The Effect of Prostaglandin F2 Analog Treatment on the Immunoexpression of Fibrosis-Associated Factors in Patients with Glaucoma Undergoing Deep Sclerotomy
Abstract
1. Introduction
2. Results
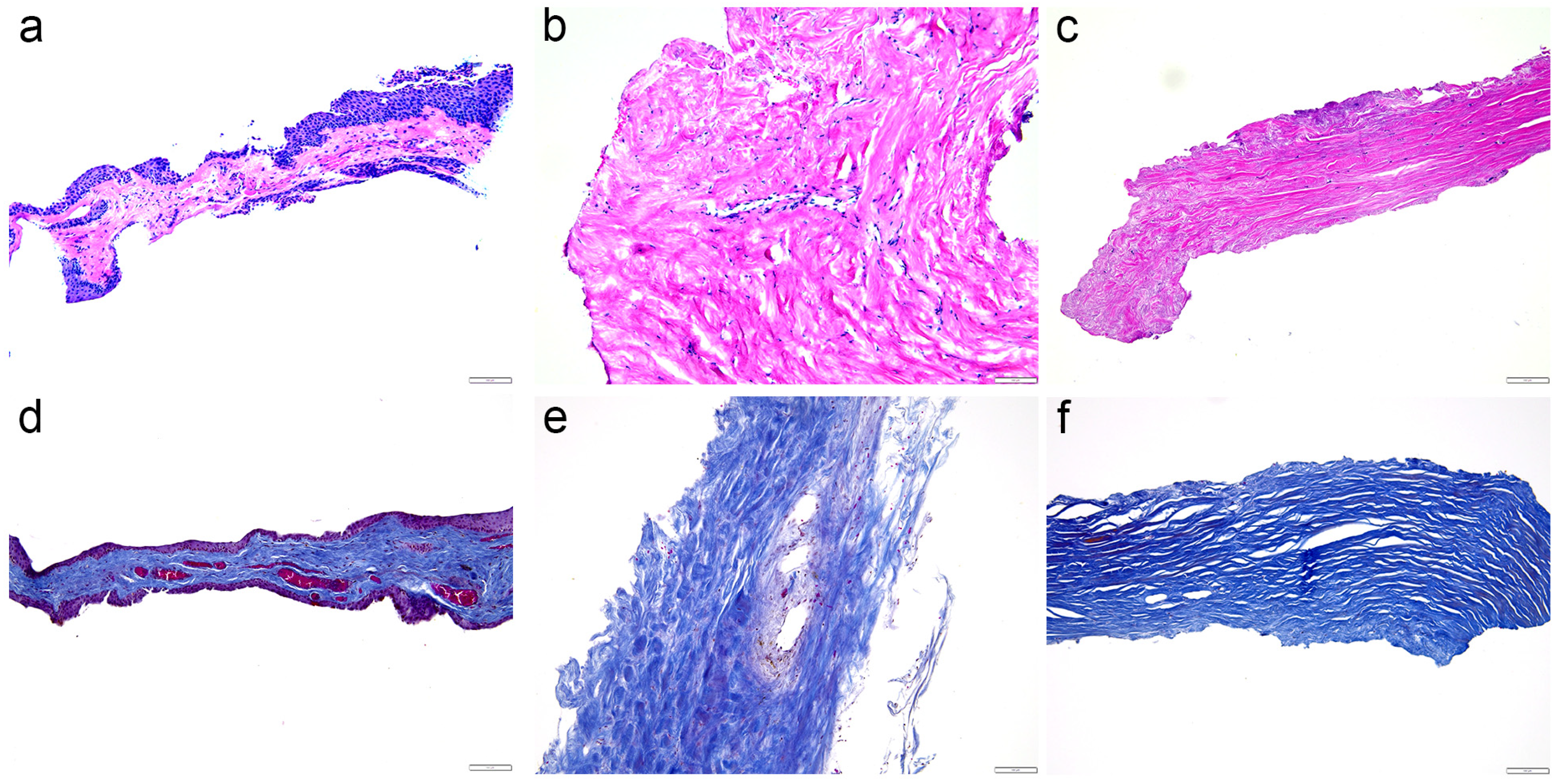
2.1. Hematoxylin and Eosin Staining and Mallory Staining
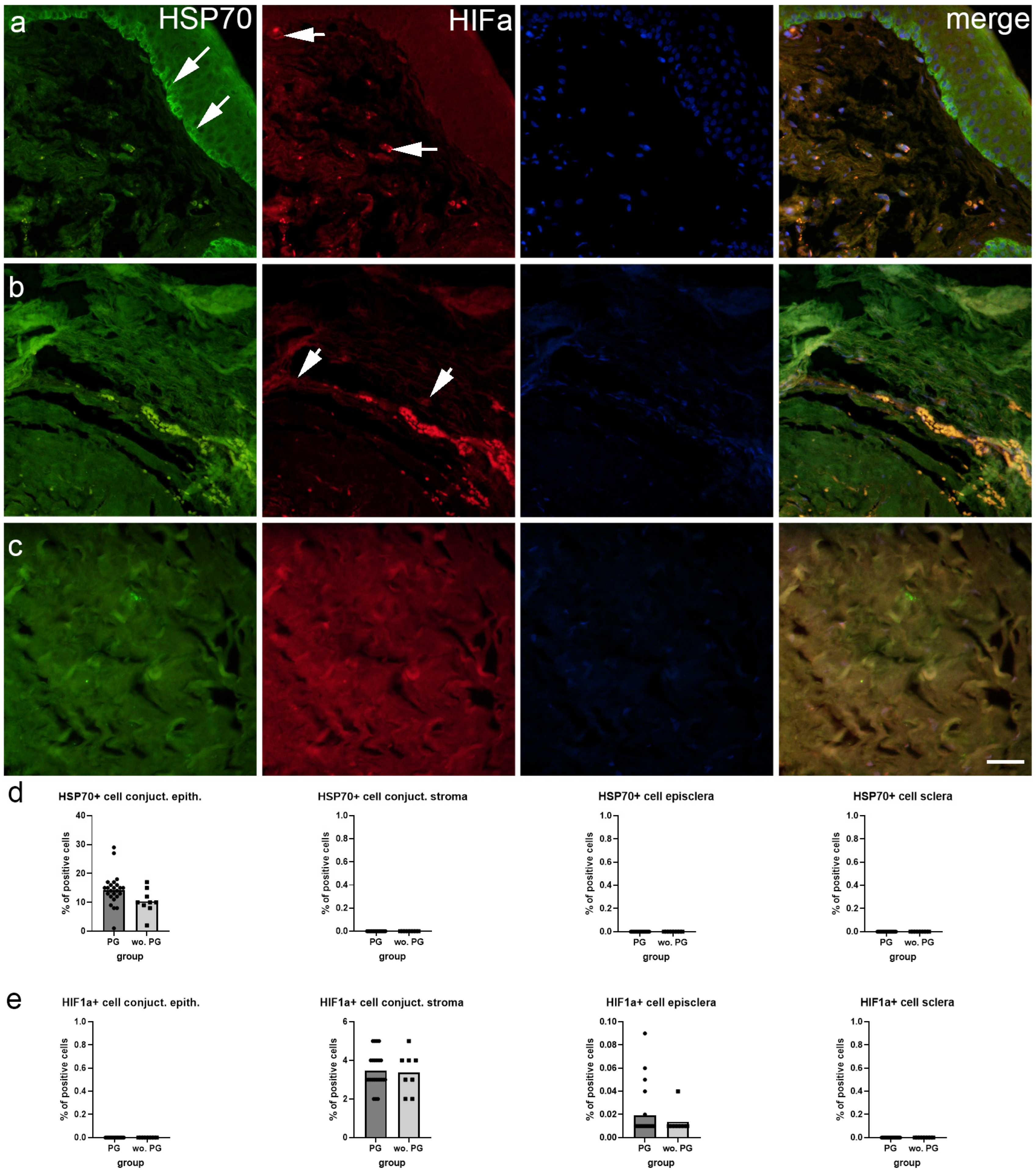
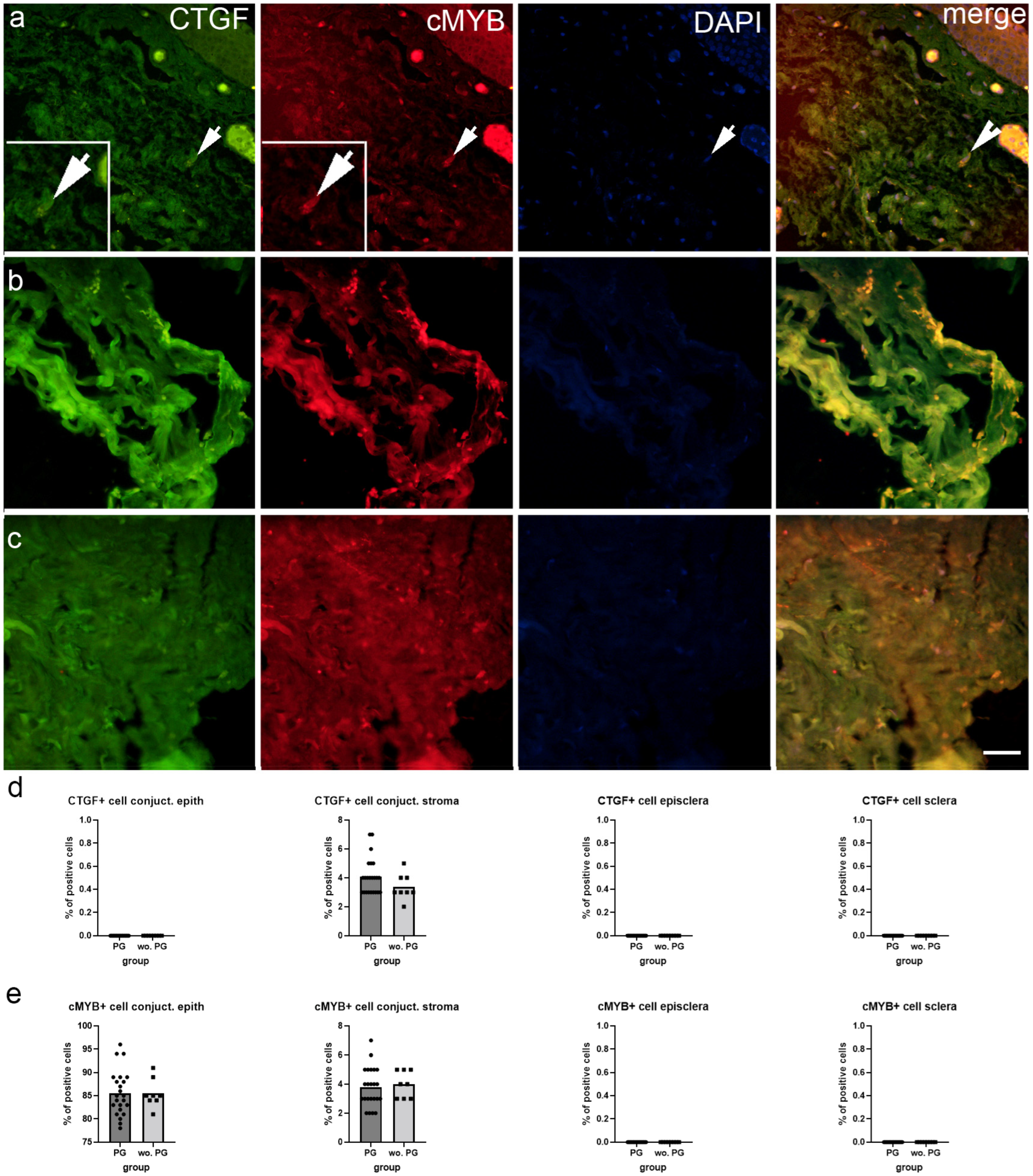
2.2. Immunofluorescence Staining
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Tissue Collection and Basic Staining Procedures
4.3. Double Immunofluorescence
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.Y.; Hu, W.N.; Cai, W.T.; Jin, H.Z.; Yu, D.H.; Sun, J.H.; Yu, J. Effectiveness and Safety of Trabeculectomy along with Amniotic Membrane Transplantation on Glaucoma: A Systematic Review. J. Ophthalmol. 2020, 2020, 3949735. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Kitano-Izutani, A.; Tomoyose, K.; Reinach, P.S. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015, 15 (Suppl. S1), 157. [Google Scholar] [CrossRef]
- Kalala, A.; Gillmann, K.; Mermoud, A. Prospective evaluation of penetrating deep sclerectomy in advanced open-angle glaucoma: Filtration surgery adapted to resource scarcity in developing countries. J. Fr. D’ophtalmologie 2020, 43, 228–236. [Google Scholar] [CrossRef]
- Kozobolis, V.; Kalogianni, E.; Sideroudi, H. Penetrating deep sclerectomy in primary open-angle and pseudoexfoliative glaucoma. Eur. J. Ophthalmol. 2020, 30, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Mermoud, A.; Schnyder, C.C. Nonpenetrating filtering surgery in glaucoma. Curr. Opin. Ophthalmol. 2000, 11, 151–157. [Google Scholar] [CrossRef]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br. J. Ophthalmol. 2017, 101, 130–195. [CrossRef]
