Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation
Abstract
1. Introduction
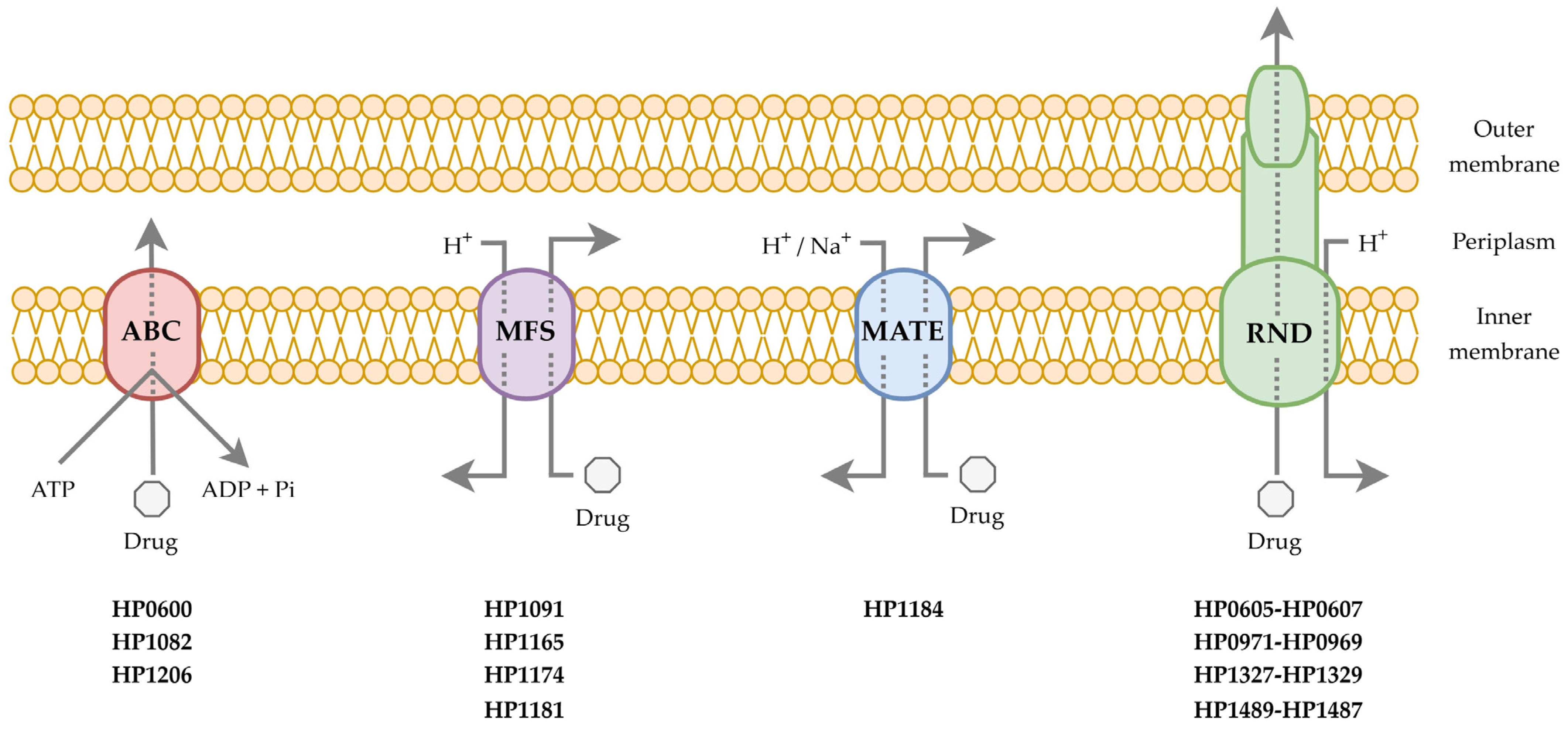
2. Characteristics of Efflux Pumps
3. Involvement of Efflux Pumps in Resistance to Antimicrobial Compounds
3.1. The RND Superfamily
3.1.1. HefABC (HP0605-HP0607)
3.1.2. HefDEF (HP0971-HP0969)
3.1.3. HefGHI (HP1327-HP1329)
3.1.4. HP1489-HP1487
3.2. The MFS Superfamily
3.2.1. HP1174 (GluP)
3.2.2. HP1165 (TetA)
3.2.3. HP1181
3.3. The ABC Superfamily
3.3.1. HP1082 (MsbA)
3.3.2. HP0600 (SpaB)
3.4. The MATE Superfamily
| Superfamily | Efflux Pump | Substrates | Association with MDR | Additional Biological Activities | References |
|---|---|---|---|---|---|
| RND | HP0605-HP0607 (HefABC) | penicillin G, piperacillin, amoxicillin, ceftriaxone, cefotaxime, gentamycin, tetracycline, erythromycin, clarithromycin, clindamycin, novobiocin, metronidazole, bile salts, ceragenins, ethidium bromide | + | Transport of cholesterol molecules and cholesterol-dependent changes in the cell membrane fluidity | [33,34,35,36,37,38,39,40,46] |
| HP0971-HP0969 (HefDEF) | heavy metal ions (Cd2+, Zn2+, Ni2+), clarithromycin, metronidazole, levofloxacin | + | Modulation of urease activity and a role in the gastric colonization | [33,34,38,40,41,47,48,49] | |
| HP1327-HP1329 (HefGHI) | heavy metal ions (Cu2+), levofloxacin | ND | ND | [33,34,49,50] | |
| HP1489-HP1487 | ethidium bromide | ND | Stabilization of the flagellar motor and a role in the motility | [33,34,40,51] | |
| MFS | HP1091 (KgtP) | ND | ND | ND | [33,34] |
| HP1165 (TetA) | tetracycline | ND | ND | [33,34,55] | |
| HP1174 (GluP) | amoxicillin, ampicillin, piperacillin, tetracycline, metronidazole, furazolidone | + | Transport of simple sugars and a role in the maintenance of the energy balance | [34,52,53,54] | |
| HP1181 | amoxicillin, ampicillin, tetracycline, metronidazole, ciprofloxacin, ethidium bromide, levofloxacin | ND | ND | [33,34,56] | |
| ABC | HP0600 (SpaB) | clarithromycin, chloramphenicol, rifampicin, ofloxacin, levofloxacin, metronidazole | + | ND | [33,34,59] |
| HP1082 (MsbA) | clarithromycin, erythromycin, chloramphenicol, rifampicin, ofloxacin, novobiocin, glutaraldehyde | + | Transport and biogenesis of lipopolysaccharide | [33,34,58,59] | |
