Structure and Properties of Poly(Ethylene-co-vinyl Acetate) Nanocomposites with Dual-Functionalized Dolomite Nanoparticles
Abstract
1. Introduction
2. Result and Discussion
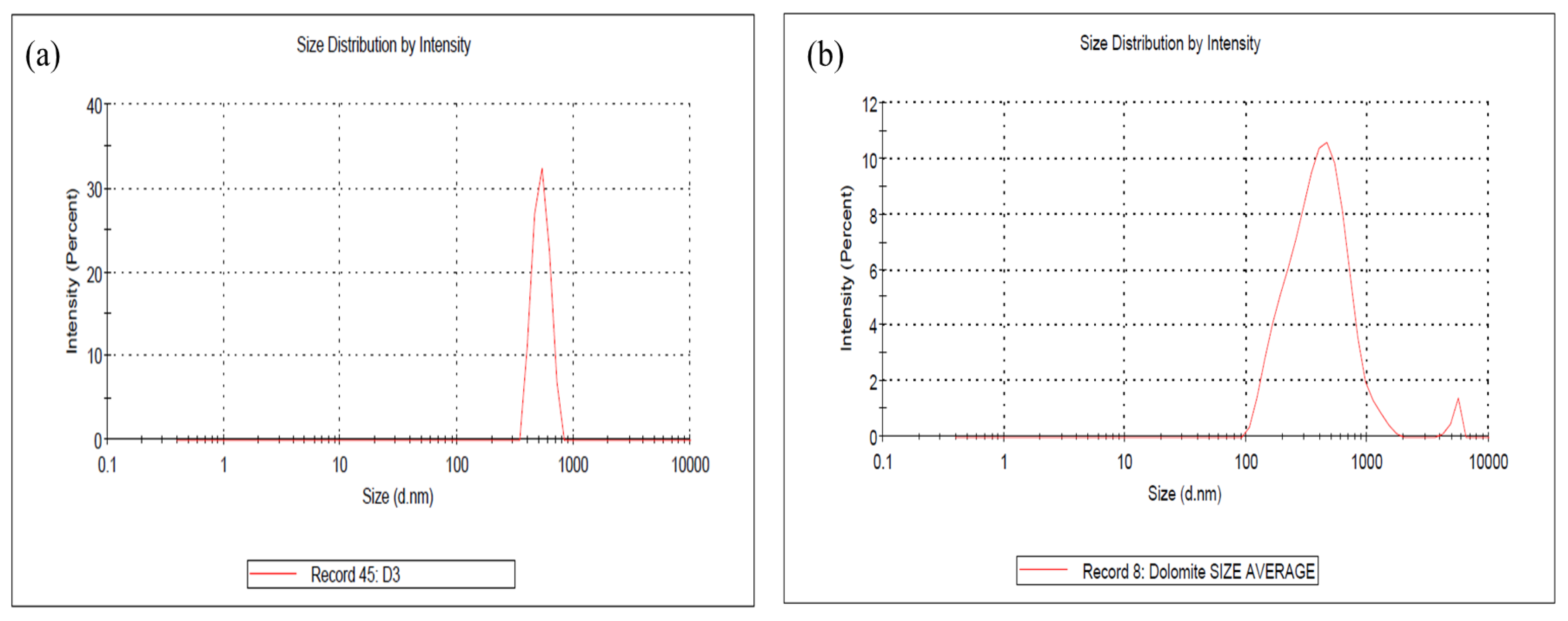
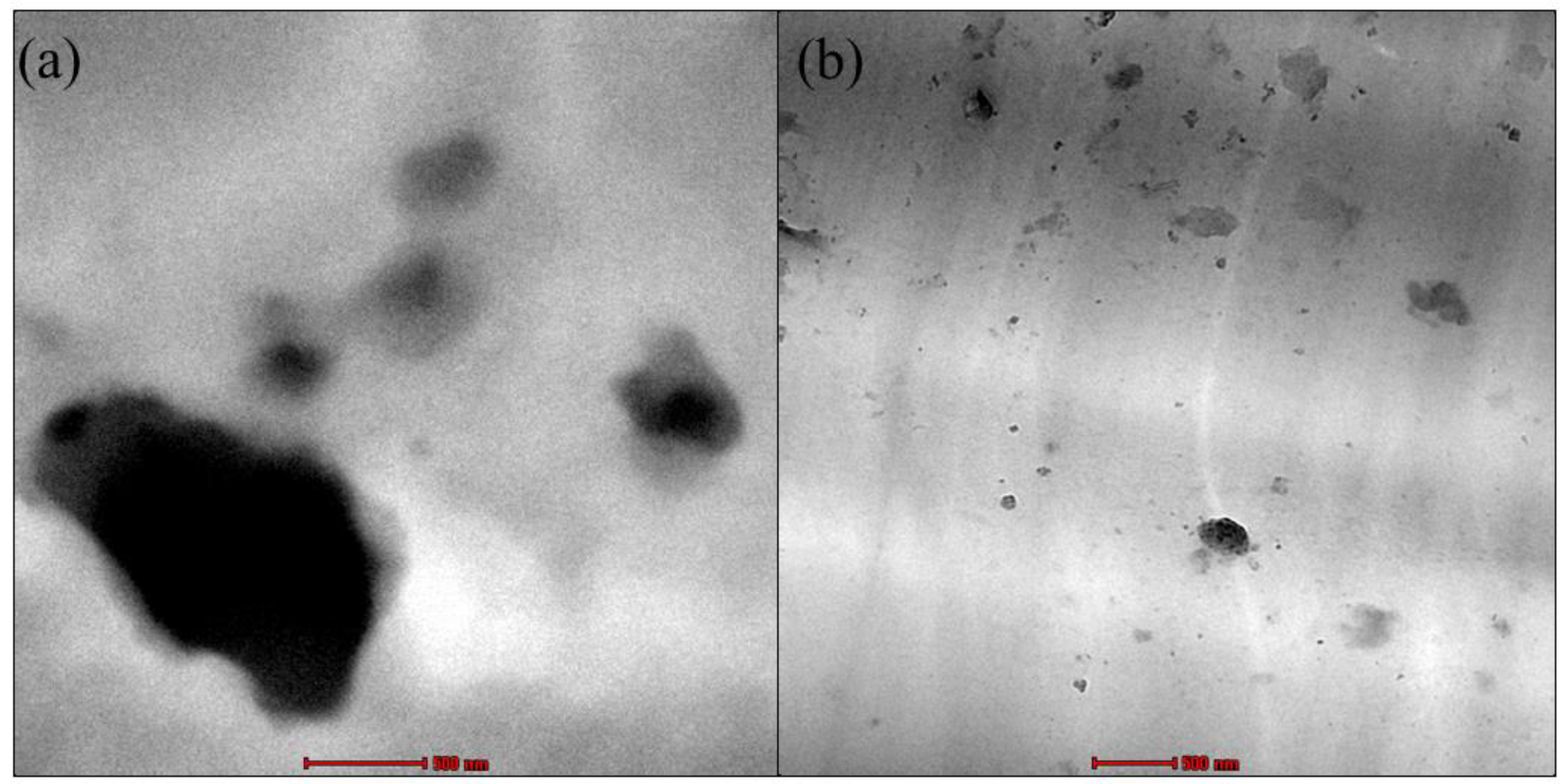
2.1. Characterization of of Raw Dolomite (RD), Dolomite Micron Particles (DMPs) and Dolomite Nanoparticles (DNPs)
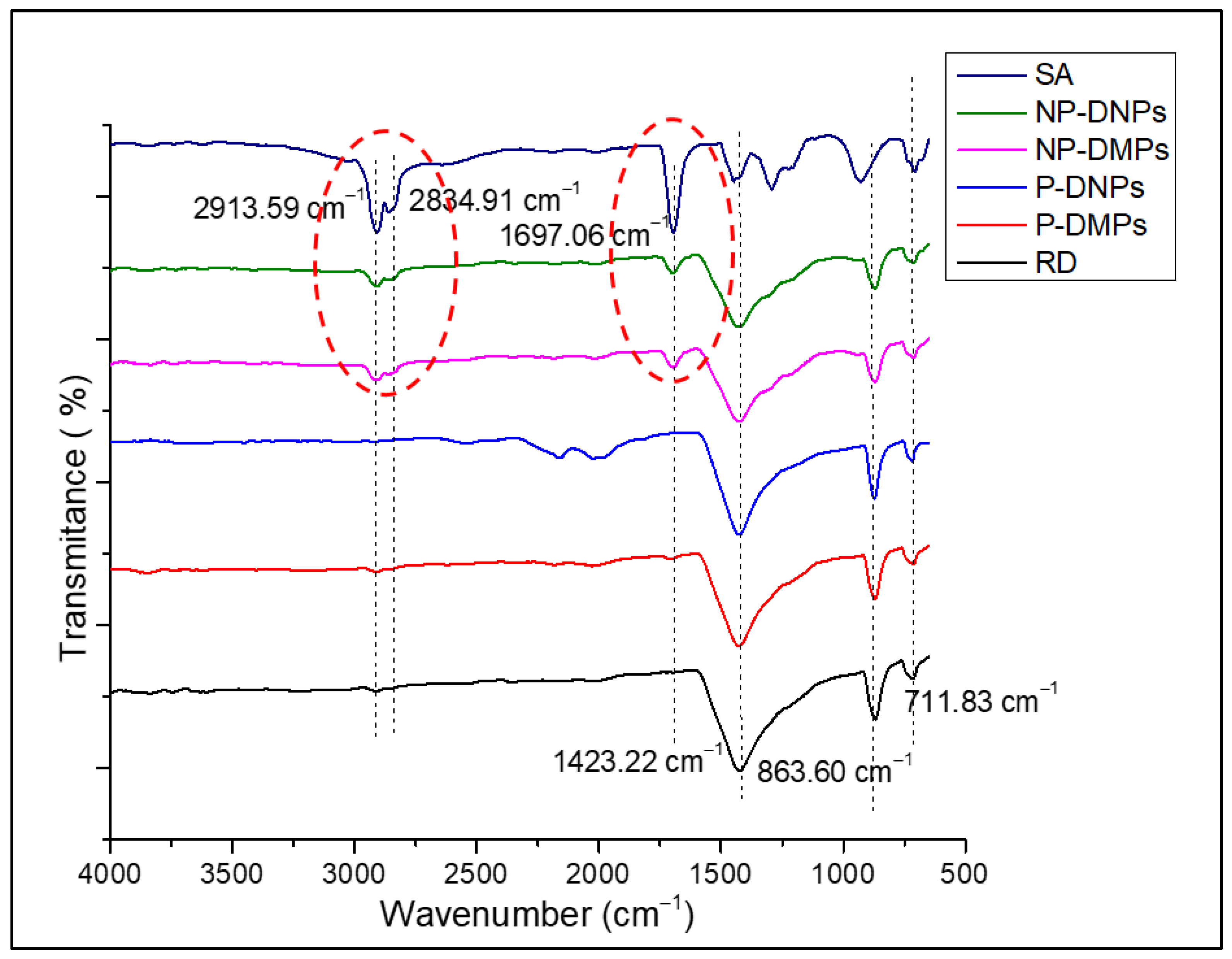
2.2. Characterization of NP-DMPs and NP-DNPs
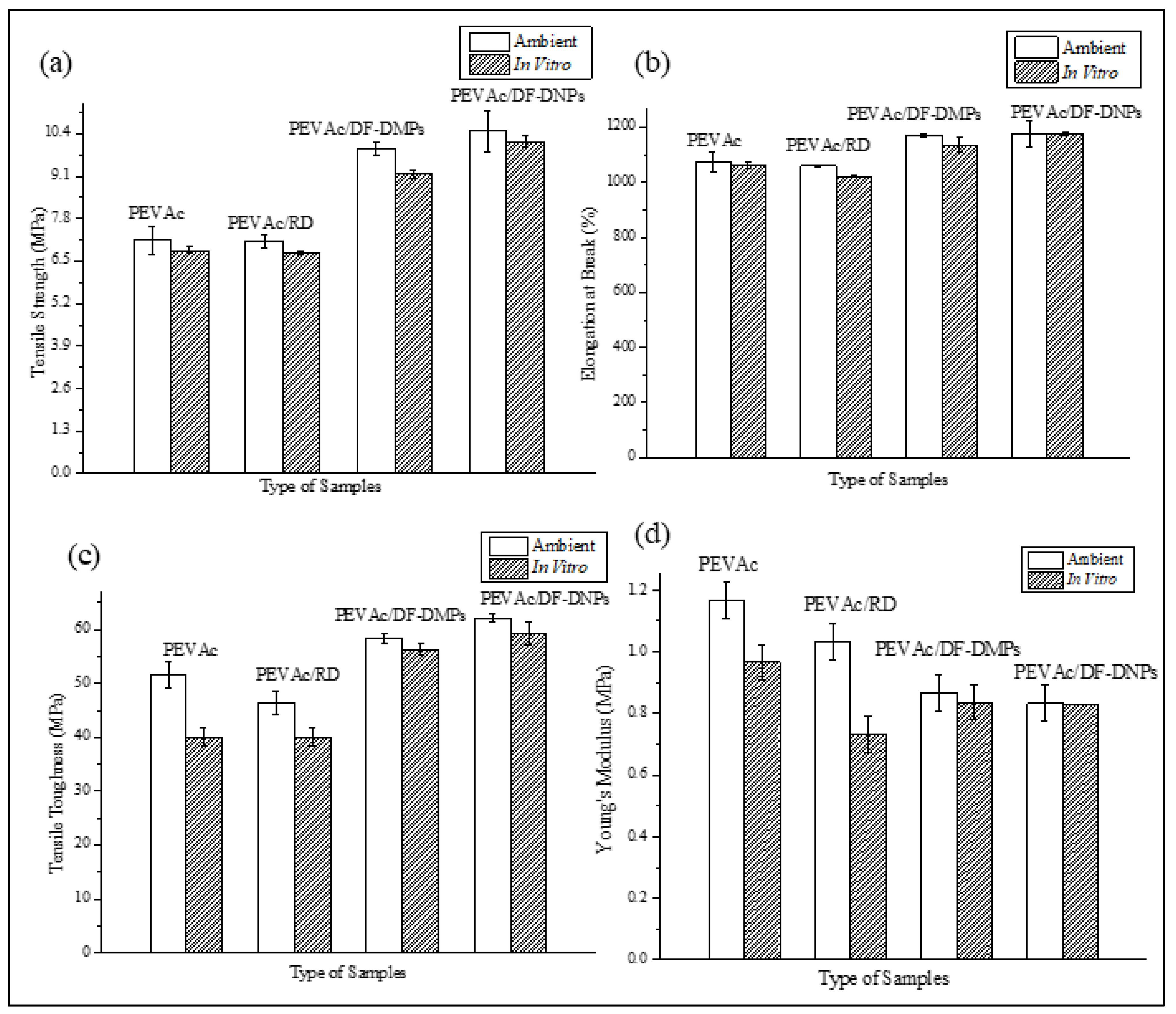
2.3. Comparison of the Tensile Properties of Neat PEVAc, PEVAc Composite, and PEVAc Nanocomposite
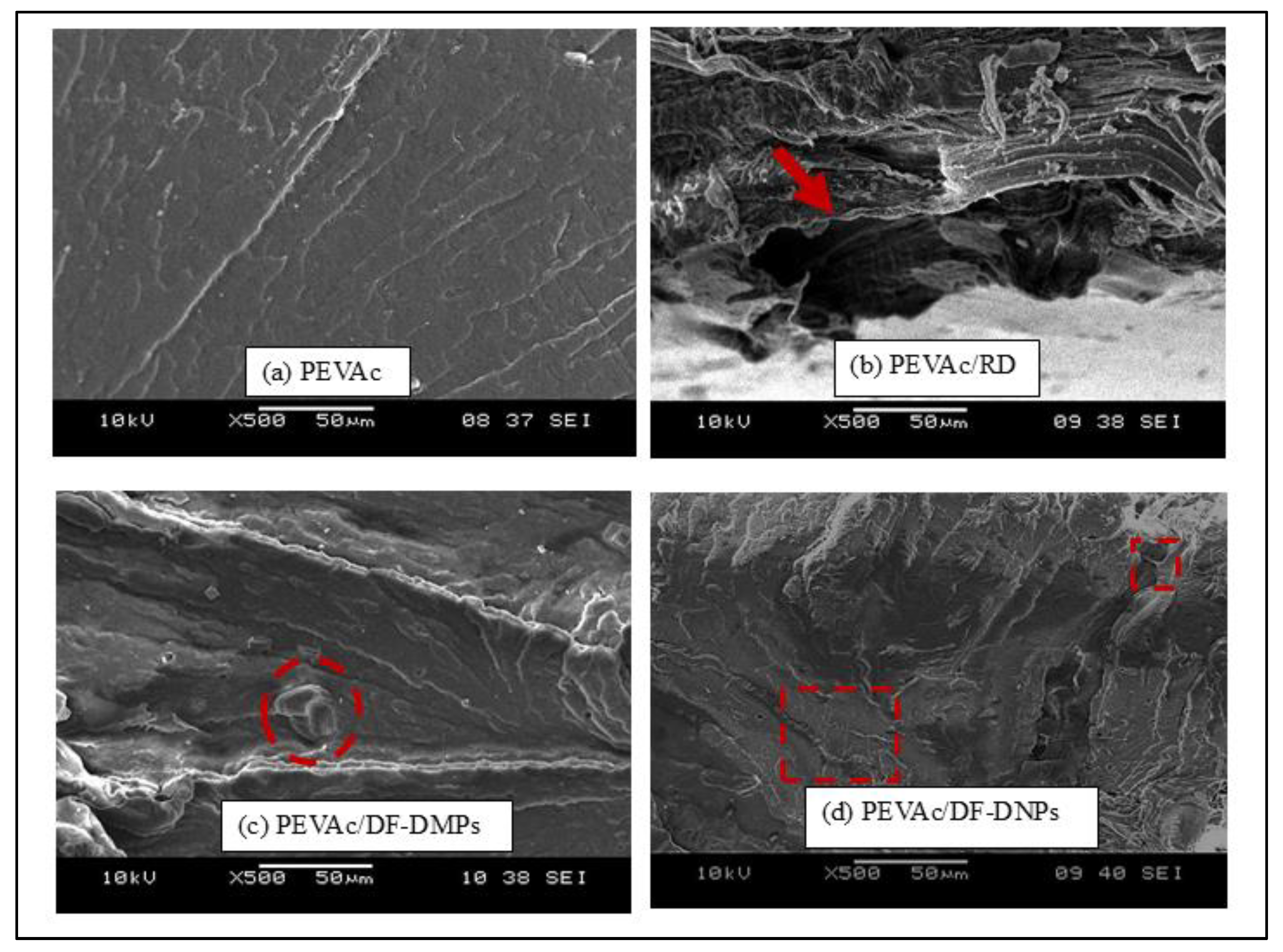
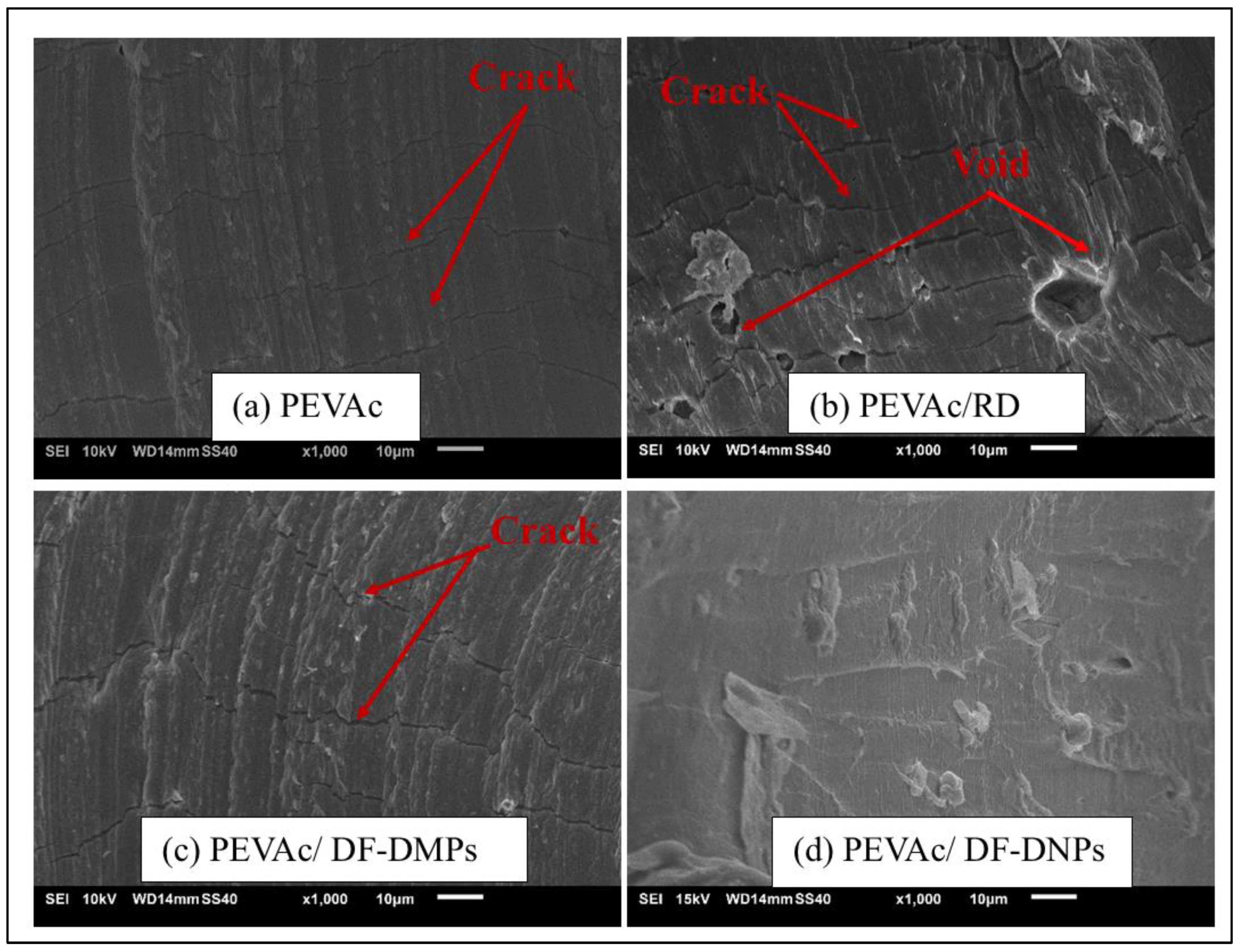
2.4. Structure and Morphology Analysis of Neat PEVAc, PEVAc Composite, and PEVAc Nanocomposite
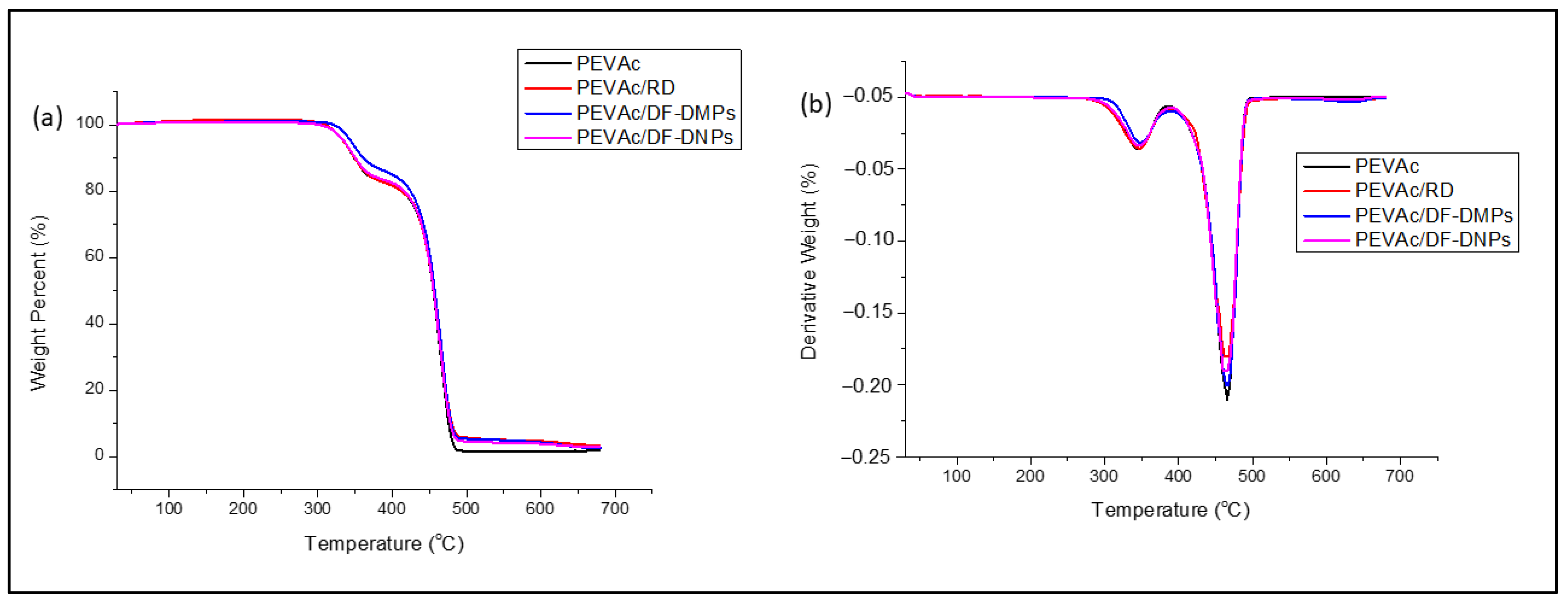
2.5. Thermal Properties of the Neat PEVAc, PEVAc Composite and PEVAc Nanocomposite
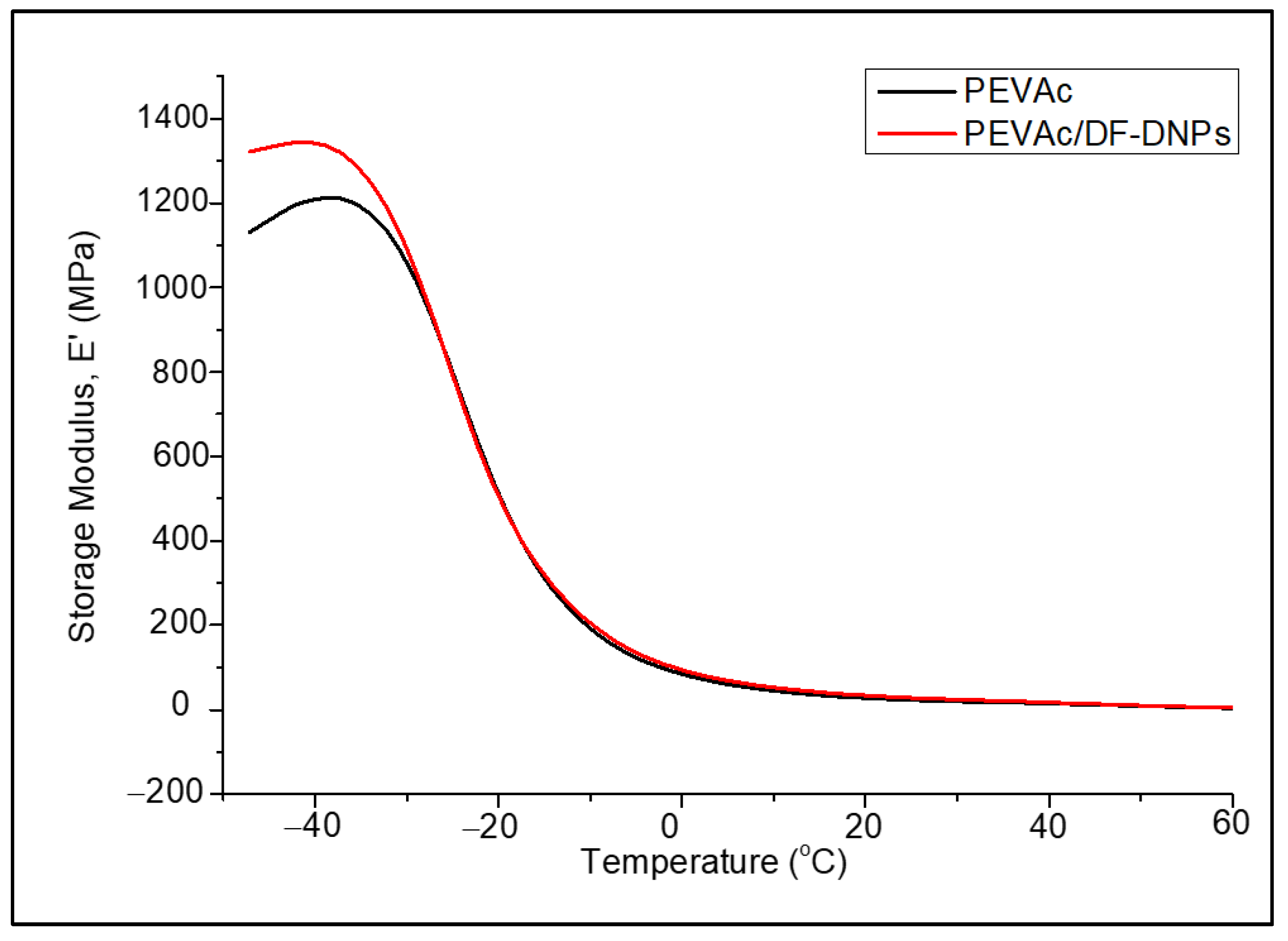
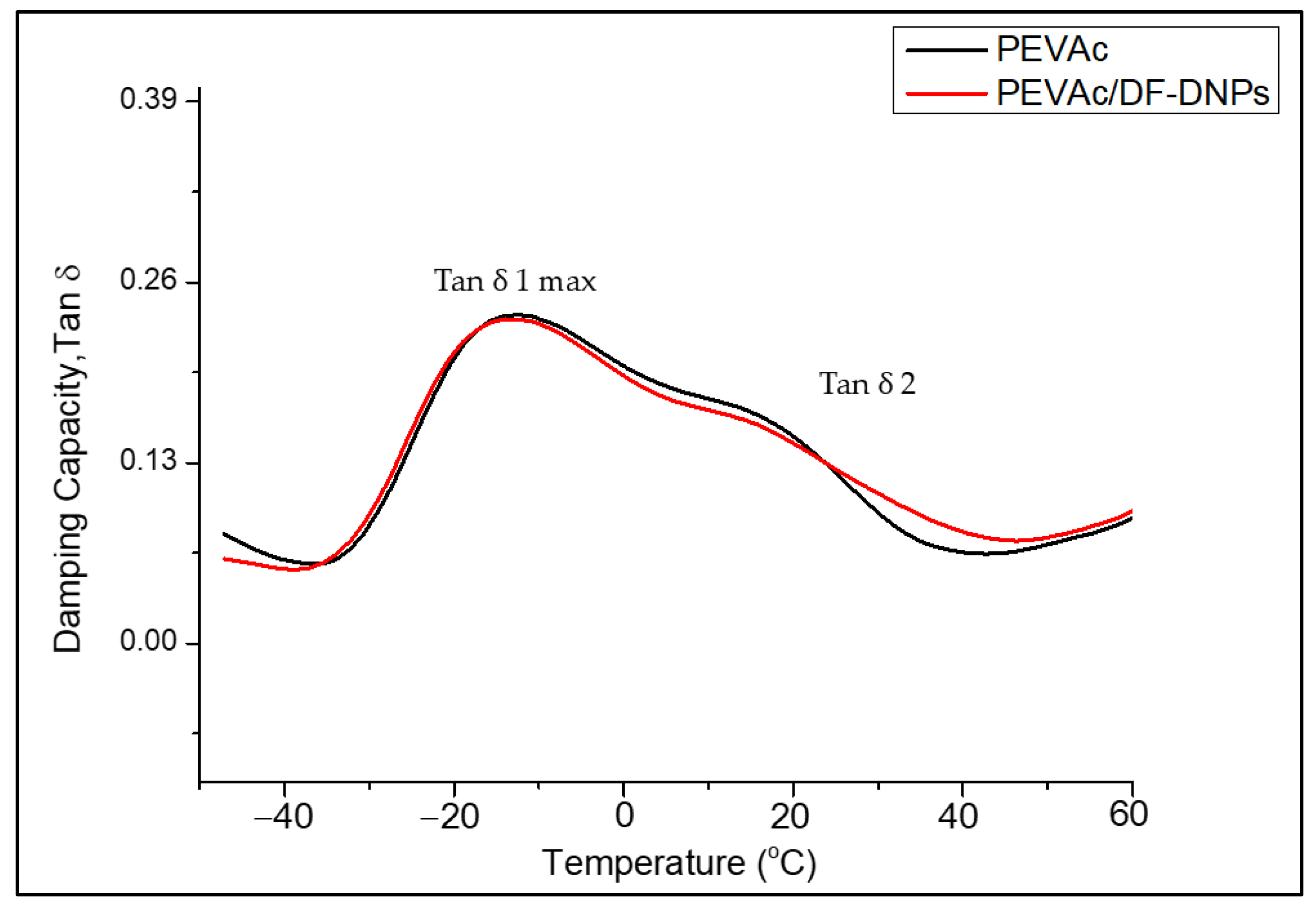
2.6. Biostability Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparations of Dual-Functionalized Dolomite Filler
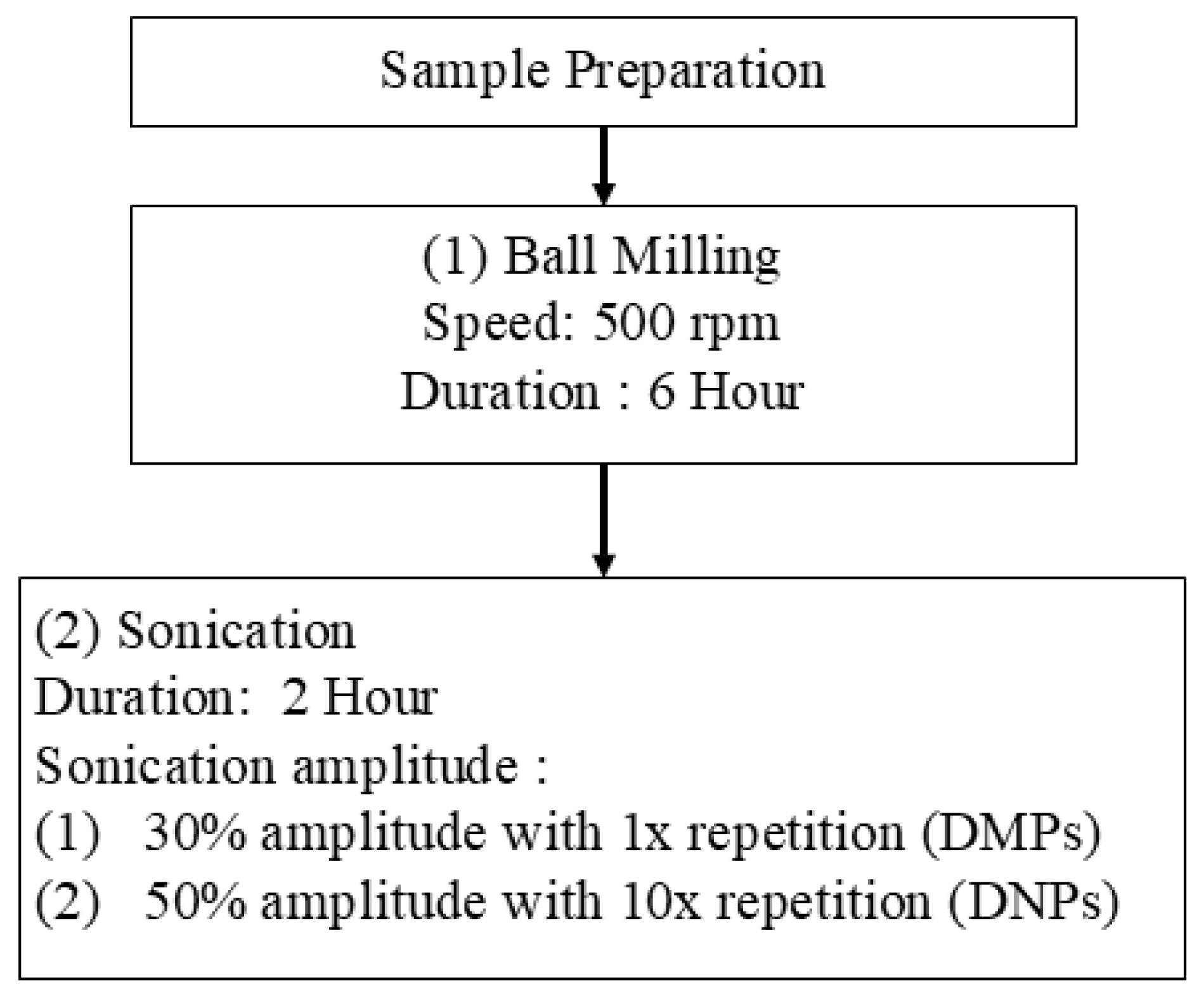
3.2.1. Preparation of P-DMPs and P-DNPs via Ball Milling and Ultrasonication
3.2.2. Preparation of NP-DMPs and NP-DNPs
3.3. Preparations of Neat PEVAc, PEVAc Composite and PEVAc Nanocomposites
3.4. Characterization
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Transmission Electron Microscopy (TEM)
3.4.3. Particle Size Analyzer (PSA)
3.4.4. X-Ray Diffractometer (XRD)
3.4.5. Fourier Transform-Infrared Spectroscopy (FTIR)
3.4.6. Contact Angle Analysis
3.4.7. Thermogravimetry Analysis (TGA)
3.4.8. Tensile Analysis
3.4.9. Differential Scanning Calorimetry Analysis (DSC)
3.4.10. Dynamic Mechanical Thermal Analysis (DMTA)
3.4.11. Biostability Analysis
3.4.12. Water Resistance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Bi, B.; Liu, J.; Yang, S.; Zhou, L.; Lu, L.; Wang, Y.; Xu, F.; Huang, R. Halogen-free intumescent flame-retardant ethylene-vinyl acetate copolymer system based on organic montmorillonite and graphene nanosheets. J. Appl. Polym. Sci. 2018, 135, 46361. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Experimental evaluation of a diesel engine fuelled by pyrolysis oils produced from low-density polyethylene and ethylene–vinyl acetate plastics. Fuel Process. Technol. 2017, 161, 125–131. [Google Scholar] [CrossRef]
- Barbour, M.; Wood, N.J.; Maddocks, S.; Grady, H.J.; Collins, A.M. Functionalization of ethylene vinyl acetate with antimicrobial chlorhexidine hexametaphosphate nanoparticles. Int. J. Nanomed. 2014, 9, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Saadat, E. Pharmaceutical implants: From concept to commercialization. In Advances in Drug Delivery Systems for Healthcare; Mehta, P.P., Parihar, A., Eds.; Institute of Physics Publishing: Bristol, UK, 2024; pp. 1–42. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Jeong, J.; Kim, S.J. Emerging encapsulation technologies for long-term reliability of microfabricated implantable devices. Micromachines 2019, 10, 508. [Google Scholar] [CrossRef]
- Seok, S. Polymer-based biocompatible packaging for implantable devices: Packaging method, materials, and reliability simulation. Micromachines 2021, 12, 1020. [Google Scholar] [CrossRef]
- Fink, J.K. Handbook of Engineering and Specialty Thermoplastics, Polyolefins and Styrenics; Wiley-Scrivener: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chauhan, N.P.S.; Jangid, N.K.; Kaur, N.; Rathore, B.S.; Gholipourmalekabadi, M.; Mozafari, M. Medicines: Polymers. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Maytin, M.; Epstein, L.M.; Ellenbogen, K.A. (Eds.) Implantable Devices: Design, Manufacturing, and Malfunction, An Issue of Cardiac Electrophysiology Clinics; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Feldman, D. Polyolefin, olefin copolymers and polyolefin polyblend nanocomposites. J. Macromol. Sci. Part A 2016, 53, 651–658. [Google Scholar] [CrossRef]
