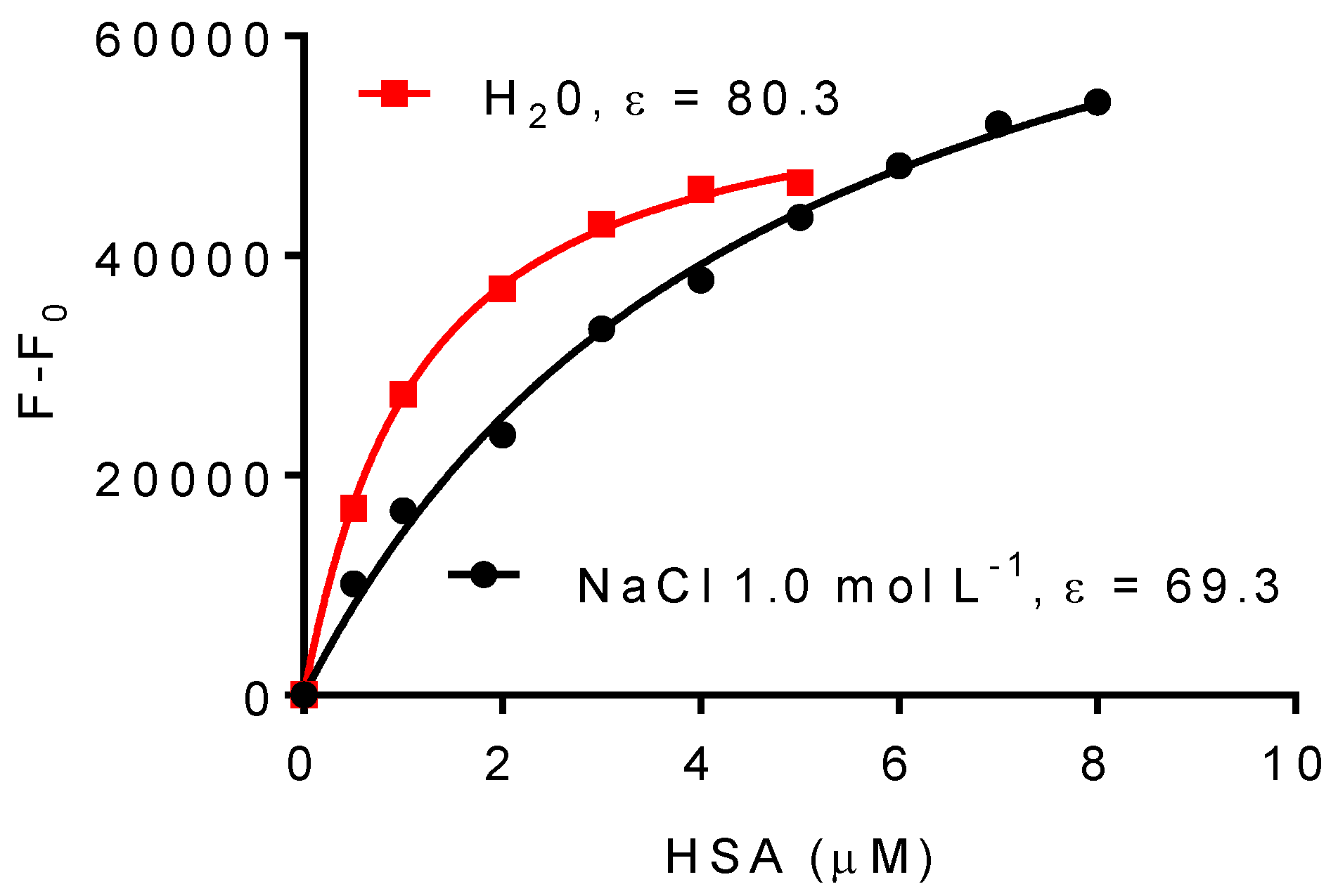
2.3. Effect of NaCl on the Association Constant of DPH
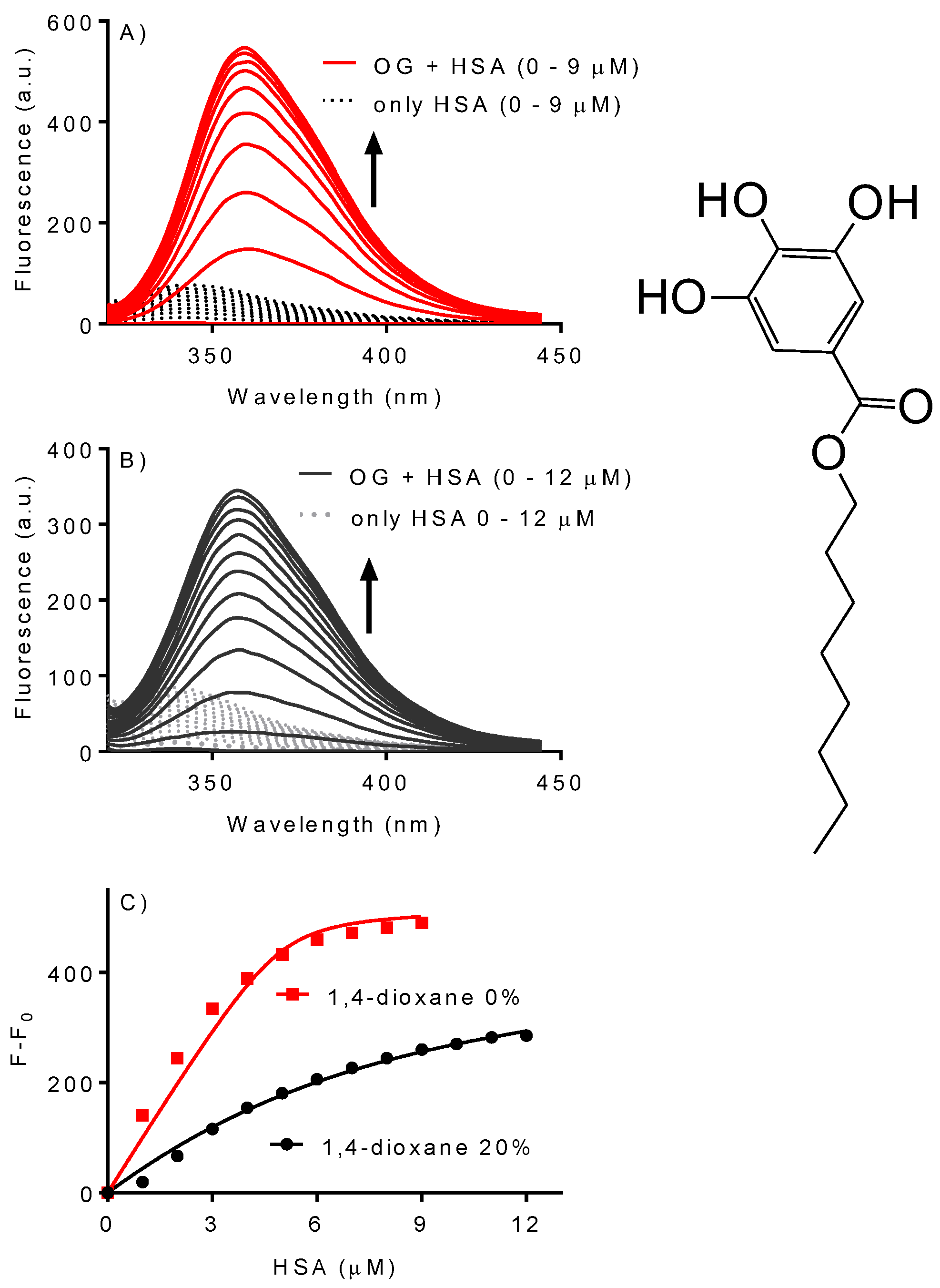
2.4. Effect of 1,4-Dioxane on the Association Constant of Octyl Gallate
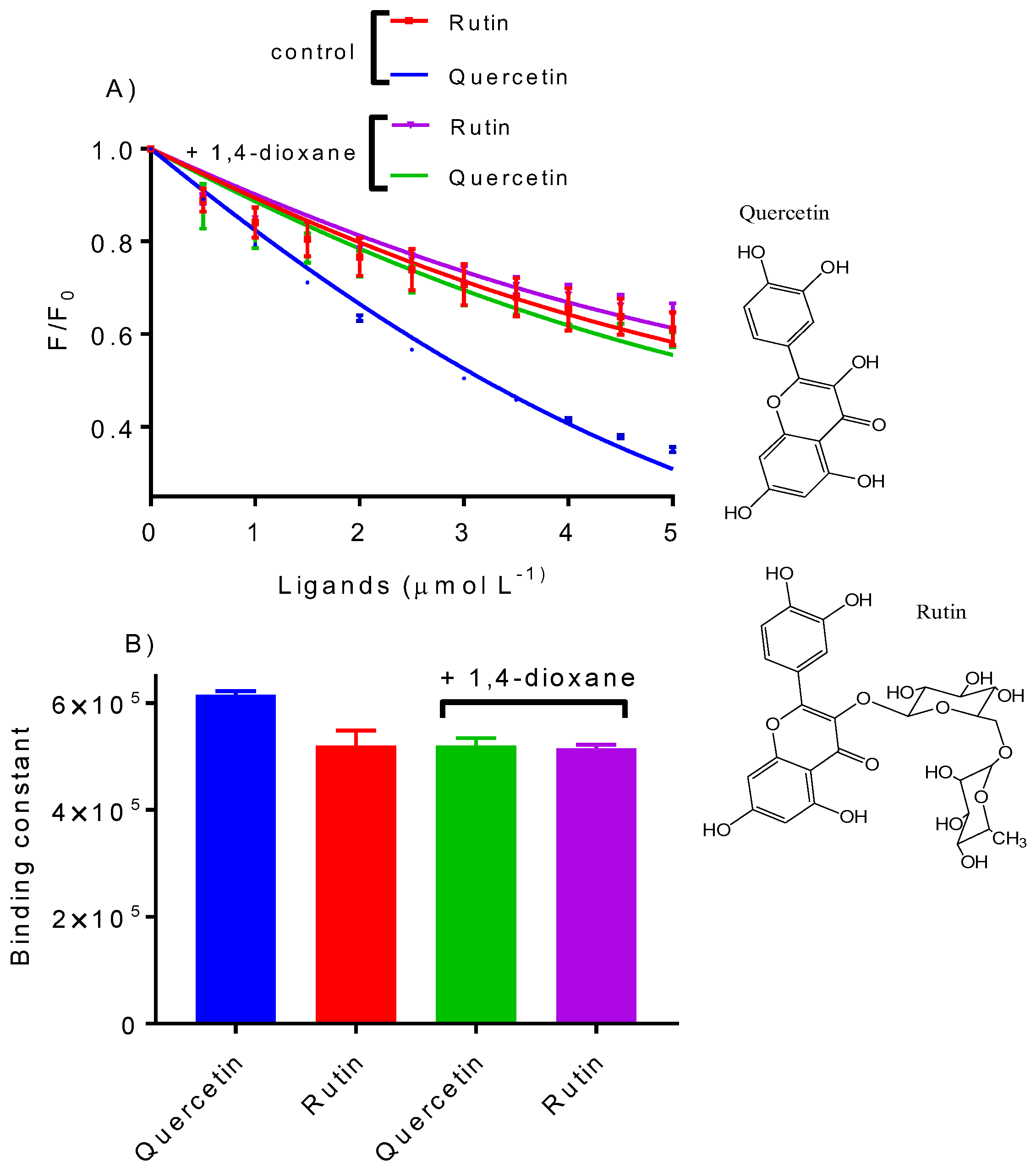
2.5. Quercetin Versus Rutin as Ligands of HSA
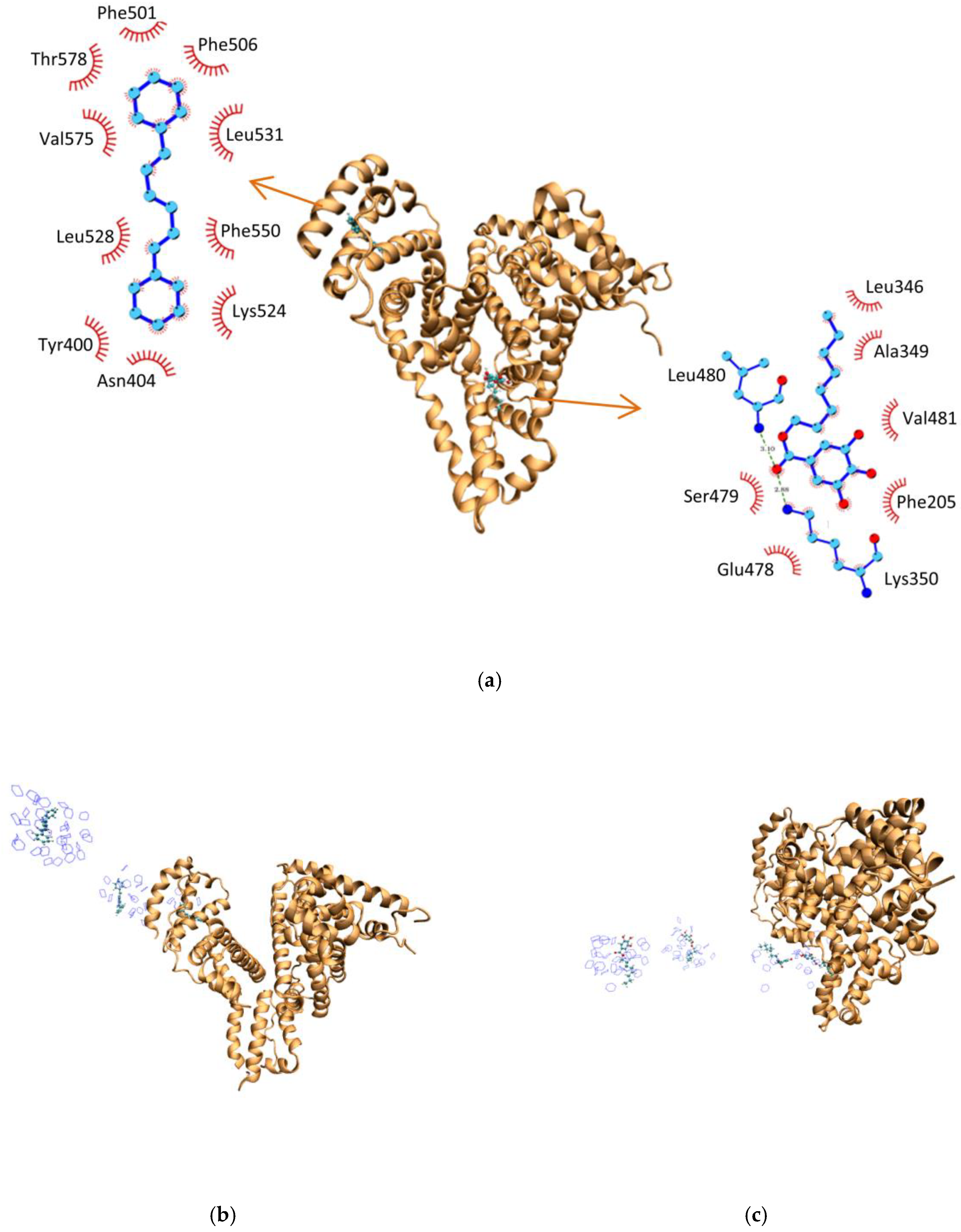
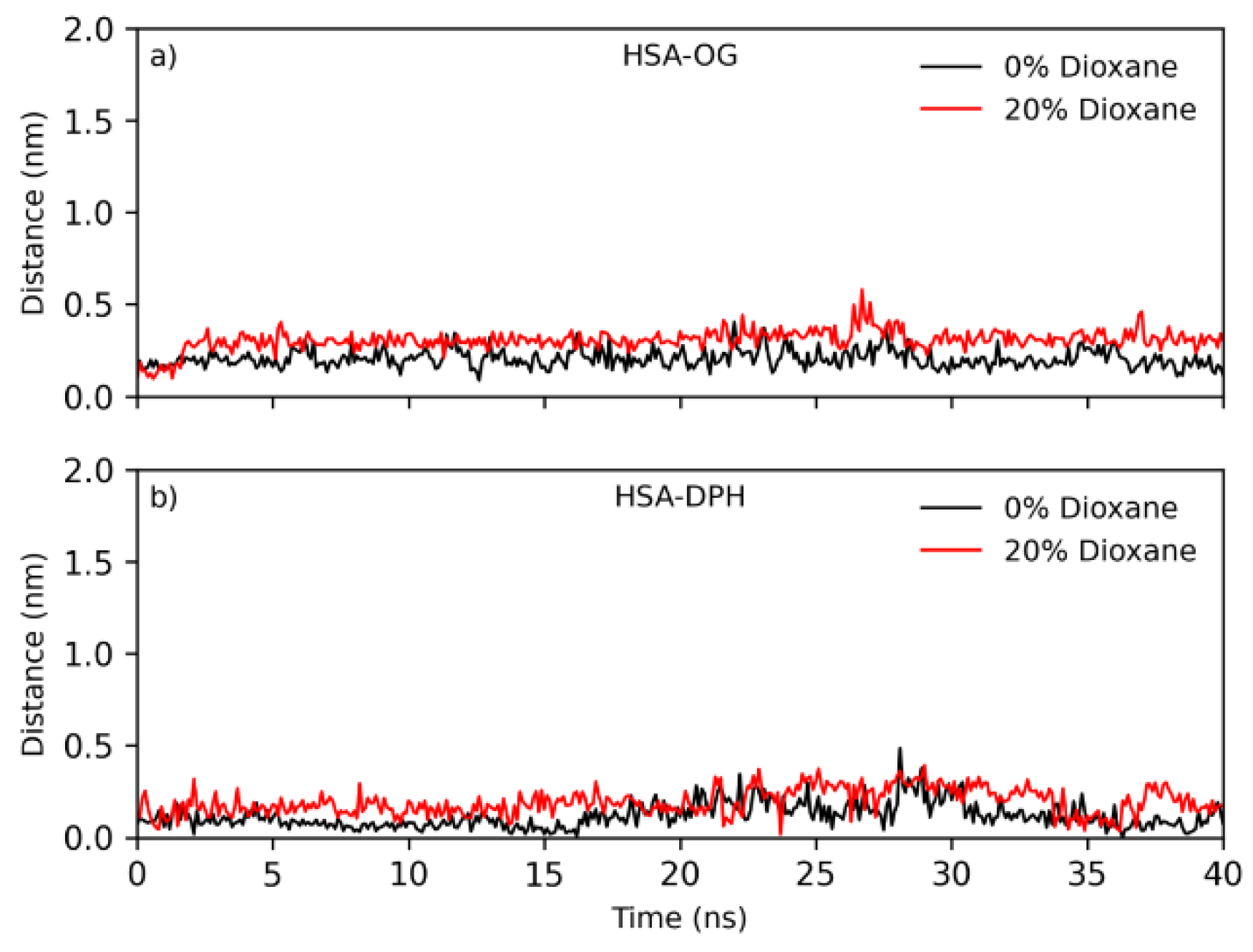
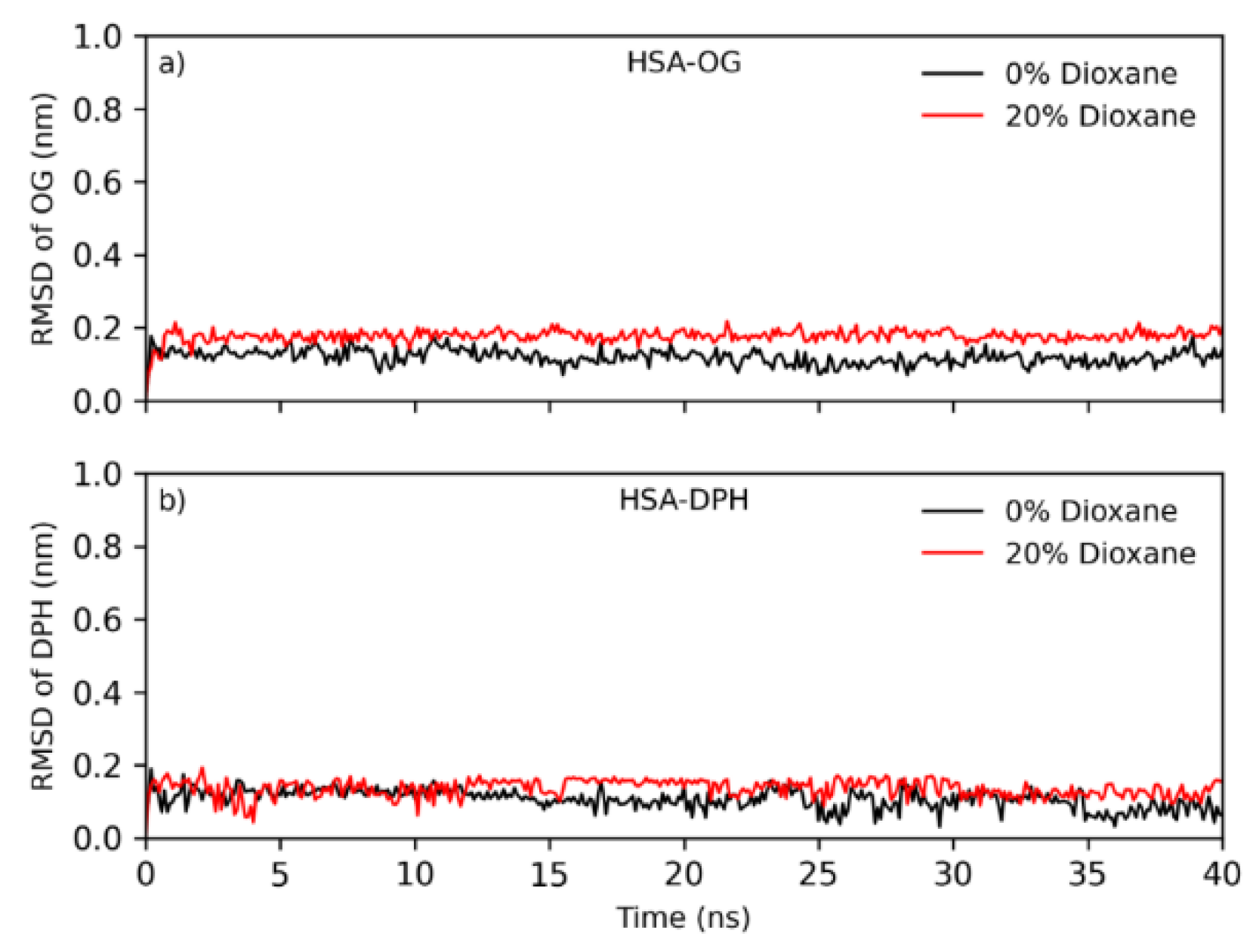
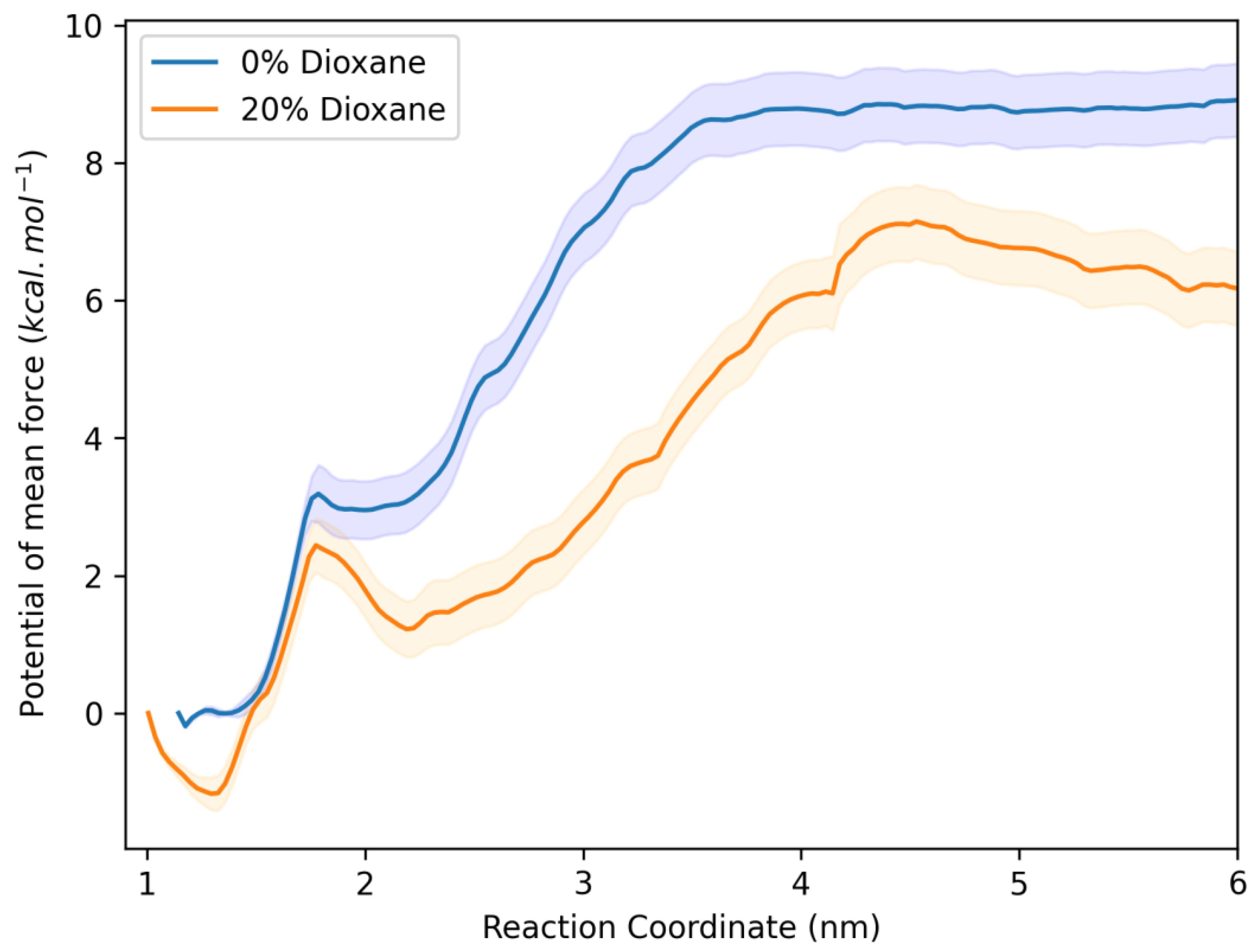
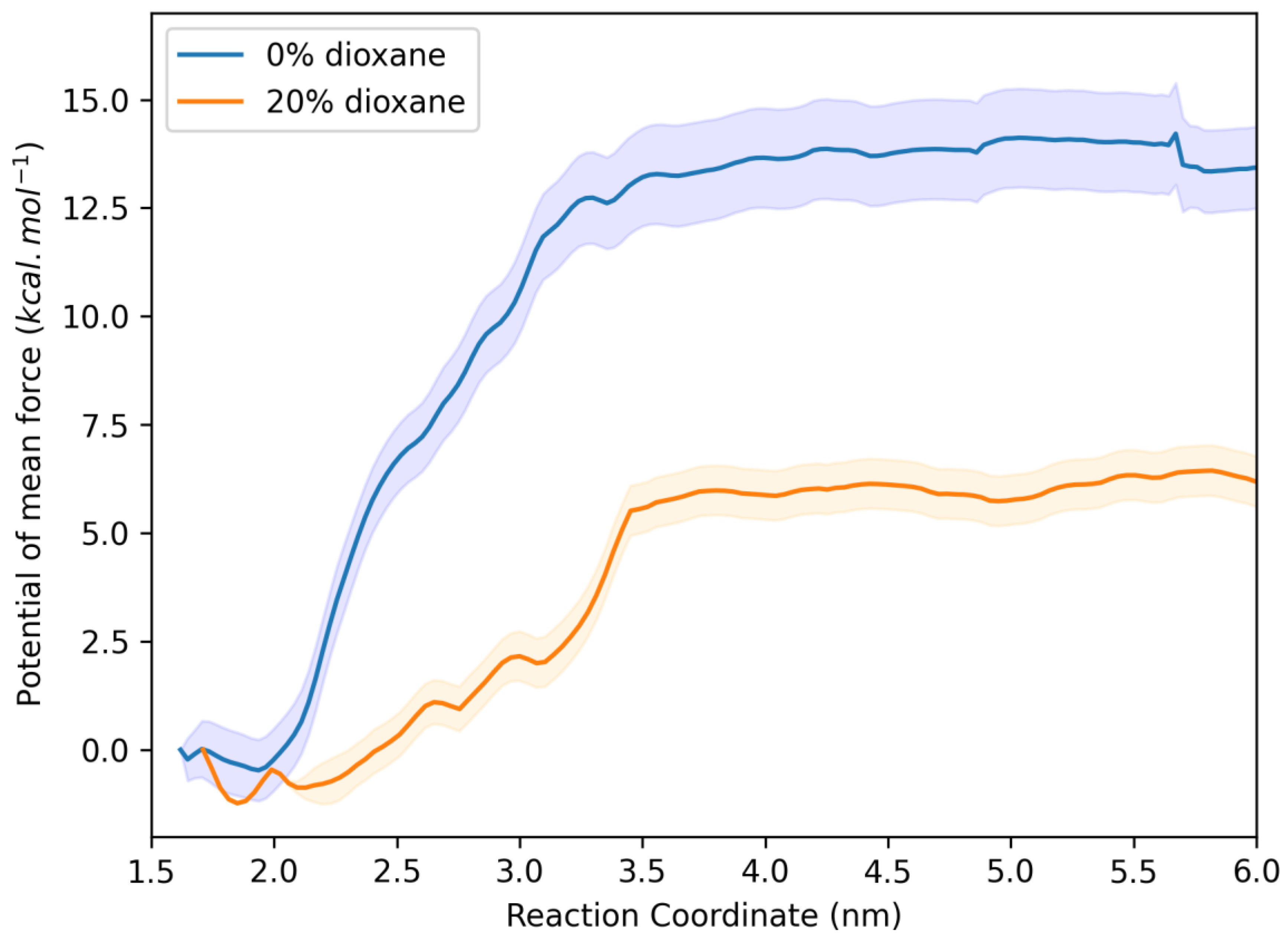
2.6. Molecular Dynamics Studies
3. Materials and Methods
3.1. Reagents
3.2. Measurement of Binding Constants
3.3. Circular Dichroism and Rayleigh Scattering Assays
3.4. Time-Resolved Fluorescence Studies
3.5. Molecular Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frayn, K.N.; Arner, P.; Yki-Järvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [PubMed]
- Nishi, K.; Yamasaki, K.; Otagiri, M. Serum albumin, lipid and drug binding. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Springer: Berlin/Heidelberg, Germany, 2020; pp. 383–397. [Google Scholar]
- Ullah, A.; Kwon, H.T.; Lim, S.I. Albumin: A Multi-talented Clinical and Pharmaceutical Player. Biotechnol. Bioprocess Eng. 2022, 27, 765–787. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Pilati, D.; Howard, K.A. Albumin-based drug designs for pharmacokinetic modulation. Expert Opin. Drug Metab. Toxicol. 2020, 16, 783–795. [Google Scholar] [CrossRef]
- Leboffe, L.; di Masi, A.; Polticelli, F.; Trezza, V.; Ascenzi, P. Structural basis of drug recognition by human serum albumin. Curr. Med. Chem. 2020, 27, 4907–4931. [Google Scholar] [CrossRef]