| HP1206 (HetA) | ND | ND | ND | [33,34] | |
| MATE | HP1184 | amoxicillin, ampicillin, tetracycline, metronidazole, ciprofloxacin, ethidium bromide | ND | ND | [34,40,56] |
4. Involvement of Metabolic Transporters in Resistance to Antimicrobial Compounds
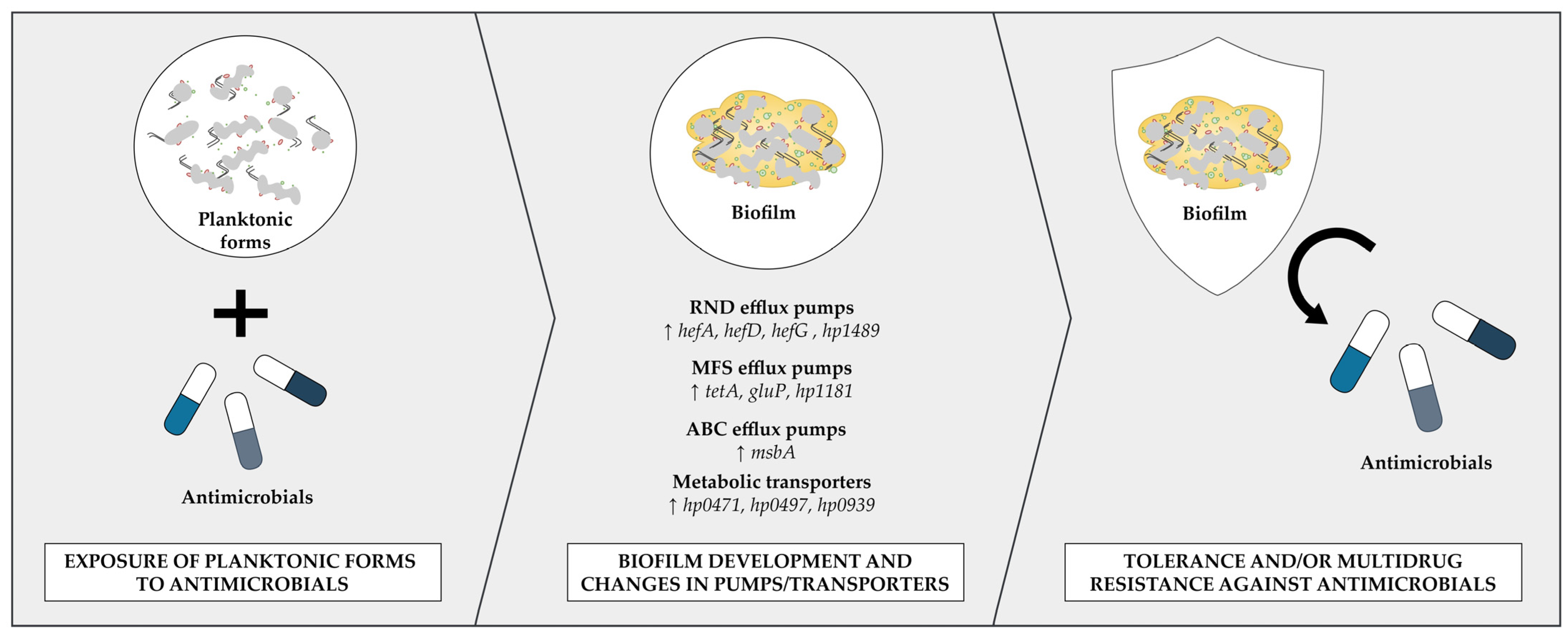
5. Participation of Efflux Pumps in Biofilm Formation
6. Inhibition of Efflux Pumps as a New Therapeutic Path
7. Conclusions
Funding
Conflicts of Interest
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yuan, C.; Zhou, S.; Lu, J.; Zeng, M.; Cai, X.; Song, H. Helicobacter pylori Infection: A Dynamic Process from Diagnosis to Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1257817. [Google Scholar] [CrossRef] [PubMed]
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular Basis for Colonization and Survival in Gastric Environment and Resistance to Antibiotics. A Short Review. Infect. Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, M.; Rothenbacher, D.; Brenner, H. Acquisition of Helicobacter pylori Infection in Early Childhood: Independent Contributions of Infected Mothers, Fathers, and Siblings. Am. J. Gastroenterol. 2009, 104, 182–189. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bang, C.S.; Gong, E.J. Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications. J. Korean Med. Sci. 2024, 39, e44. [Google Scholar] [CrossRef]
- Liang, B.; Yuan, Y.; Peng, X.J.; Liu, X.L.; Hu, X.K.; Xing, D.M. Current and Future Perspectives for Helicobacter pylori Treatment and Management: From Antibiotics to Probiotics. Front. Cell. Infect. Microbiol. 2022, 12, 1740. [Google Scholar] [CrossRef]
- Graham, D.Y. Transitioning of Helicobacter pylori Therapy from Trial and Error to Antimicrobial Stewardship. Antibiotics 2020, 9, 671. [Google Scholar] [CrossRef]
- Graham, D.Y.; Liou, J.M. Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship. Clin. Gastroenterol. Hepatol. 2021, 20, 973–983. [Google Scholar] [CrossRef]
- Brandstaeter, S.; Fuchs, S.L.; Aydin, R.C.; Cyron, C.J. Mechanics of the Stomach: A Review of an Emerging Field of Biomechanics. GAMM-Mitteilungen 2019, 42, e201900001. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shao, Y.; Yan, J.; Ye, G. Antibiotic Resistance in Helicobacter pylori: From Potential Biomolecular Mechanisms to Clinical Practice. J. Clin. Lab. Anal. 2023, 37, e24885. [Google Scholar] [CrossRef] [PubMed]