- Razeghinejad, M.R. The Effect of Latanaprost on Intraocular Inflammation and Macular Edema. Ocul. Immunol. Inflamm. 2019, 27, 181–188. [Google Scholar] [CrossRef]
- Ichhpujani, P.; Katz, L.J.; Hollo, G.; Shields, C.L.; Shields, J.A.; Marr, B.; Eagle, R.; Alvim, H.; Wizov, S.S.; Acheampong, A.; et al. Comparison of human ocular distribution of bimatoprost and latanoprost. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2012, 28, 134–145. [Google Scholar] [CrossRef]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Helin, M.; Ronkko, S.; Puustjarvi, T.; Terasvirta, M.; Ollikainen, M.; Uusitalo, H. Conjunctival inflammatory cells and their predictive role for deep sclerectomy in primary open-angle glaucoma and exfoliation glaucoma. J. Glaucoma 2011, 20, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, M.; Voykov, B.; Klemm, M.; Gross, U.; Schultheiss, H.P.; Spitzer, M.S.; Casagrande, M. Scleral Inflammation around Collector Channels in Eyes with Primary Open-Angle Glaucoma. Ocul. Immunol. Inflamm. 2020, 29, 1338–1344. [Google Scholar] [CrossRef]
- Mizdrak, M.; Filipovic, N.; Vukojevic, K.; Capkun, V.; Mizdrak, I.; Durdov, M.G. Prognostic value of connective tissue growth factor and c-Myb expression in IgA nephropathy and Henoch-Schonlein purpura-A pilot immunohistochemical study. Acta Histochem. 2020, 122, 151479. [Google Scholar] [CrossRef]
- Wang, W.H.; Deng, A.J.; He, S.G. A key role of microRNA-26a in the scar formation after glaucoma filtration surgery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 831–837. [Google Scholar] [CrossRef]