- de Carvalho Bertozo, L.; Tadeu, H.C.; Sebastian, A.; Maszota-Zieleniak, M.; Samsonov, S.A.; Ximenes, V.F. Role for Carboxylic Acid Moiety in NSAIDs: Favoring the Binding at Site II of Bovine Serum Albumin. Mol. Pharm. 2024, 21, 2501–2511. [Google Scholar] [CrossRef]
- Van de Sande, L.; Cosyns, S.; Willaert, W.; Ceelen, W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: A review. Drug Deliv. 2020, 27, 40–53. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging albumin-binding anticancer drugs for tumor-targeted drug delivery: Current understandings and clinical translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Sun, Q. The hydrophobic effects: Our current understanding. Molecules 2022, 27, 7009. [Google Scholar] [CrossRef]
- Dragan, A.I.; Read, C.M.; Crane-Robinson, C. Enthalpy–entropy compensation: The role of solvation. Eur. Biophys. J. 2017, 46, 301–308. [Google Scholar] [CrossRef]
- Schönbeck, C.; Holm, R. Exploring the origins of enthalpy–Entropy compensation by calorimetric studies of cyclodextrin complexes. J. Phys. Chem. B 2019, 123, 6686–6693. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Anusiem, A.C.; Biltonen, R.L. Enthalpy-entropy compensation and heat capacity changes for protein-ligand interactions: General thermodynamic models and data for the binding of nucleotides to ribonuclease A. Biochemistry 1983, 22, 3884–3896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-J.; Kou, S.-B.; Hu, L.; Li, L.; Shi, J.-H.; Jiang, S.-L. Exploring binding interaction of baricitinib with bovine serum albumin (BSA): Multi-spectroscopic approaches combined with theoretical calculation. J. Mol. Liq. 2022, 354, 118831. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Borlan, R.; Focsan, M.; Oniga, O.; Bogdan, M.; Vlase, L.; Oniga, I.; Marc, G. Antioxidant Activity Evaluation and Assessment of the Binding Affinity to HSA of a New Catechol Hydrazinyl-Thiazole Derivative. Antioxidants 2022, 11, 1245. [Google Scholar] [CrossRef]