- Ramesh, M.; Rajeshkumar, L.N.; Srinivasan, N.; Kumar, D.V.; Balaji, D. Influence of filler material on properties of fiber-reinforced polymer composites: A review. E-Polym. 2022, 22, 898–916. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Senthilkumar, K.; Chandrasekar, M.; Subramaniam, S.; Rangappa, S.M.; Siengchin, S.; Rajini, N. Influence of Fillers on the Thermal and Mechanical Properties of Biocomposites: An Overview. In Biofibers and Biopolymers for Biocomposites; Khan, A., Rangappa, S.M., Siengchin, S., Asiri, A.M., Eds.; Springer: Cham, Switzerland; pp. 111–134.
- Yuan, B.; Bao, C.; Qian, X.; Jiang, S.; Wen, P.; Xing, W.; Song, L.; Liew, K.M.; Hu, Y. Synergetic Dispersion Effect of Graphene Nanohybrid on the Thermal Stability and Mechanical Properties of Ethylene Vinyl Acetate Copolymer Nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 1143–1149. [Google Scholar] [CrossRef]
- Osman, A.F.; Hamid, A.R.A.; Rakibuddin, M.; Weng, G.K.; Ananthakrishnan, R.; Ghani, S.A.; Mustafa, Z. Hybrid silicate nanofillers: Impact on morphology and performance of EVA copolymer uponin vitrophysiological fluid exposure. J. Appl. Polym. Sci. 2016, 134, 44640. [Google Scholar] [CrossRef]
- Fauzi, A.A.A.; Osman, A.F.; Abdullah, M.A.A.; Mandal, S.; Ananthakrishan, R. Ethylene vinyl acetate nanocomposites with hybrid silicate nanofillers of destabilized natural and commercial bentonites and organomontmorillonites. J. Vinyl Addit. Technol. 2019, 25, 396–411. [Google Scholar] [CrossRef]
- Fitri, T.F.M.; Osman, A.F.; Alosime, E.M.; Othman, R.; Hashim, F.; Abdullah, M.A.A. Biomedical PEVA Nanocomposite with Dual Clay Nanofiller: Cytotoxicity, Mechanical Properties, and Biostability. Polymers 2021, 13, 4345. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tanaka, K.; Chujo, Y. Synthesis of POSS Derivatives Having Dual Types of Alkyl Substituents and Their Application as a Molecular Filler for Low-Refractive and Highly Durable Materials. Bull. Chem. Soc. Jpn. 2017, 90, 205–209. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA. Polymers 2018, 10, 1332. [Google Scholar] [CrossRef]
- Gunji, N.; Komori, Y.; Yoshitake, H. Double functionalization with mercaptopropyl and vinyl groups of the surface of silica nanoparticles and its application to tire rubber. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 351–356. [Google Scholar] [CrossRef]
- Ran, S.; Xie, J.; Li, C.; Qin, H.; Chen, Z.; Wang, X.; Yu, Y.; Wang, S.; Xiong, C. Polydopamine-assisted silver-coated spherical boron nitride as dual functional filler for thermal management and electromagnetic interference shielding. Diam. Relat. Mater. 2023, 135, 109856. [Google Scholar] [CrossRef]
- Song, K.; Xu, H.; Xie, K.; Yang, Y. Keratin-Based Biocomposites Reinforced and Cross-Linked with Dual-Functional Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2017, 5, 5669–5678. [Google Scholar] [CrossRef]
- Cao, Z.-F.; Chen, P.; Yang, F.; Wang, S.; Zhong, H. Transforming structure of dolomite to enhance its ion-exchange capacity for copper(II). Colloids Surf A Physicochem. Eng. Asp. 2018, 539, 201–208. [Google Scholar] [CrossRef]
- Azza, N.N.N.; Ong, H.L.; Noorina, H.J.; Akil, H.M.; Ting, S.S. Analysis of Ground Dolomite: Effect of Grinding Time on the Production of Submicron Particles. Appl. Mech. Mater. 2014, 679, 145–148. [Google Scholar] [CrossRef]
- Moreschi, E.; Hernandes, L.; Dantas, J.A.; da Silva, M.A.R.C.P.; Casaroto, A.R.; Bersani-Amado, C.A. Effect of dolomite on the repair of bone defects in rats: Histological study. Histol. Histopathol. 2010, 25, 1547–1556. [Google Scholar] [CrossRef]
