Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
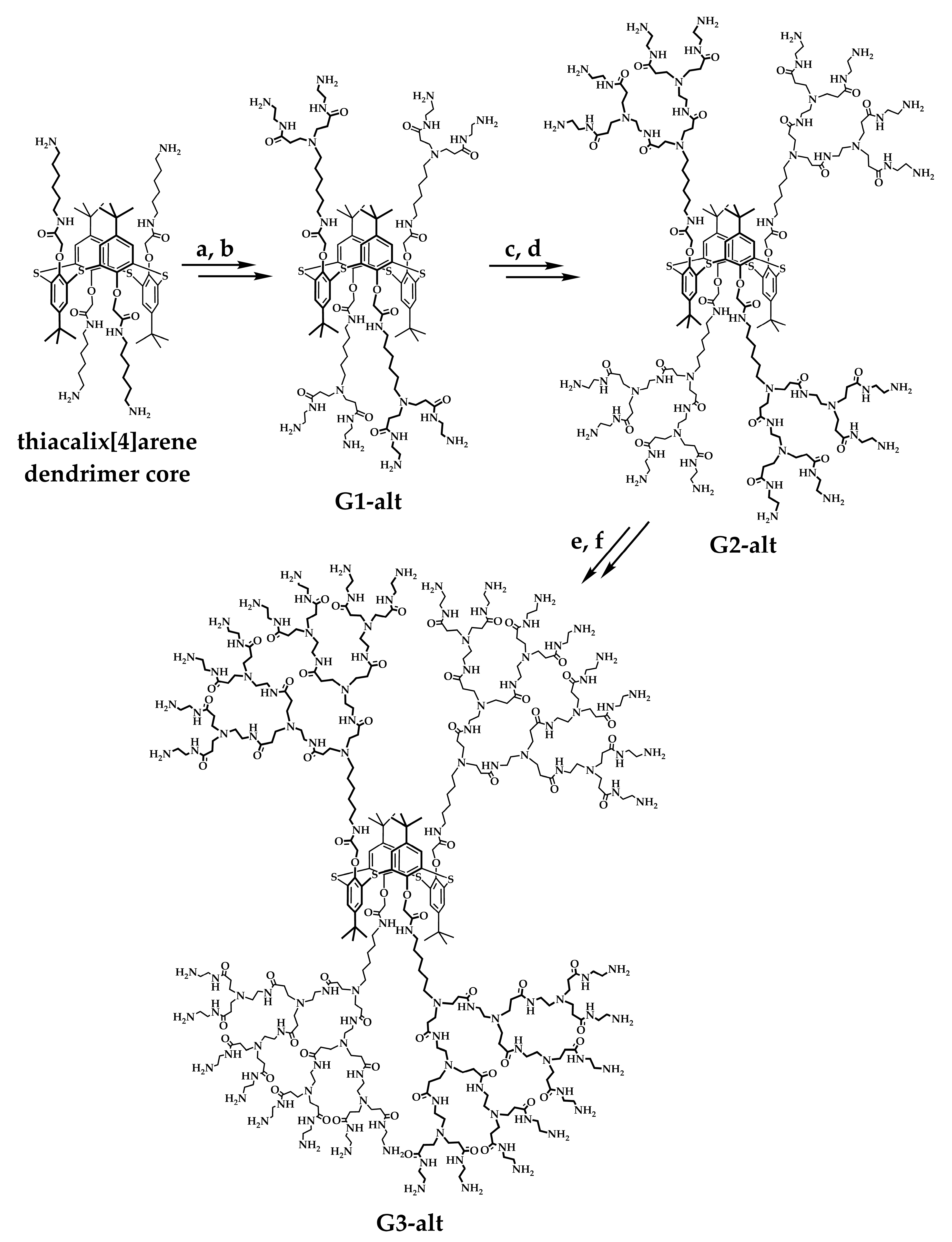
2.1. Design, Synthesis, and Self-Association of PAMAM-Calix-Dendrimers
2.2. The Study of Supramolecular Systems Based on PAMAM-Calix-Dendrimers and siRNA
2.2.1. Surface Charge of the Dendrimer/siRNA Complexes
2.2.2. Hydrodynamic Diameter of the Dendrimer/siRNA Complexes
2.2.3. Evaluation of the Dendrimer/siRNA Complexes and Their Stability
2.3. Biological Activity of Dendrimer/siRNA Complexes
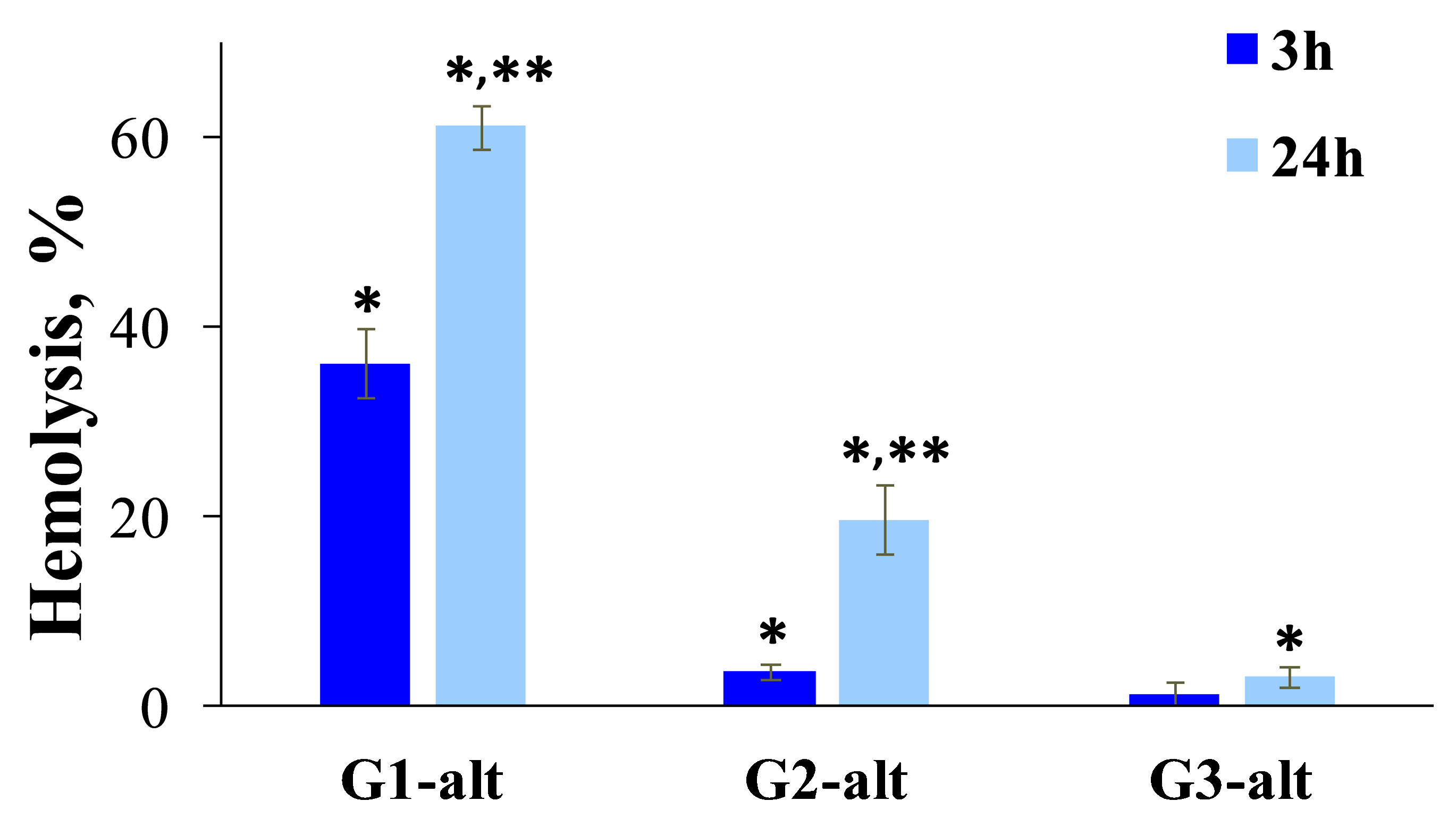
2.3.1. Toxic Effects of PAMAM-Calix-Dendrimers and PAMAM Dendrimers on Human Peripheral Blood Cells
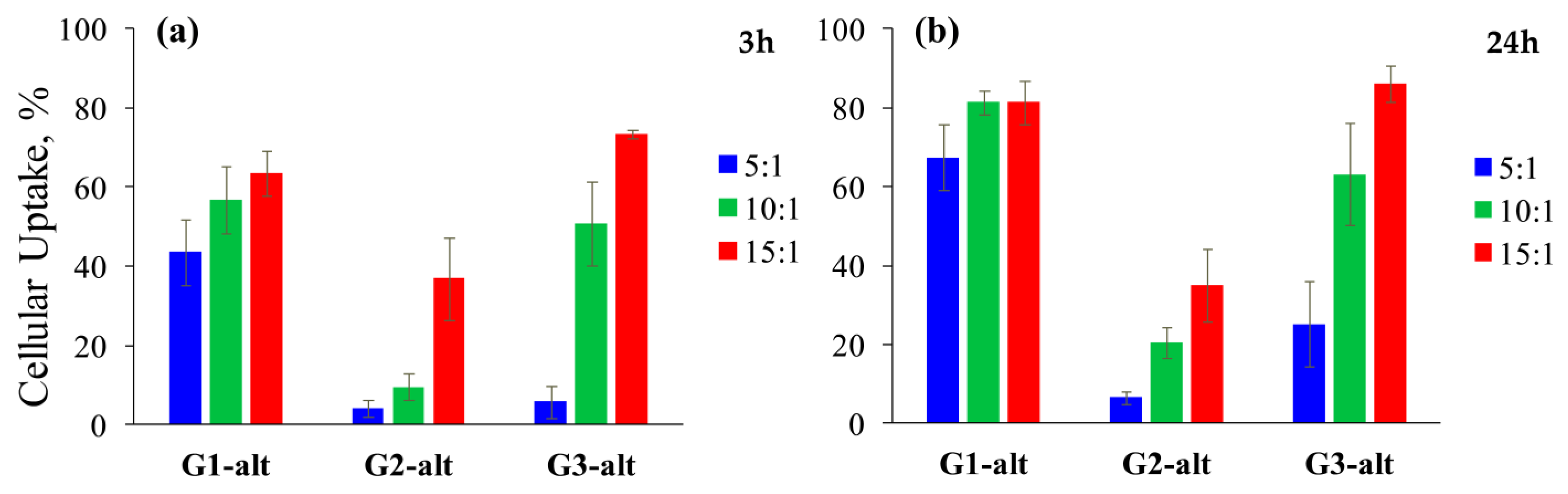
2.3.2. Cellular Uptake of the Dendrimer/siRNA Complexes by HeLa Cells
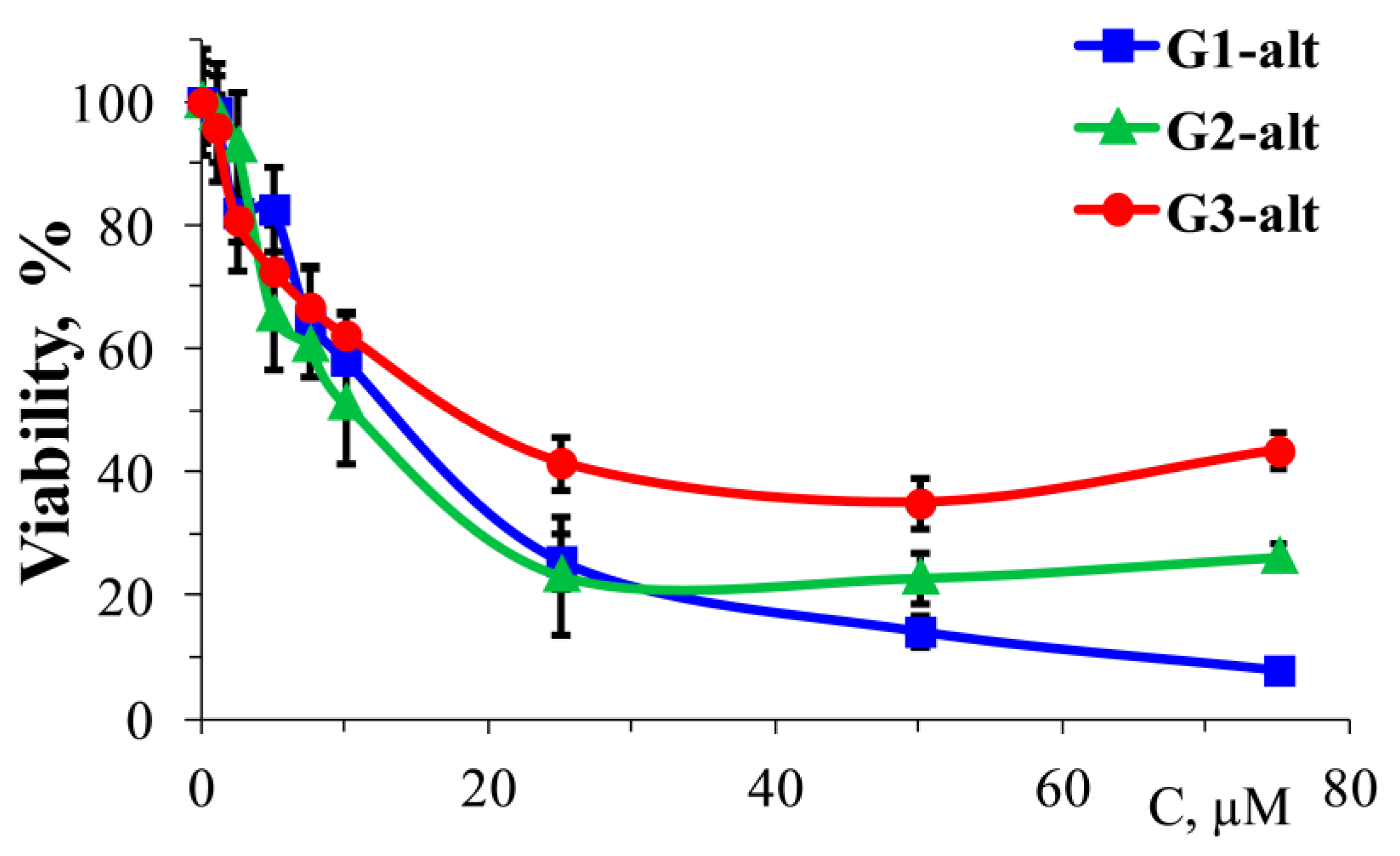
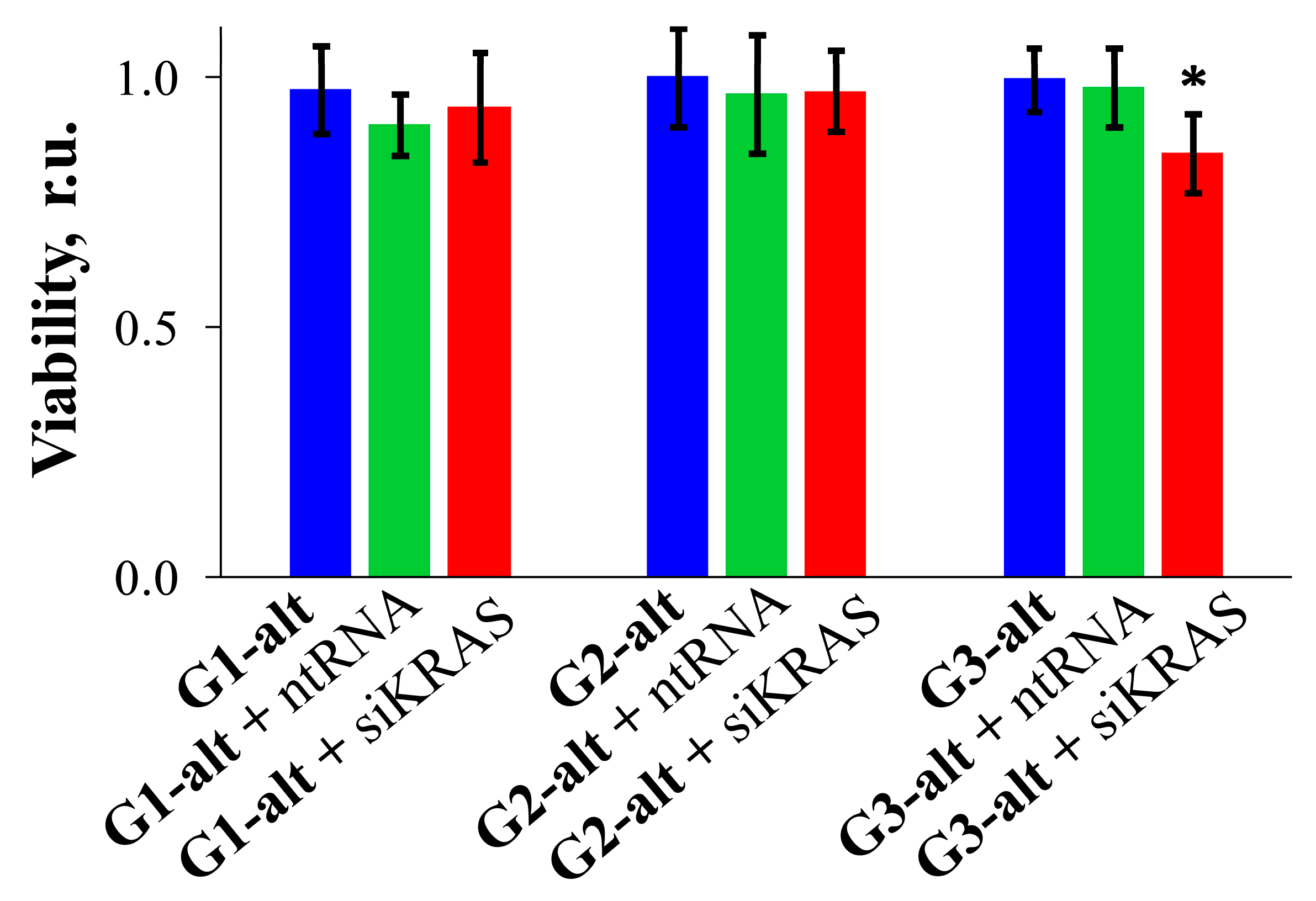
2.3.3. Cytotoxic Effect of Dendrimers and Their Complexes with siRNAs on HeLa Cells
3. Materials and Methods
3.1. Synthesis
3.2. Small Interfering RNAs
3.3. Molecular Modeling
3.4. Dendrimer/siRNA Complex Preparation
3.5. Dynamic Light Scattering (DLS)
3.5.1. Self-Assembly of PAMAM-Calix-Dendrimers
3.5.2. Aggregation of the Dendrimers with siRNA
3.6. Zeta-Potential of the Dendrimer/siRNA Complexes
3.7. One-Dimensional Agarose Gel Electrophoresis
3.8. Hemolysis
3.9. Peripheral Blood Mononuclear Cell (PBMC) Inhibition
3.10. Cell Line Cultures
3.11. Cytotoxicity Studies
3.12. Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/health-topics/cancer (accessed on 25 October 2024).
- Baik, C.S.; Rubin, E.H.; Forde, P.M.; Mehnert, J.M.; Collyar, D.; Butler, M.O.; Dixon, E.L.; Chow, L.Q.M. Immuno-Oncology Clinical Trial Design: Limitations, Challenges, and Opportunities. Clin. Cancer Res. 2017, 23, 4992–5002. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for Poorly Water-Soluble Anticancer Drugs—Barriers of Translation and Solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef]
- Srivastava, S.; Koay, E.J.; Borowsky, A.D.; De Marzo, A.M.; Ghosh, S.; Wagner, P.D.; Kramer, B.S. Cancer Overdiagnosis: A Biological Challenge and Clinical Dilemma. Nat. Rev. Cancer 2019, 19, 349–358. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 205031212110343. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Gao, Y.; Song, C.; Ouyang, Z.; Li, C.; Mignani, S.; Majoral, J.P.; Shi, X.; Shen, M. Impact of Molecular Rigidity on the Gene Delivery Efficiency of Core–Shell Tecto Dendrimers. J. Mater. Chem. B 2021, 9, 6149–6154. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, 175. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; ElMallah, M.K.; Flotte, T.R. Gene Therapy 2017: Progress and Future Directions. Clin. Transl. Sci. 2017, 10, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.; Jia, H.; Kandachi, K. China Approves First Gene Therapy. Nat. Biotechnol. 2004, 22, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. A Brief History of RNAi: The Silence of the Genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Novina, C.D.; Sharp, P.A. The RNAi Revolution. Nature 2004, 430, 161–164. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small Interfering RNAs (siRNAs) in Cancer Therapy: A Nano-Based Approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef]