- Nai, X.; Chen, Y.; Hao, S.; Liu, M.; Zhang, Q.; Liu, J.; Li, M.; Kong, J. Temperature, pH and additives effects on the binding of Caffeic acid phenethyl ester to the native state of bovine serum albumin. J. Chem. Thermodyn. 2022, 168, 106724. [Google Scholar] [CrossRef]
- de Carvalho Bertozo, L.; Maszota-Zieleniak, M.; Bolean, M.; Ciancaglini, P.; Samsonov, S.A.; Ximenes, V.F. Binding of fluorescent dansyl amino acids in albumin: When access to the protein cavity is more important than the strength of binding. Dye. Pigment. 2021, 188, 109195. [Google Scholar] [CrossRef]
- Singh, S.; Gopi, P.; Pandya, P. Structural aspects of formetanate hydrochloride binding with human serum albumin using spectroscopic and molecular modeling techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121618. [Google Scholar] [CrossRef]
- Singh, I.R.; Yesylevskyy, S.O.; Mitra, S. Dietary polyphenols inhibit plasma protein arabinosylation: Biomolecular interaction of genistein and ellagic acid with serum albumins. Biophys. Chem. 2021, 277, 106651. [Google Scholar] [CrossRef]
- de Carvalho Bertozo, L.; Fernandes, A.J.F.C.; Yoguim, M.I.; Bolean, M.; Ciancaglini, P.; Ximenes, V.F. Entropy-driven binding of octyl gallate in albumin: Failure in the application of temperature effect to distinguish dynamic and static fluorescence quenching. J. Mol. Recognit. 2020, 33, e2840. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Deng, N.; Zhang, P.; Cieplak, P.; Lai, L. Elucidating the energetics of entropically driven protein–ligand association: Calculations of absolute binding free energy and entropy. J. Phys. Chem. B 2011, 115, 11902–11910. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kellogg, G.E. Hydrophobicity-shake flasks, protein folding and drug discovery. Curr. Top. Med. Chem. 2010, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Doweyko, A.M.; Johnson, S.R. A novel method to simulate the hydrophobic effect and its application to the ranking of host/guest complexes. J. Chem. Inf. Model. 2006, 46, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, M.D.; Westwood, I.M.; Barbeau, O.; Rowlands, M.; Dobson, S.; Jones, A.M.; Jeganathan, F.; Burke, R.; Kadi, N.; Workman, P. Exploiting protein conformational change to optimize adenosine-derived inhibitors of HSP70. J. Med. Chem. 2016, 59, 4625–4636. [Google Scholar] [CrossRef]
- Poojari, C.; Wilkosz, N.; Lira, R.B.; Dimova, R.; Jurkiewicz, P.; Petka, R.; Kepczynski, M.; Róg, T. Behavior of the DPH fluorescence probe in membranes perturbed by drugs. Chem. Phys. Lipids 2019, 223, 104784. [Google Scholar] [CrossRef]
- Alves, A.C.; Ribeiro, D.; Horta, M.; Lima, J.L.F.C.; Nunes, C.; Reis, S. The daunorubicin interplay with mimetic model membranes of cancer cells: A biophysical interpretation. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 941–948. [Google Scholar] [CrossRef]
- Makwana, P.K.; Jethva, P.N.; Roy, I. Coumarin 6 and 1,6-diphenyl-1,3,5-hexatriene (DPH) as fluorescent probes to monitor protein aggregation. Analyst 2011, 136, 2161–2167. [Google Scholar] [CrossRef]
- Bryl, K. Fluorescence resonance energy transfer (FRET) as a spectroscopic ruler for the investigation of protein induced lipid membrane curvature: Bacteriorhodopsin and bacteriorhodopsin analogs in model lipid membranes. Appl. Spectrosc. 2023, 77, 187–199. [Google Scholar] [CrossRef]