- Bao, H.; Jiang, K.; Meng, K.; Liu, W.; Liu, P.; Du, Y.; Wang, D. TGF-beta2 induces proliferation and inhibits apoptosis of human Tenon capsule fibroblast by miR-26 and its targeting of CTGF. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 104, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, R.; Hu, N.; Shi, J.; Zhang, J.; Jiang, L.; Jiang, H.; Guan, H. Association of frizzled-related protein (MFRP) and heat shock protein 70 (HSP70) single nucleotide polymorphisms with primary angle closure in a Han Chinese population: Jiangsu Eye Study. Mol. Vis. 2013, 19, 128–134. [Google Scholar] [PubMed]
- Gui, D.; Li, Y.; Chen, X.; Gao, D.; Yang, Y.; Li, X. HIF1 signaling pathway involving iNOS, COX2 and caspase9 mediates the neuroprotection provided by erythropoietin in the retina of chronic ocular hypertension rats. Mol. Med. Rep. 2015, 11, 1490–1496. [Google Scholar] [CrossRef]
- Diebold, Y.; Garcia-Posadas, L. Is the Conjunctiva a Potential Target for Advanced Therapy Medicinal Products? Pharmaceutics 2021, 13, 1140. [Google Scholar] [CrossRef]
- Vannas, S.; Teir, H. Histologic observations of the structure of the sclera in glaucomatous human eyes. Am. J. Ophthalmol. 1960, 49, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Belmares, R.; Raychaudhuri, U.; Maansson, S.; Clark, A.F. Histological investigation of human glaucomatous eyes: Extracellular fibrotic changes and galectin 3 expression in the trabecular meshwork and optic nerve head. Clin. Anat. 2018, 31, 1031–1049. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog. Retin. Eye Res. 2002, 21, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Towler, H.M.; Calder, V.L. The immunomodulatory role of human conjunctival epithelial cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3906–3910. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Chapman, E. Function, Therapeutic Potential, and Inhibition of Hsp70 Chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol./Hematol. 2006, 59, 15–26. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Context specificity of the EMT transcriptional response. Nat. Commun. 2020, 11, 2142. [Google Scholar] [CrossRef]