- Tai, W.; Gao, X. Noncovalent Tagging of siRNA with Steroids for Transmembrane Delivery. Biomaterials 2018, 178, 720–727. [Google Scholar] [CrossRef]
- Cao, S.; Lin, C.; Li, X.; Liang, Y.; Saw, P.E. TME-Responsive Multistage Nanoplatform for SiRNA Delivery and Effective Cancer Therapy. Int. J. Nanomed. 2021, 16, 5909–5921. [Google Scholar] [CrossRef]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and Clinical Development of siRNA-Based Therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef]
- Querfeld, C.; Pacheco, T.; Foss, F.M.; Halwani, A.S.; Porcu, P.; Seto, A.G.; Ruckman, J.; Landry, M.L.; Jackson, A.L.; Pestano, L.A.; et al. Preliminary Results of a Phase 1 Trial Evaluating MRG-106, a Synthetic MicroRNA Antagonist (LNA AntimiR) of MicroRNA-155, in Patients with CTCL. Blood 2016, 128, 1829. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking Down Barriers: Advances in SiRNA Delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, J.; Li, Z.; Tang, G. Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy. Int. J. Mol. Sci. 2019, 20, 2097. [Google Scholar] [CrossRef] [PubMed]
- Seidi, K.; Neubauer, H.A.; Moriggl, R.; Jahanban-Esfahlan, R.; Javaheri, T. Tumor Target Amplification: Implications for Nano Drug Delivery Systems. J. Control. Release 2018, 275, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of Nanotechnology in Drug Delivery to the Central Nervous System. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Qin, S.Y.; Zhang, A.Q.; Cheng, S.X.; Rong, L.; Zhang, X.Z. Drug Self-Delivery Systems for Cancer Therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.W.; Park, B.J.; Han, H.K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Touchot, N.; Flume, M. Early Insights from Commercialization of Gene Therapies in Europe. Genes 2017, 8, 78. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular Chemotherapy Based on Host–Guest Molecular Recognition: A Novel Strategy in the Battle against Cancer With a Bright Future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Ghitman, J.; Voicu, S.I. Controlled Drug Delivery Mediated by Cyclodextrin-Based Supramolecular Self-Assembled Carriers: From Design to Clinical Performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrin Superstructures for Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103650. [Google Scholar] [CrossRef]
- Fan, X.; Guo, X. Development of Calixarene-Based Drug Nanocarriers. J. Mol. Liq. 2021, 325, 115246. [Google Scholar] [CrossRef]