- Vita, N.A.; Anderson, S.M.; LaFleur, M.D.; Lee, R.E. Targeting Helicobacter pylori for Antibacterial Drug Discovery with Novel Therapeutics. Curr. Opin. Microbiol. 2022, 70, 102203. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef]
- Moss, S.F.; Shah, S.C.; Tan, M.C.; El-Serag, H.B. Evolving Concepts in Helicobacter pylori Management. Gastroenterology 2024, 166, 267–283. [Google Scholar] [CrossRef]
- Gisbert, J.P. Empirical or Susceptibility-Guided Treatment for Helicobacter pylori Infection? A Comprehensive Review. Therap. Adv. Gastroenterol. 2020, 13, 1756284820968736. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 Years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Dascălu, R.I.; Bolocan, A.; Păduaru, D.N.; Constantinescu, A.; Mitache, M.M.; Stoica, A.D.; Andronic, O. Multidrug Resistance in Helicobacter pylori Infection. Front. Microbiol. 2023, 14, 1128497. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Kandilarov, N.; Markovska, R.; Mitov, I. Multidrug Resistance in Helicobacter pylori: Current State and Future Directions. Expert Rev. Clin. Pharmacol. 2019, 12, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Elshenawi, Y.; Hu, S.; Hathroubi, S. Biofilm of Helicobacter pylori: Life Cycle, Features, and Treatment Options. Antibiotics 2023, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Grande, R.; Migdał, P.; Paluch, E.; Gościniak, G. Biofilm Formation as a Complex Result of Virulence and Adaptive Responses of Helicobacter pylori. Pathogens 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, K.A.; Effendi, W.I.; Alfaray, R.I.; Malaty, H.M.; Yamaoka, Y.; Mifthussurur, M. Molecular Mechanisms of Biofilm Formation in Helicobacter pylori. Antibiotics 2024, 13, 976. [Google Scholar] [CrossRef]
- Krzyżek, P.; Grande, R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens 2020, 9, 184. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef]
- Thakur, V.; Uniyal, A.; Tiwari, V. A Comprehensive Review on Pharmacology of Efflux Pumps and Their Inhibitors in Antibiotic Resistance. Eur. J. Pharmacol. 2021, 903, 174151. [Google Scholar] [CrossRef]