- Chadli, L.; Sotthewes, B.; Li, K.; Andersen, S.N.; Cahir-McFarland, E.; Cheung, M.; Cullen, P.; Dorjee, A.; de Vries-Bouwstra, J.K.; Huizinga, T.W.J.; et al. Identification of regulators of the myofibroblast phenotype of primary dermal fibroblasts from early diffuse systemic sclerosis patients. Sci. Rep. 2019, 9, 4521. [Google Scholar] [CrossRef]
- Oglesby, E.N.; Tezel, G.; Cone-Kimball, E.; Steinhart, M.R.; Jefferys, J.; Pease, M.E.; Quigley, H.A. Scleral fibroblast response to experimental glaucoma in mice. Mol. Vis. 2016, 22, 82–99. [Google Scholar] [PubMed]
- Li, K.; Zhao, J.; Wang, M.; Niu, L.; Wang, Y.; Li, Y.; Zheng, Y. The Roles of Various Prostaglandins in Fibrosis: A Review. Biomolecules 2021, 11, 789. [Google Scholar] [CrossRef]
- Kalouche, G.; Beguier, F.; Bakria, M.; Melik-Parsadaniantz, S.; Leriche, C.; Debeir, T.; Rostene, W.; Baudouin, C.; Vige, X. Activation of Prostaglandin FP and EP2 Receptors Differently Modulates Myofibroblast Transition in a Model of Adult Primary Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1816–1825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5 (Suppl. S1), S24. [Google Scholar] [CrossRef] [PubMed]
- George, O.L.; Ness, S.A. Situational awareness: Regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers 2014, 6, 2049–2071. [Google Scholar] [CrossRef]
- Racetin, A.; Juric, M.; Filipovic, N.; Solic, I.; Kosovic, I.; Glavina Durdov, M.; Kunac, N.; Zekic Tomas, S.; Saraga, M.; Soljic, V.; et al. Expression and localization of DAB1 and Reelin during normal human kidney development. Croat. Med. J. 2019, 60, 521–531. [Google Scholar] [CrossRef]