- Basilotta, R.; Mannino, D.; Filippone, A.; Casili, G.; Prestifilippo, A.; Colarossi, L.; Raciti, G.; Esposito, E.; Campolo, M. Role of Calixarene in Chemotherapy Delivery Strategies. Molecules 2021, 26, 3963. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Makhmutova, L.I.; Usachev, K.S.; Islamov, D.R.; Mostovaya, O.A.; Nazarova, A.A.; Kizhnyaev, V.N.; Stoikov, I.I. Towards Universal Stimuli-Responsive Drug Delivery Systems: Pillar[5]arenes Synthesis and Self-Assembly into Nanocontainers With Tetrazole Polymers. Nanomaterials 2021, 11, 947. [Google Scholar] [CrossRef]
- Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Santra, S.; Majee, A.; Ranu, B.C. Pillararenes as Promising Carriers for Drug Delivery. Int. J. Mol. Sci. 2023, 24, 5167. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Davies, N.L.; James, K. Comparison of Diffusion Coefficients for Matched Pairs of Macrocyclic and Linear Molecules Over a Drug-Like Molecular Weight Range. Org. Biomol. Chem. 2011, 9, 7727. [Google Scholar] [CrossRef]
- Mallinson, J.; Collins, I. Macrocycles in New Drug Discovery. Future Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef]
- Bono, N.; Pennetta, C.; Sganappa, A.; Giupponi, E.; Sansone, F.; Volonterio, A.; Candiani, G. Design and Synthesis of Biologically Active Cationic Amphiphiles Built on the Calix[4]arene Scaffold. Int. J. Pharm. 2018, 549, 436–445. [Google Scholar] [CrossRef]
- Gasparello, J.; Manicardi, A.; Casnati, A.; Corradini, R.; Gambari, R.; Finotti, A.; Sansone, F. Efficient Cell Penetration and Delivery of Peptide Nucleic Acids by an Argininocalix[4]arene. Sci. Rep. 2019, 9, 3036. [Google Scholar] [CrossRef]
- Galukhin, A.; Imatdinov, I.; Osin, Y. p-tert-Butylthiacalix[4]arenes Equipped With Guanidinium Fragments: Aggregation, Cytotoxicity, and DNA Binding Abilities. RSC Adv. 2016, 6, 32722–32726. [Google Scholar] [CrossRef]
- Rodik, R.V.; Klymchenko, A.S.; Mely, Y.; Kalchenko, V.I. Calixarenes and Related Macrocycles as Gene Delivery Vehicles. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 189–200. [Google Scholar] [CrossRef]
- Mironova, D.; Makarov, E.; Bilyukova, I.; Akyol, K.; Sultanova, E.; Evtugyn, V.; Davletshin, D.; Gilyazova, E.; Bulatov, E.; Burilov, V.; et al. Aggregation, Cytotoxicity and DNA Binding in a Series of Calix[4]arene Amphiphile Containing Aminotriazole Groups. Pharmaceuticals 2023, 16, 699. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, O.; Padnya, P.; Shiabiev, I.; Mukhametzyanov, T.; Stoikov, I. PAMAM-calix-dendrimers: Synthesis and Thiacalixarene Conformation Effect on DNA Binding. Int. J. Mol. Sci. 2021, 22, 11901. [Google Scholar] [CrossRef] [PubMed]
- Burilov, V.; Makarov, E.; Mironova, D.; Sultanova, E.; Bilyukova, I.; Akyol, K.; Evtugyn, V.; Islamov, D.; Usachev, K.; Mukhametzyanov, T.; et al. Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding. Int. J. Mol. Sci. 2022, 23, 14889. [Google Scholar] [CrossRef]