- Li, M.-H.; Manathunga, L.; London, E.; Raleigh, D.P. The Fluorescent Dye 1,6-Diphenyl-1,3,5-hexatriene Binds to Amyloid Fibrils Formed by Human Amylin and Provides a New Probe of Amylin Amyloid Kinetics. Biochemistry 2021, 60, 1964–1970. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Åkerlöf, G.; Short, O.A. The dielectric constant of dioxane—Water mixtures between 0 and 80. J. Am. Chem. Soc. 1936, 58, 1241–1243. [Google Scholar] [CrossRef]
- Critchfield, F.E.; Gibson, J.A., Jr.; Hall, J.L. Dielectric constant for the dioxane—Water system from 20 to 35°. J. Am. Chem. Soc. 1953, 75, 1991–1992. [Google Scholar] [CrossRef]
- Van Holde, K.E.; Sun, S.F. Bovine serum albumin in water-dioxane mixtures. J. Am. Chem. Soc. 1962, 84, 66–72. [Google Scholar] [CrossRef]
- Khan, M.S.; Althobaiti, M.S.; Almutairi, G.S.; Alokail, M.S.; Altwaijry, N.; Alenad, A.M.; Al-Bagmi, M.S.; Alafaleq, N.O. Elucidating the binding and inhibitory potential of p-coumaric acid against amyloid fibrillation and their cytotoxicity: Biophysical and docking analysis. Biophys. Chem. 2022, 291, 106823. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Pavan, N.M.; Jones, A.M.; Ximenes, V.F. Solvent-Induced Lag Phase during the Formation of Lysozyme Amyloid Fibrils Triggered by Sodium Dodecyl Sulfate: Biophysical Experimental and In Silico Study of Solvent Effects. Molecules 2023, 28, 6891. [Google Scholar] [CrossRef]
- Barrow, D.A.; Lentz, B.R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys. J. 1985, 48, 221–234. [Google Scholar] [CrossRef]
- Fernandes, A.J.F.C.; de Carvalho Bertozo, L.; Povinelli, A.P.R.; Zazeri, G.; de Souza, A.R.; Morgon, N.H.; Ximenes, V.F. 4-Dimethylamino-beta-nitrostyrene, a fluorescent solvatochromic probe to estimate the apparent dielectric constant in serum albumin: Experimental and molecular dynamics studies. J. Photochem. Photobiol. A Chem. 2022, 433, 114197. [Google Scholar] [CrossRef]