- Racetin, A.; Raguz, F.; Durdov, M.G.; Kunac, N.; Saraga, M.; Sanna-Cherchi, S.; Soljic, V.; Martinovic, V.; Petricevic, J.; Kostic, S.; et al. Immunohistochemical expression pattern of RIP5, FGFR1, FGFR2 and HIP2 in the normal human kidney development. Acta Histochem. 2019, 121, 531–538. [Google Scholar] [CrossRef]
- Vukojevic, K.; Skobic, H.; Saraga-Babic, M. Proliferation and differentiation of glial and neuronal progenitors in the development of human spinal ganglia. Differ. Res. Biol. Divers. 2009, 78, 91–98. [Google Scholar] [CrossRef]

| Antibodies | Catalog Number | Host | Dilution | Source |
|---|---|---|---|---|
| Anti-HSP70 | ab31010 | Rabbit | 1:100 | Abcam (Cambridge, UK) |
| HIF1a | sc-13515 | Mouse | 1:200 | Santa Cruz Bt. (Dallas, TX, USA) |
| Anti-Snail | ab53519 | Goat | 1:500 | Abcam (Cambridge, UK) |
| Anti-Smooth Muscle Actin | M0851 | Mouse | 1:300 | Dako, Glostrup, Denmark |
| CTGF | sc-14939 | Goat | 1:200 | Santa Cruz Bt. (Dallas, TX, USA) |
| c-Myb | sc-7874 | Rabbit | 1:200 | Santa Cruz Bt. (Dallas, TX, USA) |
| Alexa Fluor®488 AffiniPure Anti-Goat lgG (H+L) | 705-545-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. Baltimore, PA, USA |
| Anti-Rabbit IgG, Alexa Fluor® 488, | 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. (Baltimore, PA, USA) |
| Rhodamine Red™-X (RRX) AffiniPure Anti-Mouse IgG (H+L) | 715-295-151 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. Baltimore, PA, USA |
| Anti-Rabbit IgG, Alexa Fluor® 488, | 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. (Baltimore, PA, USA) |