- Padnya, P.L.; Andreyko, E.A.; Mostovaya, O.A.; Rizvanov, I.K.; Stoikov, I.I. The Synthesis of New Amphiphilic p-tert-Butylthiacalix[4]arenes Containing Peptide Fragments and Their Interaction with DNA. Org. Biomol. Chem. 2015, 13, 5894–5904. [Google Scholar] [CrossRef]
- Gasparello, J.; Lomazzi, M.; Papi, C.; D’Aversa, E.; Sansone, F.; Casnati, A.; Donofrio, G.; Gambari, R.; Finotti, A. Efficient Delivery of microRNA and antimiRNA Molecules Using an Argininocalix[4]arene Macrocycle. Mol. Ther.-Nucleic Acids 2019, 18, 748–763. [Google Scholar] [CrossRef]
- Krošl, I.; Otković, E.; Nikšić-Franjić, I.; Colasson, B.; Reinaud, O.; Višnjevac, A.; Piantanida, I. Impact of Positive Charge and Ring-Size on the Interactions of Calixarenes with DNA, RNA and Nucleotides. New J. Chem. 2022, 46, 6860–6869. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Kashapova, N.; Ziganshina, A.; Salnikov, V.; Sapunova, A.; Voloshina, A.; Zakharova, L. Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene. Int. J. Mol. Sci. 2023, 24, 7911. [Google Scholar] [CrossRef]
- Lim, C.C.; Chia, L.Y.; Kumar, P.V. Dendrimer-Based Nanocomposites for the Production of RNA Delivery Systems. OpenNano 2023, 13, 100173. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging Non-Viral Vectors for Gene Delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical Applications of Dendrimers: A Tutorial. Chem. Soc. Rev. 2010, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Fard, N.T.; Panahi, H.A.; Moniri, E.; Soltani, E.R.; Mahdavijalal, M. Stimuli-Responsive Dendrimers as Nanoscale Vectors in Drug and Gene Delivery Systems: A Review Study. J. Polym. Environ. 2024, 32, 4959–4985. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs Via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Shiabiev, I.E.; Stoikov, I.I. Novel Lactide Derivatives of p-tert-Butylthiacalix[4]arene: Directed Synthesis and Molecular Recognition of Catecholamines. J. Mol. Liq. 2021, 327, 114806. [Google Scholar] [CrossRef]
- Padnya, P.; Mostovaya, O.; Ovchinnikov, D.; Shiabiev, I.; Pysin, D.; Akhmedov, A.; Mukhametzyanov, T.; Lyubina, A.; Voloshina, A.; Petrov, K.; et al. Combined Antimicrobial Agents Based on Self-Assembled PAMAM-calix-dendrimers/Lysozyme Nanoparticles: Design, Antibacterial Properties and Cytotoxicity. J. Mol. Liq. 2023, 389, 122838. [Google Scholar] [CrossRef]
- Mostovaya, O.; Shiabiev, I.; Pysin, D.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Padnya, P.; Stoikov, I. PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding. Pharmaceutics 2022, 14, 2748. [Google Scholar] [CrossRef]
- Mostovaya, O.; Shiabiev, I.; Ovchinnikov, D.; Pysin, D.; Mukhametzyanov, T.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Khannanov, A.; Kutyreva, M.; et al. PAMAM-Calix-Dendrimers: Third Generation Synthesis and Impact of Generation and Macrocyclic Core Conformation on Hemotoxicity and Calf Thymus DNA Binding. Pharmaceutics 2024, 16, 1379. [Google Scholar] [CrossRef]