- Dias, G.F.; Alves, F.B.T.; de Andrade, A.V.C.; Ferreira, A.M.; Dos Santos, F.A. Toxicity assessment and chemical properties of dolomite for use in dentistry. Rev. Odontol. Univ. Cid. São Paulo 2018, 30, 33–46. [Google Scholar] [CrossRef][Green Version]
- Malaysia, M.U.T.; Saidi, M.A.A.; Mazlan, F.S.; Hassan, A.; Rashid, R.A.; Rahmat, A.R. Flammability, Thermal and Mechanical Properties of Polybutylene Terephthalate/Dolomite Composites. J. Phys. Sci. 2019, 30, 175–189. [Google Scholar] [CrossRef]
- Ridhwan, J.; Noimam, N.; Salleh, M.M.; Sam, S.; Musa, L.; Yahya, N.N. The Effect of Different Sizes “Batu Reput” (Dolomite) as a Filler in SMR L and ENR-50. Adv. Mat. Res. 2013, 795, 383–387. [Google Scholar] [CrossRef]
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film Through Sonication Process. Polymers 2021, 13, 274. [Google Scholar] [CrossRef]
- Bakar, S.S.S.; Zakaria, N.S.; Wahab, J.A.; Salleh, M.A.A.M. Batu Reput Filled Recycled Polypropylene Eco-polymer Composites. Bdg. Indones. Adv. Environ. Biol. 2013, 7, 3596–3600. [Google Scholar]
- Saba, N.; Tahir, P.M.; Jawaid, M. A Review on Potentiality of Nano Filler/Natural Fiber Filled Polymer Hybrid Composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Przekop, R.E.; Jakubowska, P.; Sztorch, B.; Kozera, R.; Dydek, K.; Jałbrzykowski, M.; Osiecki, T.; Marciniak, P.; Martyła, A.; Kloziński, A.; et al. Opoka—Sediment Rock as New Type of Hybrid Mineral Filler for Polymer Composites. AppliedChem 2021, 1, 90–110. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review of the mechanical and thermal properties of graphene and its hybrid polymer nanocomposites for structural applications. J. Mater. Sci. 2018, 54, 5992–6026. [Google Scholar] [CrossRef]
- Matykiewicz, D.; Barczewski, M.; Mousa, M.S.; Sanjay, M.R.; Siengchin, S. Impact Strength of Hybrid Epoxy–Basalt Composites Modified with Mineral and Natural Fillers. ChemEngineering 2021, 5, 56. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, X.; Li, Z.; Zheng, S.; Wang, Z. Effects of rotation speed and media density on particle size distribution and structure of ground calcium carbonate in a planetary ball mill. Adv. Powder Technol. 2015, 26, 505–510. [Google Scholar] [CrossRef]
- Mio, H.; Kano, J.; Saito, F.; Kaneko, K. Effects of rotational direction and rotation-to-revolution speed ratio in planetary ball milling. Mater. Sci. Eng. A 2002, 332, 75–80. [Google Scholar] [CrossRef]
- Alex, T.; Kumar, R.; Roy, S.; Mehrotra, S. Mechanically induced reactivity of gibbsite: Part 1. Planetary milling. Powder Technol. 2014, 264, 105–113. [Google Scholar] [CrossRef]
- Alex, T.; Kumar, R.; Roy, S.; Mehrotra, S. Stirred Bead Mill Grinding of Gibbsite: Surface and Morphological Changes. Adv. Powder Technol. 2008, 19, 483–491. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B.; Blanchet, P. Ultrasonication Technique: A Method for Dispersing Nanoclay in Wood Adhesives. J. Nanomater. 2013, 2013, 341897. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q. Preparation of conductive polyaniline/nanosilica particle composites through ultrasonic irradiation. J. Appl. Polym. Sci. 2003, 87, 1811–1817. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, K.; Nishimiya, N. Effect of amplitude and frequency of ultrasonic irradiation on morphological characteristics control of calcium carbonate. Ultrason. Sonochem. 2010, 17, 617–620. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Rouxel, D.; Hadji, R.; Vincent, B.; Fort, Y. Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason. Sonochem. 2011, 18, 382–388. [Google Scholar] [CrossRef]
- Derkani, M.H.; Fletcher, A.J.; Fedorov, M.; Abdallah, W.; Sauerer, B.; Anderson, J.; Zhang, Z.J. Mechanisms of Surface Charge Modification of Carbonates in Aqueous Electrolyte Solutions. Colloids Interfaces 2019, 3, 62. [Google Scholar] [CrossRef]