| Rhodamine Red™-X (RRX) AffiniPure Donkey Anti-Rabbit IgG (H+L) | 711-295-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. I (Baltimore, PA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanić, R.; Vukojević, K.; Filipović, N.; Benzon, B.; Ogorevc, M.; Kunac, N.; Čanović, S.; Kovačević, P.; Paradžik Šimunović, M.; Konjevoda, S. The Effect of Prostaglandin F2 Analog Treatment on the Immunoexpression of Fibrosis-Associated Factors in Patients with Glaucoma Undergoing Deep Sclerotomy. Int. J. Mol. Sci. 2024, 25, 12618. https://doi.org/10.3390/ijms252312618
Stanić R, Vukojević K, Filipović N, Benzon B, Ogorevc M, Kunac N, Čanović S, Kovačević P, Paradžik Šimunović M, Konjevoda S. The Effect of Prostaglandin F2 Analog Treatment on the Immunoexpression of Fibrosis-Associated Factors in Patients with Glaucoma Undergoing Deep Sclerotomy. International Journal of Molecular Sciences. 2024; 25(23):12618. https://doi.org/10.3390/ijms252312618
Chicago/Turabian StyleStanić, Robert, Katarina Vukojević, Natalija Filipović, Benjamin Benzon, Marin Ogorevc, Nenad Kunac, Samir Čanović, Petra Kovačević, Martina Paradžik Šimunović, and Suzana Konjevoda. 2024. "The Effect of Prostaglandin F2 Analog Treatment on the Immunoexpression of Fibrosis-Associated Factors in Patients with Glaucoma Undergoing Deep Sclerotomy" International Journal of Molecular Sciences 25, no. 23: 12618. https://doi.org/10.3390/ijms252312618
APA StyleStanić, R., Vukojević, K., Filipović, N., Benzon, B., Ogorevc, M., Kunac, N., Čanović, S., Kovačević, P., Paradžik Šimunović, M., & Konjevoda, S. (2024). The Effect of Prostaglandin F2 Analog Treatment on the Immunoexpression of Fibrosis-Associated Factors in Patients with Glaucoma Undergoing Deep Sclerotomy. International Journal of Molecular Sciences, 25(23), 12618. https://doi.org/10.3390/ijms252312618