- Pavan, N.M.; Martins, L.M.; Augusto, L.C.; da Silva-Filho, L.C.; Ximenes, V.F. Development of fluorescent azapentalenes to study the reactivity of hypochlorous acid and chloramines in micellar systems. J. Mol. Liq. 2022, 365, 120137. [Google Scholar] [CrossRef]
- Hasted, J.B.; Ritson, D.M.; Collie, C.H. Dielectric properties of aqueous ionic solutions. Parts I and II. J. Chem. Phys. 1948, 16, 1–21. [Google Scholar] [CrossRef]
- Bramley, A. Dielectric constant of aqueous solutions of sodium chloride. J. Franklin Inst. 1928, 205, 649–657. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, H. Octyl gallate: An antioxidant demonstrating selective and sensitive fluorescent property. Food Chem. 2017, 219, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, L.; Ma, L.; Liu, S.; Zhou, H.; Zhang, Y.; Guo, T.; Liu, W.; Dai, H.; Yu, Y. Fluorescence spectroscopic investigation of competitive interactions between quercetin and aflatoxin B1 for binding to human serum albumin. Toxins 2019, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, N.A.; Wani, T.A.; Bakheit, A.H.; Zargar, S. Multi-spectroscopic investigation, molecular docking and molecular dynamic simulation of competitive interactions between flavonoids (quercetin and rutin) and sorafenib for binding to human serum albumin. Int. J. Biol. Macromol. 2020, 165, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Niether, D.; Koenig, B.W.; Lohstroh, W.; Zamponi, M.; Jalarvo, N.H.; Wiegand, S.; Fitter, J.; Stadler, A.M. Strong adverse contribution of conformational dynamics to streptavidin–biotin binding. J. Phys. Chem. B 2019, 124, 324–335. [Google Scholar] [CrossRef]