- Abdullah, C.A.C.; Albert, E.L.; Lau, G.E.; Zarin, M.A.; Yusoff, M.Z.M. Effect of Thermal Treatment on Physical Properties of Malaysian Dolomitic Limestone. Int. J. Electroact. Mater. 2022, 10, 18–23. [Google Scholar]
- Mroczkowska-Szerszeń, M.; Orzechowski, M. Infrared spectroscopy methods in reservoir rocks analysis—Semiquantitative approach for carbonate rocks. Naft. -Gaz. 2018, 74, 802–812. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Sonmez, M.; Juganaru, M.; Ficai, A.; Ficai, D.; Oprea, O.; Gurau, D.; Alexandrescu, L.; Stelescu, M.D.; Georgescu, M.; Nituica, M.; et al. Dolomite surface modification with titanium and silicon precursors and its morphostructural and thermal characterisation. In Proceedings of the 8th International Conference on Advanced Materials and Systems, Bucharest, Romania, 1–3 October 2020. [Google Scholar] [CrossRef]
- Shahraki, B.; Mehrabi, B.; Dabiri, R. Thermal behavior of Zefreh dolomite mine (Central Iran). J. Min. Metall. Sect. B Metall. 2009, 45, 35–44. [Google Scholar] [CrossRef]
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTIR): A fast method for identifying Heinrich events in IODP Site U1308. Mar. Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Chong, L.K.; Osman, A.F.; Fauzi, A.A.A.; Alrashdi, A.A.; Halim, K.A.A. The Mechanical and Thermal Properties of Poly(ethylene-co-vinyl acetate) (PECoVA) Composites with Pristine Dolomite and Organophilic Microcrystalline Dolomite (OMCD). Polymers 2021, 13, 3034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, B.; Lv, M.; Zhu, Y.; Gao, J. Fabrication and characterization of novel shape-stabilized stearic acid composite phase change materials with tannic-acid-templated mesoporous silica nanoparticles for thermal energy storage. RSC Adv. 2017, 7, 15625–15631. [Google Scholar] [CrossRef]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Fitri, T.F.M.; Osman, A.F.; Othman, R.; Mustafa, Z. Incorporation of Hybrid Pre-Dispersed Organo-Montmorillonite/Destabilized Bentonite Nanofillers for Improving Tensile Strength of PEVA Copolymer with 40% Vinyl Acetate Composition. Mater. Sci. Forum 2020, 1010, 118–123. [Google Scholar] [CrossRef]
- El-Mezayen, A. Mineralogical and chemical studies on some minerals used in pharmaceutical industries in egypt. Al-Azhar J. Pharm. Sci. 2014, 50, 181–190. [Google Scholar] [CrossRef][Green Version]
- Tanahashi, M. Development of Fabrication Methods of Filler/Polymer Nanocomposites: With Focus on Simple Melt-Compounding-Based Approach Without Surface Modification of Nanofillers. Materials 2010, 3, 1593–1619. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T.; Francis, T. Effect of cellulose whisker content on the properties of poly(ethylene-co-vinyl acetate)/cellulose composites. Carbohydr. Polym. 2013, 95, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Soheilmoghaddam, M.; Adelnia, H.; Bidsorkhi, H.C.; Sharifzadeh, G.; Wahit, M.U.; Akos, N.I.; Yussuf, A.A. Development of Ethylene-Vinyl Acetate Composites Reinforced with Graphene Platelets. Macromol. Mater. Eng. 2016, 302, 1600260. [Google Scholar] [CrossRef]
- Gaston, F.; Dupuy, N.; Marque, S.R.; Barbaroux, M.; Dorey, S. FTIR study of ageing of γ-irradiated biopharmaceutical EVA based film. Polym. Degrad. Stab. 2016, 129, 19–25. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Ferreira, E.d.S.B.; Siqueira, D.D.; Filho, E.A.d.S.; Araújo, E.M. Additivation of the ethylene–vinyl acetate copolymer (EVA) with maleic anhydride (MA) and dicumyl peroxide (DCP): The impact of styrene monomer on cross-linking and functionalization. Polym. Bull. 2021, 79, 7323–7346. [Google Scholar] [CrossRef]
- Hamid, A.R.A.; Osman, A.F.; Mustafa, Z.; Mandal, S.; Ananthakrishnan, R. Tensile, fatigue and thermomechanical properties of poly(ethylene-co-vinyl acetate) nanocomposites incorporating low and high loadings of pre-swelled organically modified montmorillonite. Polym. Test. 2020, 85, 106426. [Google Scholar] [CrossRef]