- Kumawat, M.; Nabi, B.; Daswani, M.; Viquar, I.; Pal, N.; Sharma, P.; Tiwari, S.; Sarma, D.K.; Shubham, S.; Kumar, M. Role of Bacterial Efflux Pump Proteins in Antibiotic Resistance across Microbial Species. Microb. Pathog. 2023, 181, 106182. [Google Scholar] [CrossRef]
- Henderson, P.J.F.; Maher, C.; Elbourne, L.D.H.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological Functions of Bacterial “Multidrug” Efflux Pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Yang, F.; Chi, W.; Ding, L.; Liu, T.; Zhu, F.; Ji, D.; Zhou, J.; Fang, Y.; et al. Antimicrobial Resistance Patterns and Genetic Elements Associated with the Antibiotic Resistance of Helicobacter pylori Strains from Shanghai. Gut Pathog. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Alfaray, R.I.; Saruuljavkhlan, B.; Fauzia, K.A.; Torres, R.C.; Thorell, K.; Dewi, S.R.; Kryukov, K.A.; Matsumoto, T.; Akada, J.; Vilaichone, R.K.; et al. Global Antimicrobial Resistance Gene Study of Helicobacter pylori: Comparison of Detection Tools, ARG and Efflux Pump Gene Analysis, Worldwide Epidemiological Distribution, and Information Related to the Antimicrobial-Resistant Phenotype. Antibiotics 2023, 12, 1118. [Google Scholar] [CrossRef]
- Hashemi, S.J.; Sheikh, A.F.; Goodarzi, H.; Yadyad, M.J.; Seyedian, S.S.; Aslani, S.; Assarzadegan, M.A. Genetic Basis for Metronidazole and Clarithromycin Resistance in Helicobacter pylori Strains Isolated from Patients with Gastroduodenal Disorders. Infect. Drug Resist. 2019, 12, 535. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zheng, P.Y.; Yang, P.C. Efflux Pump Gene hefA of Helicobacter pylori Plays an Important Role in Multidrug Resistance. World J. Gastroenterol. 2008, 14, 5222. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.Q.; Zheng, P.Y.; Tang, F.A.; Yang, P.C. Influence of Efflux Pump Inhibitors on the Multidrug Resistance of Helicobacter pylori. World J. Gastroenterol. 2010, 16, 1279–1284. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; An, Y.; Li, Z.; Liu, D.; Yong, X. The Crosstalk between Efflux Pump and Resistance Gene Mutation in Helicobacter pylori. Gut Microbes 2024, 16, 2379439. [Google Scholar] [CrossRef]