- Baklouti, L.; Cheriaa, N.; Mahouachi, M.; Abidi, R.; Kim, J.S.; Kim, Y.; Vicens, J. Calixarene-Based Dendrimers. A Timely Review. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 1–7. [Google Scholar] [CrossRef]
- Khan, K.; Badshah, S.; Ahmad, N.; Rashid, H.; Mabkhot, Y. Inclusion Complexes of a New Family of Non-Ionic Amphiphilic Dendrocalix[4]arene and Poorly Water-Soluble Drugs Naproxen and Ibuprofen. Molecules 2017, 22, 783. [Google Scholar] [CrossRef]
- Rahimi, M.; Karimian, R.; Mostafidi, E.; Bahojb Noruzi, E.; Taghizadeh, S.; Shokouhi, B.; Kafil, H.S. Highly Branched Amine-Functionalized p-Sulfonatocalix[4]arene Decorated With Human Plasma Proteins As a Smart, Targeted, and Stealthy Nano-Vehicle for the Combination Chemotherapy of MCF7 Cells. New J. Chem. 2018, 42, 13010–13024. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision A.02. Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular Modeling to Study Dendrimers for Biomedical Applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef]
- Lee, I.; Athey, B.D.; Wetzel, A.W.; Meixner, W.; Baker, J.R. Structural Molecular Dynamics Studies on Polyamidoamine Dendrimers for a Therapeutic Application: Effects of pH and Generation. Macromolecules 2002, 35, 4510–4520. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional Supramolecular Systems: Design and Applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Bormashenko, E.; Fedorets, A.A.; Frenkel, M.; Dombrovsky, L.A.; Nosonovsky, M. Clustering and Self-Organization in Small-Scale Natural and Artificial Systems. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190443. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic Peptide Self-Assembly for Functional Materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Sultanaev, V.; Yakimova, L.; Nazarova, A.; Sedov, I.; Mostovaya, O.; Mukhametzyanov, T.; Davletshin, D.; Takuntseva, D.; Gilyazova, E.; Bulatov, E.; et al. Pillar[5]arene/Albumin Biosupramolecular Systems for Simultaneous Native Protein Preservation and Encapsulation of a Water-Soluble Substrate. J. Mater. Chem. B 2024, 12, 3103–3114. [Google Scholar] [CrossRef] [PubMed]
- Rest, C.; Kandanelli, R.; Fernández, G. Strategies to Create Hierarchical Self-Assembled Structures Via Cooperative Non-Covalent Interactions. Chem. Soc. Rev. 2015, 44, 2543–2572. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, A.; Yakimova, L.; Mostovaya, O.; Kulikova, T.; Mikhailova, O.; Evtugyn, G.; Ganeeva, I.; Bulatov, E.; Stoikov, I. Encapsulation of the Quercetin with Interpolyelectrolyte Complex Based on Pillar[5]arenes. J. Mol. Liq. 2022, 368, 120807. [Google Scholar] [CrossRef]