- D’Amelia, R.P.; Gentile, S.; Nirode, W.F.; Huang, L. Quantitative Analysis of Copolymers and Blends of Polyvinyl Acetate (PVAc) Using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Dutta, R.; Dhar, S.; Baruah, K.; Dutta, N.; Doley, S.; Sedai, P.; Dolui, S.; Ray, B.; Karmakar, B. Removal of organic solvents and oils from wastewater by absorption with crosslinked poly (ethylene-co-vinyl acetate) modified by cetyl alcohol. J. Water Process Eng. 2022, 49, 103073. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Şimşek, S.; Ulusoy, H.I.; Özer, A. Synthesis and characterization of a polyacrylamide-dolomite based new composite material for efficient removal of uranyl ions. J. Radioanal. Nucl. Chem. 2020, 324, 317–330. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Wang, B.; La Plante, E.C.; Pignatelli, I.; Krishnan, N.M.A.; Le Pape, Y.; Neithalath, N.; Bauchy, M.; Sant, G. The effect of irradiation on the atomic structure and chemical durability of calcite and dolomite. Npj Mater. Degrad. 2019, 3, 36. [Google Scholar] [CrossRef]
- Choi, S.-S.; Chung, Y.Y. Simple test method for determination of contribution level of crosslink density by crystalline structure of poly(ethylene-co-vinyl acetate) compound. Polym. Test. 2019, 77, 105928. [Google Scholar] [CrossRef]
- Choi, S.S.; Chung, Y.Y. Change of Crystalline Properties of Poly(ethylene-co-vinyl acetate) According to the Microstructures. Elastomers Compos. 2021, 56, 92–99. [Google Scholar] [CrossRef]
- Doumeng, M.; Berthet, F.; Delbé, K.; Marsan, O.; Denape, J.; Chabert, F. Effect of size, concentration, and nature of fillers on crystallinity, thermal, and mechanical properties of polyetheretherketone composites. J. Appl. Polym. Sci. 2021, 139, 51574. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Azlina, W.A.K.G.W.; Amran, M.S.M.; Taufiq-Yap, Y.H. Preparation and Characterization of Malaysian Dolomites as a Tar Cracking Catalyst in Biomass Gasification Process. J. Energy 2013, 2013, 791582. [Google Scholar] [CrossRef]
- Mantilaka, M.; Karunaratne, D.; Rajapakse, R.; Pitawala, H. Precipitated calcium carbonate/poly(methyl methacrylate) nanocomposite using dolomite: Synthesis, characterization and properties. Powder Technol. 2013, 235, 628–632. [Google Scholar] [CrossRef]
- Jing, Y.; Nai, X.; Dang, L.; Zhu, D.; Wang, Y.; Dong, Y.; Li, W. Reinforcing polypropylene with calcium carbonate of different morphologies and polymorphs. Sci. Eng. Compos. Mater. 2017, 25, 745–751. [Google Scholar] [CrossRef]
- Osman, A.F.; Fitri, T.F.M.; Rakibuddin, M.; Hashim, F.; Johari, S.A.T.T.; Ananthakrishnan, R.; Ramli, R. Pre-dispersed organo-montmorillonite (organo-MMT) nanofiller: Morphology, cytocompatibility and impact on flexibility, toughness and biostability of biomedical ethyl vinyl acetate (EVA) copolymer. Mater. Sci. Eng. C 2017, 74, 194–206. [Google Scholar] [CrossRef]
- Osman, A.F.; Kalo, H.; Hassan, M.S.; Hong, T.W.; Azmi, F. Pre-dispersing of montmorillonite nanofiller: Impact on morphology and performance of melt compounded ethyl vinyl acetate nanocomposites. J. Appl. Polym. Sci. 2015, 133, 43204. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Thomann, R.; Mülhaupt, R. Synthesis and thermal behaviour of layered silicate–EVA nanocomposites. Polymers 2001, 42, 4501–4507. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Thongraar, R.; Saravari, O. Preparation and Properties of PVC/EVA/Organomodified Montmorillonite Nanocomposites. J. Reinf. Plast. Compos. 2008, 27, 431–442. [Google Scholar] [CrossRef]
- Ni, L.; Mao, Y.; Liu, Y.; Cai, P.; Jiang, X.; Gao, X.; Cheng, X.; Chen, J. Synergistic reinforcement of waterborne polyurethane films using Palygorskite and dolomite as micro/nano-fillers. J. Polym. Res. 2020, 27, 23. [Google Scholar] [CrossRef]