- Kutschke, A.; De Jonge, B.L.M. Compound Efflux in Helicobacter pylori. Antimicrob. Agents Chemother. 2005, 49, 3009–3010. [Google Scholar] [CrossRef]
- van Amsterdam, K.; Bart, A.; van der Ende, A. A Helicobacter pylori TolC Efflux Pump Confers Resistance to Metronidazole. Antimicrob. Agents Chemother. 2005, 49, 1477–1482. [Google Scholar] [CrossRef]
- Mehrabadi, J.F.; Sirous, M.; Daryani, N.E.; Eshraghi, S.; Akbari, B.; Shirazi, M.H. Assessing the Role of the RND Efflux Pump in Metronidazole Resistance of Helicobacter pylori by RT-PCR Assay. J. Infect. Dev. Ctries. 2011, 5, 88–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsugawa, H.; Suzuki, H.; Muraoka, H.; Ikeda, F.; Hirata, K.; Matsuzaki, J.; Saito, Y.; Hibi, T. Enhanced Bacterial Efflux System Is the First Step to the Development of Metronidazole Resistance in Helicobacter pylori. Biochem. Biophys. Res. Commun. 2011, 404, 660. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, N.; Kwon, Y.H.; Nam, R.H.; Kim, J.M.; Park, J.Y.; Lee, Y.S.; Lee, D.H. RdxA, FrxA, and Efflux Pump in Metronidazole-Resistant Helicobacter pylori: Their Relation to Clinical Outcomes. J. Gastroenterol. Hepatol. 2018, 33, 681–688. [Google Scholar] [CrossRef]
- Iwamoto, A.; Tanahashi, T.; Okada, R.; Yoshida, Y.; Kikuchi, K.; Keida, Y.; Murakami, Y.; Yang, L.; Yamamoto, K.; Nishiumi, S.; et al. Whole-Genome Sequencing of Clarithromycin Resistant Helicobacter pylori Characterizes Unidentified Variants of Multidrug Resistant Efflux Pump Genes. Gut Pathog. 2014, 6, 27. [Google Scholar] [CrossRef]
- Qureshi, N.N.; Gallaher, B.; Schiller, N.L. Evolution of Amoxicillin Resistance of Helicobacter pylori In Vitro: Characterization of Resistance Mechanisms. Microb. Drug Resist. 2014, 20, 509–516. [Google Scholar] [CrossRef]
- Trainor, E.A.; Horton, K.E.; Savage, P.B.; Testerman, T.L.; McGee, D.J. Role of the HefC Efflux Pump in Helicobacter pylori Cholesterol-Dependent Resistance to Ceragenins and Bile Salts. Infect. Immun. 2011, 79, 88–97. [Google Scholar] [CrossRef]
- Stähler, F.N.; Odenbreit, S.; Haas, R.; Wilrich, J.; Van Vliet, A.H.M.; Kusters, J.G.; Kist, M.; Bereswill, S. The Novel Helicobacter pylori CznABC Metal Efflux Pump Is Required for Cadmium, Zinc, and Nickel Resistance, Urease Modulation, and Gastric Colonization. Infect. Immun. 2006, 74, 3852. [Google Scholar] [CrossRef]