- Arkas, M.; Kitsou, I.; Gkouma, A.; Papageorgiou, M. The Role of Hydrogen Bonds in the Mesomorphic Behaviour of Supramolecular Assemblies Organized in Dendritic Architectures. Liq. Cryst. Rev. 2019, 7, 60–105. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, X.; Gao, L.; Xu, D.; Wan, H.; Wang, Y.; Yan, L.T. The Entropy-Controlled Strategy in Self-Assembling Systems. Chem. Soc. Rev. 2023, 52, 6806–6837. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Esposito, R.; Paduano, L.; D’Errico, G. From Composite Molecular Structures to a Multiplicity of Supramolecular Aggregates: The Role of Intermolecular Interactions in Biosurfactant Self-Assembly. Curr. Opin. Colloid Interface Sci. 2024, 70, 101792. [Google Scholar] [CrossRef]
- Jasmine, M.J.; Kavitha, M.; Prasad, E. Effect of Solvent-Controlled Aggregation on the Intrinsic Emission Properties of PAMAM Dendrimers. J. Lumin. 2009, 129, 506–513. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging Innate Biological Properties of Nano-Drug Delivery Systems: A Focus on PAMAM Dendrimers and Their Clinical Potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review. Int. J. Nanomed. 2020, 15, 2789–2808. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Hafdi, N.; Behr, J.P.; Erbacher, P.; Peng, L. PAMAM Dendrimers for Efficient siRNA Delivery and Potent Gene Silencing. Chem. Commun. 2006, 22, 2362. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Mohd Amin, M.C.I.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM Dendrimers as Promising Nanocarriers for RNAi Therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and Synthesis of Novel Fluorescence Sensing Perylene Diimides Based on Photoinduced Electron Transfer. Dye. Pigment. 2011, 91, 332–339. [Google Scholar] [CrossRef]
- Ghosh, S.; Banthia, A.K. Fluorophore-Labeled Polyamidoamine (PAMAM) Dendritic Architectures: Synthesis and Fluorescence Sensing Properties. Supramol. Chem. 2004, 16, 487–494. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Yammine, M.; Zhou, J.; Posocco, P.; Viel, S.; Liu, C.; Ziarelli, F.; Fermeglia, M.; Pricl, S.; et al. Structurally Flexible Triethanolamine Core PAMAM Dendrimers Are Effective Nanovectors for DNA Transfection in Vitro and in Vivo to the Mouse Thymus. Bioconjugate Chem. 2011, 22, 2461–2473. [Google Scholar] [CrossRef]
- Kavyani, S.; Amjad-Iranagh, S.; Modarress, H. Aqueous Poly(Amidoamine) Dendrimer G3 and G4 Generations With Several Interior Cores at pHs 5 and 7: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2014, 118, 3257–3266. [Google Scholar] [CrossRef]
- Rodrigo, A.C.; Rivilla, I.; Pérez-Martínez, F.C.; Monteagudo, S.; Ocaña, V.; Guerra, J.; García-Martínez, J.C.; Merino, S.; Sánchez-Verdú, P.; Ceña, V.; et al. Efficient, Non-Toxic Hybrid PPV-PAMAM Dendrimer as a Gene Carrier for Neuronal Cells. Biomacromolecules 2011, 12, 1205–1213. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.C.; Zhang, L.M.; Deng, J.J.; Xie, X.Y.; Lin, D.Z.; Ren, M.; Yang, C.; Yan, L. Cationic Star-Shaped Polymer as an SiRNA Carrier for Reducing MMP-9 Expression in Skin Fibroblast Cells and Promoting Wound Healing in Diabetic Rats. Int. J. Nanomed. 