- Xie, J.; Chen, T.; Xing, B.; Liu, H.; Xie, Q.; Li, H.; Wu, Y. The thermochemical activity of dolomite occurred in dolomite–palygorskite. Appl. Clay. Sci. 2016, 119, 42–48. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Vu, T.N.; Nguyen, T.M.L.; Nguyen, T.D.; Thuc, C.N.H.; Bui, Q.B.; Colin, J.; Perré, P. Synergistic Influences of Stearic Acid Coating and Recycled PET Microfibers on the Enhanced Properties of Composite Materials. Materials 2020, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Possemiers, S.; Boone, M.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef]
- Choi, S.-S.; Chung, Y.Y. Simple analytical method for determination of microstructures of poly(ethylene-co-vinyl acetate) using the melting points. Polym. Test. 2020, 90, 106706. [Google Scholar] [CrossRef]
- Kuczyńska-Łażewska, A.; Klugmann-Radziemska, E. Influence of Fragment Size on the Time and Temperature of Ethylene Vinyl Acetate Lamination Decomposition in the Photovoltaic Module Recycling Process. Materials 2019, 12, 2857. [Google Scholar] [CrossRef]
- Arsac, A.; Carrot, C.; Guillet, J. Determination of Primary Relaxation Temperatures and Melting Points of Ethylene Vinyl Acetate Copolymers. J. Therm. Anal. Calorim. 2000, 61, 681–685. [Google Scholar] [CrossRef]
- Sabzi, M.; Jiang, L.; Atai, M.; Ghasemi, I. PLA/sepiolite and PLA/calcium carbonate nanocomposites: A comparison study. J. Appl. Polym. Sci. 2012, 129, 1734–1744. [Google Scholar] [CrossRef]
- Lasmi, S.; Zoukrami, F.; Fernández, A.M. Investigation on the physicochemical properties of poly (ethylene-co-vinyl acetate)/mesoporous silica nanocomposites: Effects of content and surface functionalization. J. Thermoplast. Compos. Mater. 2024, 37, 3185–3211. [Google Scholar] [CrossRef]
- Cubillas, P.; Anderson, M.W. Synthesis Mechanism: Crystal Growth and Nucleation. In Zeolites and Catalysis; Čejka, J., Corma, A., Zones, S., Eds.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Khonakdar, H.A. Dynamic mechanical analysis and thermal properties of LLDPE/EVA/modified silica nanocomposites. Compos. Part B Eng. 2015, 76, 343–353. [Google Scholar] [CrossRef]
- Zhou, K.; Tang, G.; Jiang, S.; Gui, Z.; Hu, Y. Combination effect of MoS2 with aluminum hypophosphite in flame retardant ethylene-vinyl acetate composites. RSC Adv. 2016, 6, 37672–37680. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Paszkiewicz, S.; Jędrzejewski, R.; Piesowicz, E.; Siemiński, J. Ethylene vinyl acetate copolymer/halloysite nanotubes nanocomposites with enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 49135. [Google Scholar] [CrossRef]
- Kutnar, A.; Widmann, R.; Brémaud, I. Preliminary studies for use of dynamic mechanical analysis (DMA) to verify intensity of thermal wood modifications. Int. Wood Prod. J. 2013, 4, 158–165. [Google Scholar] [CrossRef]
- Stark, W.; Jaunich, M. Investigation of Ethylene/Vinyl Acetate Copolymer (EVA) by thermal analysis DSC and DMA. Polym. Test. 2011, 30, 236–242. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Q. The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and Its Covalently Crosslinked Analogues (cPEVA). Polymers 2019, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Qu, B.; Wu, Q. Photoinitiated crosslinking of ethylene-vinyl acetate copolymers and characterization of related properties. Polym. Eng. Sci. 2007, 47, 1761–1767. [Google Scholar] [CrossRef]
- Osman, A.F.; Martin, D. In Vitro Tensile Properties, Hydrolytic and Oxidative Permeability of the Biomedical Thermoplastic Polyurethane (TPU)/Organohectorite Nanocomposites. Biomed. J. Sci. Tech. Res. 2018, 11, 4. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Chiu, H.-L.; Liao, Y.-C.; Pan, G.-T.; Chong, S. Hybrid nanocomposite film with enhanced moisture barrier properties. J. Taiwan Inst. Chem. Eng. 2018, 83, 168–173. [Google Scholar] [CrossRef]