- Verteramo, M.L.; Stenstrom, O.; Ignjatovic, M.M.; Caldararu, O.; Olsson, M.A.; Manzoni, F.; Leffler, H.; Oksanen, E.; Logan, D.T.; Nilsson, U.J. Interplay between conformational entropy and solvation entropy in protein–ligand binding. J. Am. Chem. Soc. 2019, 141, 2012–2026. [Google Scholar] [CrossRef]
- Syme, N.R.; Dennis, C.; Bronowska, A.; Paesen, G.C.; Homans, S.W. Comparison of entropic contributions to binding in a “hydrophilic” versus “hydrophobic” ligand− protein interaction. J. Am. Chem. Soc. 2010, 132, 8682–8689. [Google Scholar] [CrossRef]
- van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- De Vasconcelos, D.N.; Lima, A.N.; Philot, E.A.; Scott, A.L.; Ferreira Boza, I.A.; De Souza, A.R.; Morgon, N.H.; Ximenes, V.F. Methyl divanillate: Redox properties and binding affinity with albumin of an antioxidant and potential NADPH oxidase inhibitor. RSC Adv. 2019, 9, 19983–19992. [Google Scholar] [CrossRef]
- Roy, S. Fluorescence quenching methods to study protein–nucleic acid interactions. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 379, pp. 175–187. [Google Scholar]
- Graciani, F.S.; Ximenes, V.F. Investigation of human albumin-induced circular dichroism in dansylglycine. PLoS ONE 2013, 8, e76849. [Google Scholar] [CrossRef]
- Sillen, A.; Engelborghs, Y. The correct use of “average” fluorescence parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Zazeri, G.; Povinelli, A.P.R.; Le Duff, C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and Spectroscopic Analysis of Piperine-and Piperlongumine-Inspired Natural Product Scaffolds and Their Molecular Docking with IL-1β and NF-κB Proteins. Molecules 2020, 25, 2841. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An automated force field topology builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Batcho, P.F.; Case, D.A.; Schlick, T. Optimized particle-mesh Ewald/multiple-time step integration for molecular dynamics simulations. J. Chem. Phys. 2001, 115, 4003. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazeri, G.; Povinelli, A.P.R.; Bertozo, L.d.C.; Jones, A.M.; Ximenes, V.F. The Role of Medium Polarity in the Efficiency of Albumin Binding with Hydrophobic Ligands: Experimental Studies and a Molecular Dynamics Investigation. Int. J. Mol. Sci. 2024, 25, 12664. https://doi.org/10.3390/ijms252312664
Zazeri G, Povinelli APR, Bertozo LdC, Jones AM, Ximenes VF. The Role of Medium Polarity in the Efficiency of Albumin Binding with Hydrophobic Ligands: Experimental Studies and a Molecular Dynamics Investigation. International Journal of Molecular Sciences. 2024; 25(23):12664. https://doi.org/10.3390/ijms252312664
Chicago/Turabian StyleZazeri, Gabriel, Ana Paula Ribeiro Povinelli, Luiza de Carvalho Bertozo, Alan M. Jones, and Valdecir Farias Ximenes. 2024. "The Role of Medium Polarity in the Efficiency of Albumin Binding with Hydrophobic Ligands: Experimental Studies and a Molecular Dynamics Investigation" International Journal of Molecular Sciences 25, no. 23: 12664. https://doi.org/10.3390/ijms252312664
APA StyleZazeri, G., Povinelli, A. P. R., Bertozo, L. d. C., Jones, A. M., & Ximenes, V. F. (2024). The Role of Medium Polarity in the Efficiency of Albumin Binding with Hydrophobic Ligands: Experimental Studies and a Molecular Dynamics Investigation. International Journal of Molecular Sciences, 25(23), 12664. https://doi.org/10.3390/ijms252312664