2014, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; Seoud, O.E.; Giarolla, J. Dendrimers in the Context of Nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Niaz, S.; Forbes, B.; Raimi-Abraham, B.T. Exploiting Endocytosis for Non-Spherical Nanoparticle Cellular Uptake. Nanomanufacturing 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Bar, L.; Porcar, L.; Gerelli, Y.; Losada-Pérez, P. Resolving the Interactions between Hydrophilic CdTe Quantum Dots and Positively Charged Membranes at the Nanoscale. J. Colloid Interface Sci. 2025, 677, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA Cocktails as a Novel Tool to Treat Cancer Cells. Part A Mech. Interact. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-Target Inhibition of Cancer Cell Growth by SiRNA Cocktails and 5-Fluorouracil Using Effective Piperidine-Terminated Phosphorus Dendrimers. Colloids Interfaces 2017, 1, 6. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mohammadnejad, J.; Salamat, S.; Beiram Zadeh, Z.; Tanhaei, M.; Ramakrishna, S. Theranostic Polymeric Nanoparticles As a New Approach in Cancer Therapy and Diagnosis: A Review. Mater. Today Chem. 2023, 29, 101400. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Leshuk, T.; Gu, F.X. Emerging Nanomaterials for Targeting Subcellular Organelles. Nano Today 2011, 6, 478–492. [Google Scholar] [CrossRef]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.V.; Liroff, M.G.; Triplett, L.D.; Leroueil, P.R.; Mullen, D.G.; Wallace, J.M.; Meshinchi, S.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Stoichiometry and Structure of Poly(Amidoamine) Dendrimer−Lipid Complexes. ACS Nano 2009, 3, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of Fourth Generation Poly(Propyleneimine) Dendrimers on Blood Cells. J. Biomed. Mater. Res. Part A 2012, 100A, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Domański, D.M.; Klajnert, B.; Bryszewska, M. Influence of PAMAM Dendrimers on Human Red Blood Cells. Bioelectrochemistry 2004, 63, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers:: Relationship between Structure and Biocompatibility In Vitro, and Preliminary Studies on the Biodistribution of 125I-labelled Polyamidoamine Dendrimers In Vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Zhou, J.; Neff, C.P.; Liu, X.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Aboellail, T.; Huang, Y.; Du, Q.; et al. Systemic Administration of Combinatorial dsiRNAs Via Nanoparticles Efficiently Suppresses HIV-1 Infection in Humanized Mice. Mol. Ther. 2011, 19, 2228–2238. [Google Scholar] [CrossRef]
- Araújo, R.V.; Santos, S.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle Size and Surface Charge Determine Effects of PAMAM Dendrimers on Human Platelets In Vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (Amidoamine) (PAMAM) Dendrimer Mediated Delivery of Drug and PDNA/SiRNA for Cancer Therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef] [PubMed]