- Murugesan, S.; Scheibel, T. Copolymer/Clay Nanocomposites for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1908101. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Beatrice, C.A.G. Polymer Nanocomposites with Different Types of Nanofiller. In Nanocomposites—Recent Evolutions; IntechOpen: London, UK, 2019; pp. 104–127. [Google Scholar]
- Santagiuliana, G.; Picot, O.T.; Crespo, M.; Porwal, H.; Zhang, H.; Li, Y.; Rubini, L.; Colonna, S.; Fina, A.; Barbieri, E.; et al. Breaking the Nanoparticle Loading–Dispersion Dichotomy in Polymer Nanocomposites with the Art of Croissant-Making. ACS Nano 2018, 12, 9040–9050. [Google Scholar] [CrossRef]
- Osman, A.F.; Alakrach, A.M.; Kalo, H.; Azmi, W.N.W.; Hashim, F. In vitro biostability and biocompatibility of ethyl vinyl acetate (EVA) nanocomposites for biomedical applications. RSC Adv. 2015, 5, 31485–31495. [Google Scholar] [CrossRef]
- Adik, N.N.A.N.; Ong, H.L.; Akil, H.M.; Sandu, A.V.; Villagracia, A.R.; Santos, G.N. Effect of Stearic Acid on Tensile, Morphological and Thermal Analysis of Polypropylene (PP)/Dolomite (Dol) Composites. Mater. Plast. 2015, 53, 61–64. [Google Scholar]
- Guzzo, P.L.; Tino, A.A.A.; Santos, J.B. The onset of particle agglomeration during the dry ultrafine grinding of limestone in a planetary ball mill. Powder Technol. 2015, 284, 122–129. [Google Scholar] [CrossRef]
- Bessa, L.P.; Terra, N.M.; Cardoso, V.L.; Reis, M.H.M. Macro-porous dolomite hollow fibers sintered at different temperatures toward widened applications. Cem. Concr. Compos. 2017, 43, 2–16. [Google Scholar] [CrossRef]
- Cepuritis, R.; Wigum, B.; Garboczi, E.; Mørtsell, E.; Jacobsen, S. Filler from crushed aggregate for concrete: Pore structure, specific surface, particle shape and size distribution. Cem. Concr. Compos. 2014, 54, 2–16. [Google Scholar] [CrossRef]
- Díez, E.; Rodríguez, A.; Gómez, J.; Galán, J. TG and DSC as tools to analyse the thermal behaviour of EVA copolymers. J. Elastomers Plast. 2021, 53, 792–805. [Google Scholar] [CrossRef]







| Samples | Average Angle (°) |
|---|---|
| RD | 53.36 ± 0.15 |
| P-DMP | 55.12 ± 0.45 |
| P-DNPs | 57.07 ± 0.25 |
| NP-DMPs | 136.46 ± 0.32 |
| NP-DNPs | 140.18 ± 1.72 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Tensile Toughness (MPa) | Modulus of Elasticity (MPa) |
|---|---|---|---|---|
| PEVAc | 7.14 ± 0.43 | 1070.93 ± 31.12 | 51.63 ± 2.43 | 1.17 ± 0.058 |
| PEVAc/RD | 7.12 ± 0.20 | 1098.53 ± 7.74 | 46.37 ± 2.13 | 1.03 ± 0.058 |
| PEVAc/DF-DMPs | 9.96 ± 0.21 | 1168.90 ± 5.25 | 58.40 ± 0.82 | 0.87 ± 0.06 |
| PEVAc/DF-DNPs | 10.48 ± 0.63 | 1175.73 ± 49.54 | 62.12 ± 0.83 | 0.83 ± 0.06 |
| Samples and Wavenumber (cm−1) | Bands | ||
|---|---|---|---|
| PEVAc | PEVAc/RD | PEVAc/DF-DNPs | |
| 2917.08 | 2917.09 | 2916.99 | Asymmetric vibration of aliphatic (-CH2-)n |
| 2849.94 | 2849.97 | 2849.82 | Symmetric vibration of aliphatic (-CH2-)n |
| 1735.75 | 1736.16 | 1735.81 | -CO carbonyl stretching vibration of esters |
| 1462.80 | 1462.81 | 1462.52 | (-CH2-)n deformation |
| - | 1434.26 | 1434.44 | Asymmetric stretching vibration of the (CO3)2− group. |
| 1369.68 | 1369.73 | 1369.61 | (-CH2-)n wagging |
| 1237.44 | 1237.48 | 1237.28 | The asymmetric vibration of C-O-C bond |
| 1019.96 | 1020.07 | 1019.80 | C-O-C in plane stretching |
| - | 879.79 | 880.35 | Asymmetric bending vibration mode of the O-C-O bond in the (CO3)2− |
| 721.42 | 721.44 | 721.38 | CH bending (out-of-plane) |
| Samples | Crystallinity | Amorphous | Peak (2ϴ) | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| PEVAc | 17.82 | 82.18 | 21.36 | 23.51 | - |
| PEVAc/RD | 26.06 | 73.94 | 21.03 | 23.43 | 30.80 |
| PEVAc/DF-DMPs | 17.96 | 82.04 | 21.35 | 23.60 | 31.03 |
| PEVAc/DF-DNPs | 18.07 | 81.93 | 21.38 | 23.76 | 31.17 |
| Samples | Tdmax 1 (°C) | Weight Loss (%) | Tdmax 2 (°C) | Weight Loss (%) |
|---|---|---|---|---|
| PEVAc (IM) | 345.90 | 19.08 | 465.76 | 77.6 |