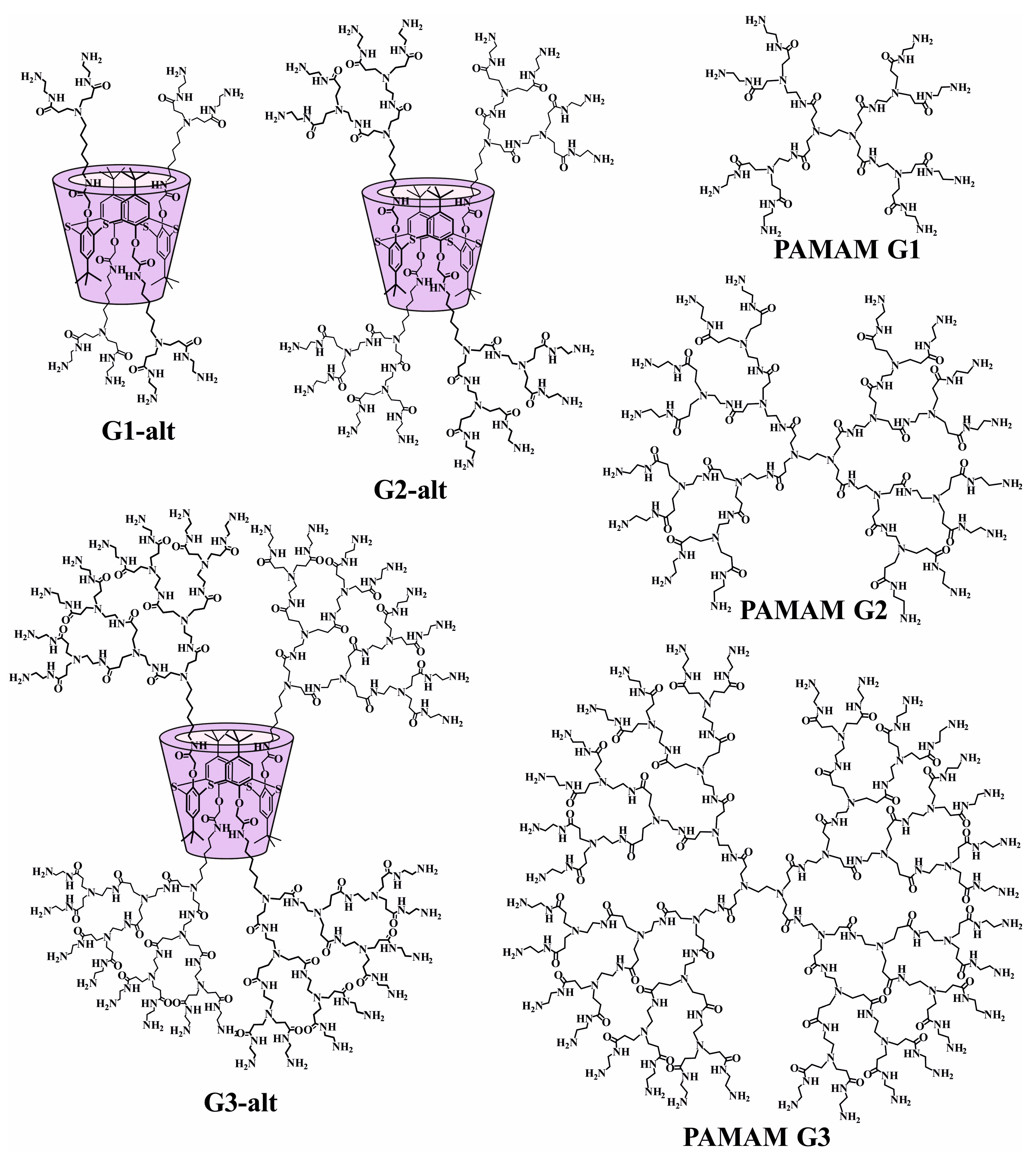
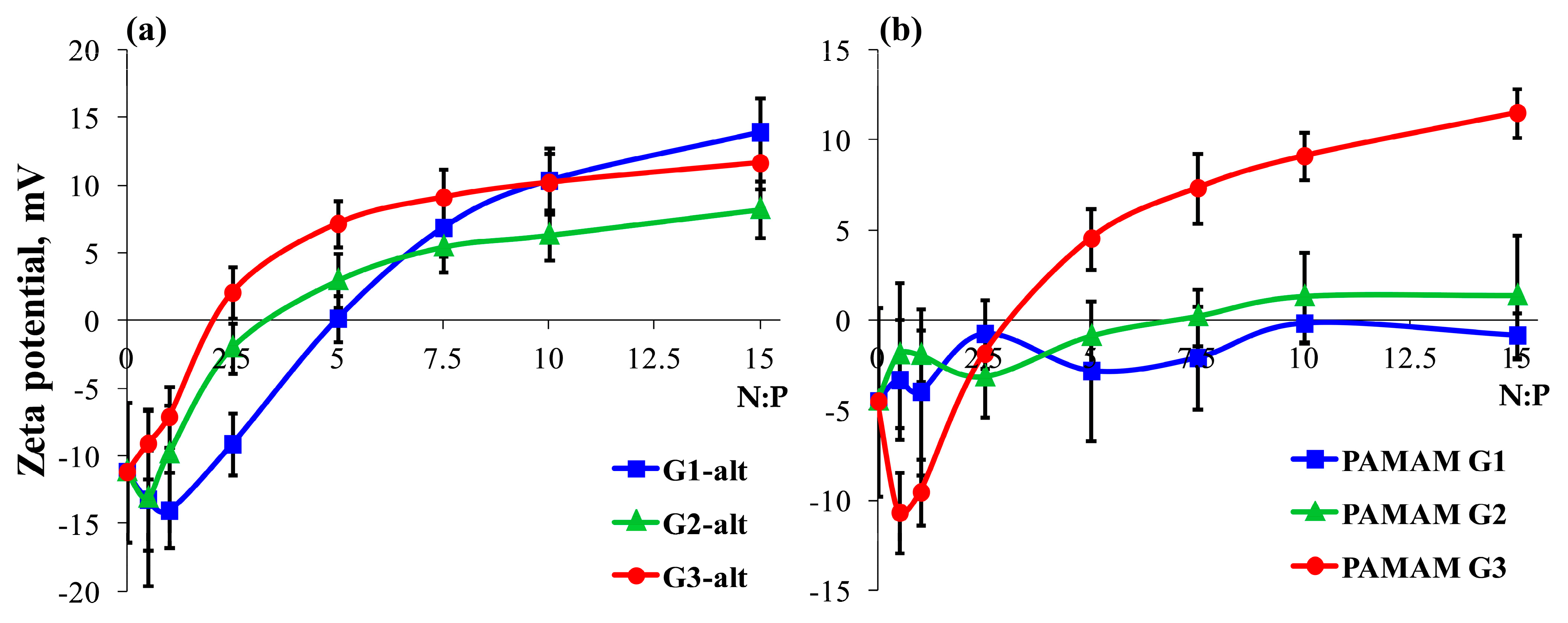
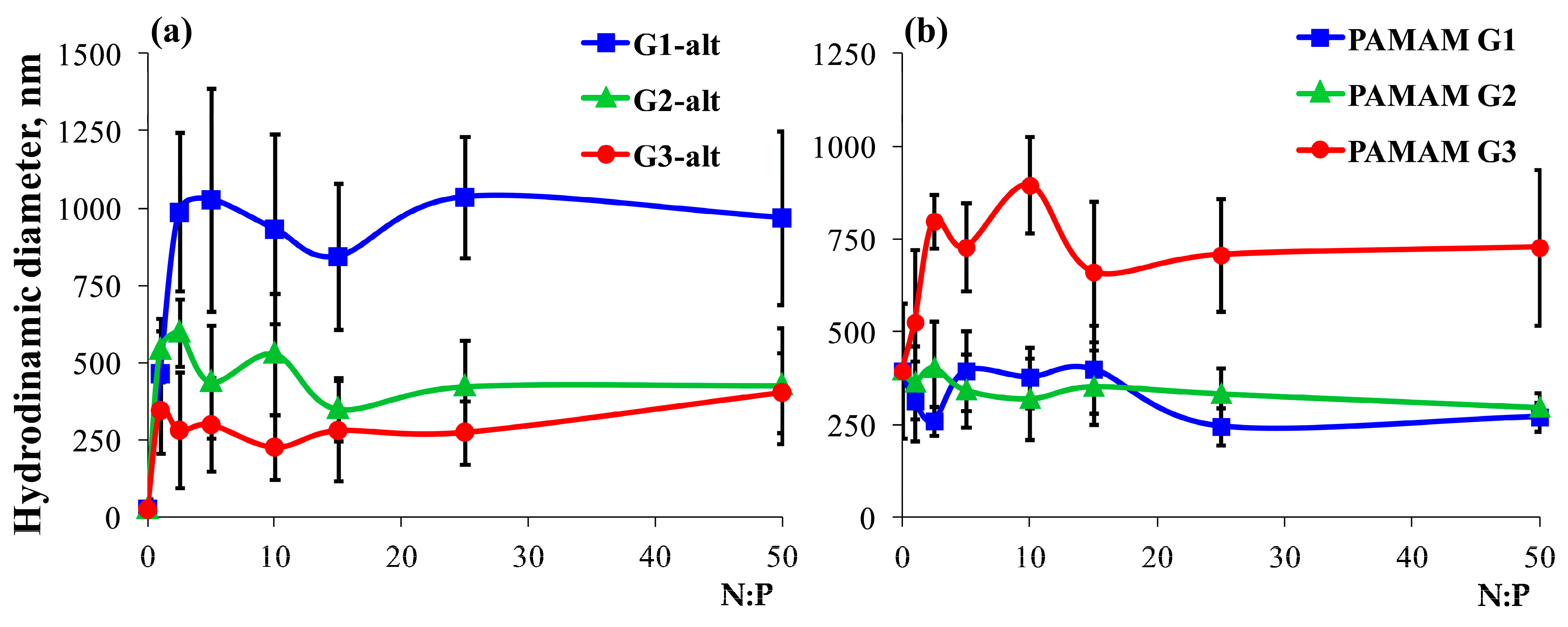
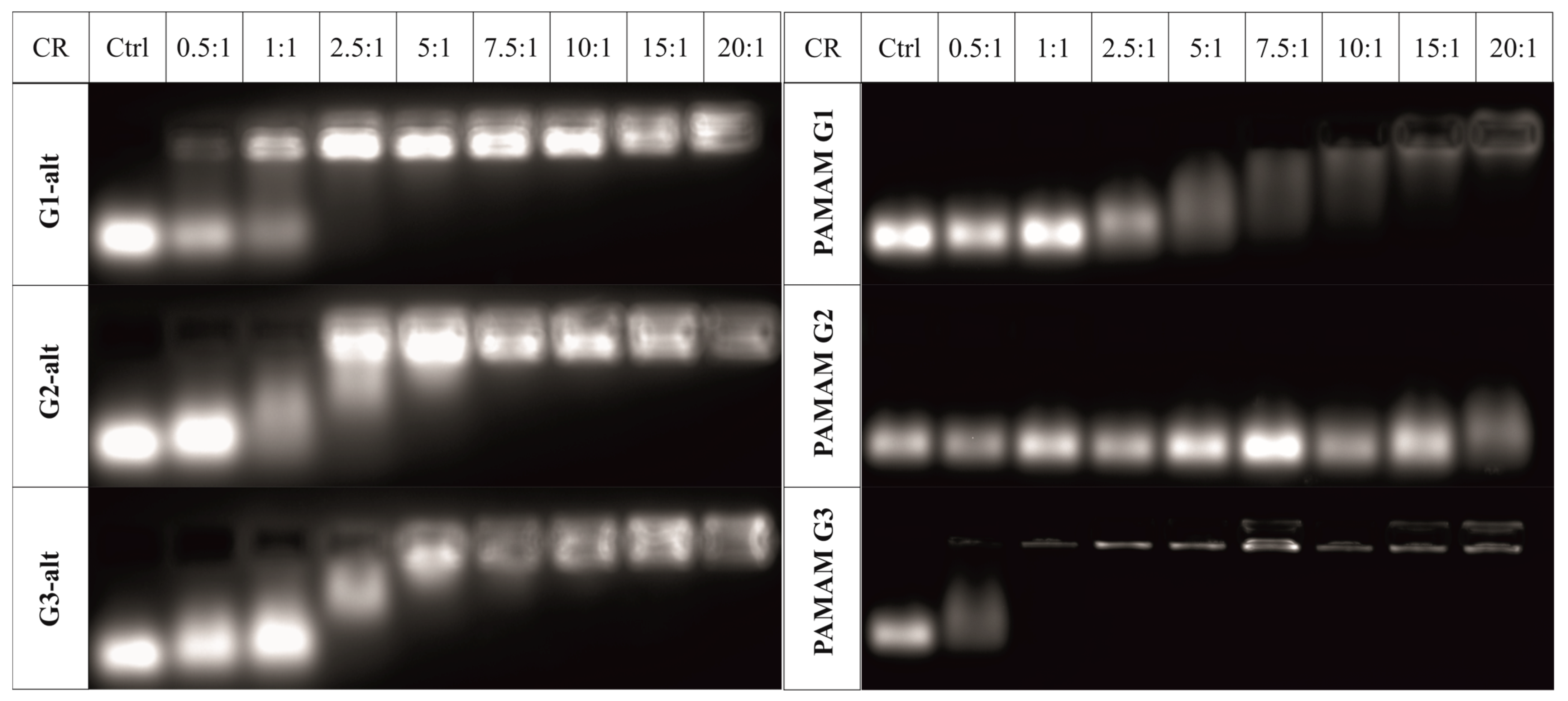

| Dendrimer | Molecular Volume (Å3) | Rg (Å) | Literature Data of Rg (Å) |
|---|---|---|---|
| G1-alt | 5260 | 10.9 | - |
| G2-alt | 11,478 | 16.3 | - |
| G3-alt | 20,900 | 22.9 | - |
| PAMAM G1 | 3926 | 10.8 | 7.03–9.4 [68] |
| PAMAM G2 | 8407 | 14.5 | 8.4–16.6 [68], 14.5 ± 0.3 (neutral pH) [69] |
| PAMAM G3 | 18,046 | 20.7 | 11.23–22.8 [68], 19.7 ± 0.3 (neutral pH) [69] |
| Concentration, µM | d, nm (PDI) | ||
|---|---|---|---|
| G1-alt | G2-alt | G3-alt | |
| 1 | 289 ± 13 (0.46 ± 0.05) | 538 ± 7 (0.25 ± 0.06) | 343 ± 44 (0.31 ± 0.05) |
| 10 | 279 ± 17 (0.32 ± 0.01) | 233 ± 39 (0.29 ± 0.02) | 197 ± 4 (0.28 ± 0.05) |
| 100 | 254 ± 36 (0.47 ± 0.09) | 242 ± 8 (0.45 ± 0.04) | 236 ± 8 (0.27 ± 0.02) |
| siRNA | Sense Sequence | Antisense Sequence |
|---|---|---|
| siKRAS | 5′-CAG CUA AUU CAG AAU CAU UdTdT-3’ | 5′-AAU GAU UCU GAA UUA GCU GdTdT-3′ |
| siBCL-2 | 5′-GCU GCA CCU GAC GCC CUU CdTdT-3’ | 5′-GAA GGG CGU CAG GUG CAG CdTdT-3′ |
| ntRNA | 5′-AAU UCU CCG AAC GUG UCA CGU dTdT-3′ | 5′-ACG UGA CAC GUU CGG AGA AUU dTdT-3′ |
| N:P Ratio Dendrimer/siRNA | Dendrimer Concentration per 1 µM siRNA, µM | ||
|---|---|---|---|
| G1 | G2 | G3 | |
| 0.5:1 | 2.6 | 1.3 | 0.7 |
| 1:1 | 5.3 | 2.6 | 1.3 |
| 2.5:1 | 2.6 | 6.6 | 3.3 |
| 5:1 | 26.3 | 13.1 | 6.6 |
| 7.5:1 | 39.4 | 26.3 | 9.8 |
| 10:1 | 52.5 | 26.3 | 13.1 |
| 15:1 | 78.8 | 39.4 | 19.7 |
| 20:1 | 105.0 | 52.5 | 26.3 |
| 25:1 | 131.3 | 65.6 | 32.8 |
| 50:1 | 262.5 | 131.3 | 65.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padnya, P.; Shiabiev, I.; Pysin, D.; Gerasimova, T.; Ranishenka, B.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Shi, X.; Shen, M.; et al. Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells. Int. J. Mol. Sci. 2024, 25, 12614. https://doi.org/10.3390/ijms252312614
Padnya P, Shiabiev I, Pysin D, Gerasimova T, Ranishenka B, Stanavaya A, Abashkin V, Shcharbin D, Shi X, Shen M, et al. Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells. International Journal of Molecular Sciences. 2024; 25(23):12614. https://doi.org/10.3390/ijms252312614
Chicago/Turabian StylePadnya, Pavel, Igor Shiabiev, Dmitry Pysin, Tatiana Gerasimova, Bahdan Ranishenka, Alesia Stanavaya, Viktar Abashkin, Dzmitry Shcharbin, Xiangyang Shi, Mingwu Shen, and et al. 2024. "Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells" International Journal of Molecular Sciences 25, no. 23: 12614. https://doi.org/10.3390/ijms252312614
APA StylePadnya, P., Shiabiev, I., Pysin, D., Gerasimova, T., Ranishenka, B., Stanavaya, A., Abashkin, V., Shcharbin, D., Shi, X., Shen, M., Nazarova, A., & Stoikov, I. (2024). Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells. International Journal of Molecular Sciences, 25(23), 12614. https://doi.org/10.3390/ijms252312614