| PEVAc/RD | 344.27 | 18.84 | 463.26 | 79.2 |
| PEVAc/DF-DMPs | 347.11 | 15.20 | 465.32 | 80.65 |
| PEVAc/DF-DNPs | 346.36 | 17.31 | 463.89 | 79.16 |
| Samples | TVAc (°C) | TmEthyl (°C) | ΔHmEthyl (J/g) | Xc (%) | TcEthyl (°C) | ΔHcEthyl (J/g) |
|---|---|---|---|---|---|---|
| PEVAc | 51.07 | 79.84 | 8.23 | 2.98 | 56.96 | 17.51 |
| PEVAc/RD | 51.97 | 79.85 | 9.88 | 3.56 | 52.19 | 23.61 |
| PEVAc/DF-DMPs | 51.57 | 80.29 | 6.43 | 2.32 | 53.89 | 13.75 |
| PEVAc/DF-DNPs | 53.93 | 80.58 | 7.92 | 2.86 | 52.12 | 25.15 |
| Samples | Tg1 (°C) (Tan ẟ Max) | Tg2 (°C) (Tan ẟ 2) |
|---|---|---|
| PEVAc | −13.52 | 18.77 |
| PEVAc/DF-DNPs | −13.45 | 17.43 |
| Weeks | Increment of Weight in Samples Upon Hydrolytic Agent (PBS Solution) (%) | |||
|---|---|---|---|---|
| PEVAc | PEVAc/RD | PEVAc/DF-DMPs | PEVAc/DF-DNPs | |
| 0 | 0 | 0 | 0 | 0 |
| 2 | 1.22 | 1.43 | 0.97 | 0.48 |
| 4 | 2.20 | 2.39 | 1.70 | 0.72 |
| 6 | 3.66 | 2.63 | 2.68 | 1.20 |
| 8 | 4.63 | 3.34 | 3.65 | 2.15 |
| 10 | 4.87 | 4.53 | 4.38 | 2.39 |
| 12 | 6.10 | 5.01 | 4.62 | 2.63 |
| Sample | Condition | Tensile Strength (MPa) | Elongation at Break (%) | Tensile Toughness (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| PEVAc | A | 7.14 ± 0.43 | 1075.73 ± 22.60 | 51.63 ± 2.42 | 1.17 ± 0.06 |
| I | 6.83 ± 0.10 | 1061.2 ± 10.81 | 48.54 ± 4.53 | 0.77 ± 0.06 | |
| Reduction (%) | 4.34 | 1.35 | 6.00 | 34.18 | |
| PEVAc/RD | A | 7.12 ± 0.20 | 1025.97 ± 1.40 | 46.38 ± 2.13 | 1.03 ± 0.058 |
| I | 6.76 ± 0.05 | 1021.63 ± 2.58 | 40.04 ± 1.78 | 0.73 ± 0.06 | |
| Reduction (%) | 5.06 | 0.42 | 13.67 | 29.13 | |
| PEVAc/DF-DMP | A | 9.96 ± 0.21 | 1168.90 ± 5.25 | 58.40 ± 0.82 | 0.87 ± 0.06 |
| I | 9.27 ± 0.12 | 1134.23 ± 26.45 | 56.26 ± 1.07 | 0.83 ± 0.06 | |
| Reduction (%) | 6.93 | 2.97 | 3.66 | 4.60 | |
| PEVAc/DF-DNP | A | 10.48 ± 0.63 | 1175.73 ± 49.54 | 62.12 ± 0.83 | 0.83 ± 0.06 |
| I | 10.16 ± 0.16 | 1174.10 ± 5.23 | 59.37 ± 2.15 | 0.80 ± 0.0000135 | |
| Reduction (%) | 3.05 | 0.13 | 4.43 | 3.61 |
| Type of Sample | Matrix (PEVAc) (wt %) | Filler (Dolomite) (wt%) | Acronym | ||
|---|---|---|---|---|---|
| PEVAc | 100 | - | |||
| Type of Filler | Weight (wt%) | PEVAc | |||
| PEVAc/RD | 97 | RD | 3% | PEVAc/RD | |
| PEVAc/(DF-DMPs) (P-DMPs + NP-DMPs) | 97 | DF-DMPs (P-DMPs + NP-DMPs) (P-DMPs:NP-DMPs = 1:3) | 3% | PEVAc/DF-DMPs | |
| P-DMPs | 0.75 | ||||
| NP-DMPs | 2.25 | ||||
| PEVAc/(DF-DNPs) (P-DNPs + NP-DNPs) | 97 | DF-DNPs (P-DNPs + NP-DNPs) (P-DNPs:NP-DNPs = 1:3) | 3% | PEVAc/DF-DNPs | |
| P-DNPs | 0.75 | ||||
| NP-DNPs | 2.25 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Fauzi, A.A.; Osman, A.F.; Alosime, E.M.; Abdul Halim, K.A.; Abdullah, M.A.A. Structure and Properties of Poly(Ethylene-co-vinyl Acetate) Nanocomposites with Dual-Functionalized Dolomite Nanoparticles. Int. J. Mol. Sci. 2024, 25, 12519. https://doi.org/10.3390/ijms252312519
Ahmad Fauzi AA, Osman AF, Alosime EM, Abdul Halim KA, Abdullah MAA. Structure and Properties of Poly(Ethylene-co-vinyl Acetate) Nanocomposites with Dual-Functionalized Dolomite Nanoparticles. International Journal of Molecular Sciences. 2024; 25(23):12519. https://doi.org/10.3390/ijms252312519
Chicago/Turabian StyleAhmad Fauzi, Asfa Amalia, Azlin Fazlina Osman, Eid M. Alosime, Khairul Anwar Abdul Halim, and Mohd Aidil Adhha Abdullah. 2024. "Structure and Properties of Poly(Ethylene-co-vinyl Acetate) Nanocomposites with Dual-Functionalized Dolomite Nanoparticles" International Journal of Molecular Sciences 25, no. 23: 12519. https://doi.org/10.3390/ijms252312519
APA StyleAhmad Fauzi, A. A., Osman, A. F., Alosime, E. M., Abdul Halim, K. A., & Abdullah, M. A. A. (2024). Structure and Properties of Poly(Ethylene-co-vinyl Acetate) Nanocomposites with Dual-Functionalized Dolomite Nanoparticles. International Journal of Molecular Sciences, 25(23), 12519. https://doi.org/10.3390/ijms252312519