- Chen, J.; Ye, L.; Jin, L.; Xu, X.; Xu, P.; Wang, X.; Li, H. Application of Next-Generation Sequencing to Characterize Novel Mutations in Clarithromycin-Susceptible Helicobacter pylori Strains with A2143G of 23S rRNA Gene. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 10. [Google Scholar] [CrossRef]
- Ye, L.; Meng, F.; Mao, X.; Zhang, Y.; Wang, J.; Liu, Y.; Zhu, W.; Gu, B.; Huang, Q. Using Next-Generation Sequencing to Analyze Helicobacter pylori Clones with Different Levofloxacin Resistances from a Patient with Eradication Failure. Medicine 2020, 99, e20761. [Google Scholar] [CrossRef]
- Waidner, B.; Melchers, K.; Ivanov, I.; Loferer, H.; Bensch, K.W.; Kist, M.; Bereswill, S. Identification by RNA Profiling and Mutational Analysis of the Novel Copper Resistance Determinants CrdA (HP1326), CrdB (HP1327), and CzcB (HP1328) in Helicobacter pylori. J. Bacteriol. 2002, 184, 6708. [Google Scholar] [CrossRef]
- Gibson, K.; Chu, J.K.; Zhu, S.; Nguyen, D.; Mrázek, J.; Liu, J.; Hoover, T.R. A Tripartite Efflux System Affects Flagellum Stability in Helicobacter pylori. Int. J. Mol. Sci. 2022, 23, 11609. [Google Scholar] [CrossRef] [PubMed]
- Psakis, G.; Saidijam, M.; Shibayama, K.; Polaczek, J.; Bettaney, K.E.; Baldwin, J.M.; Baldwin, S.A.; Hope, R.; Essen, L.O.; Essenberg, R.C.; et al. The Sodium-Dependent D-Glucose Transport Protein of Helicobacter pylori. Mol. Microbiol. 2009, 71, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cai, Y.; Chen, Z.; Gao, S.; Geng, X.; Li, Y.; Li, Y.; Jia, J.; Sun, Y. Bifunctional Enzyme SpoT Is Involved in Biofilm Formation of Helicobacter pylori with Multidrug Resistance by Upregulating Efflux Pump Hp1174 (GluP). Antimicrob. Agents Chemother. 2018, 62, e00957-18. [Google Scholar] [CrossRef] [PubMed]
- Noszka, M.; Strzałka, A.; Muraszko, J.; Kolenda, R.; Meng, C.; Ludwig, C.; Stingl, K.; Zawilak-Pawlik, A. Profiling of the Helicobacter pylori Redox Switch HP1021 Regulon Using a Multi-Omics Approach. Nat. Commun. 2023, 14, 6714. [Google Scholar] [CrossRef]
- Li, Y.; Dannelly, H.K. Inactivation of the Putative Tetracycline Resistance Gene hp1165 in Helicobacter pylori Led to Loss of Inducible Tetracycline Resistance. Arch. Microbiol. 2006, 185, 255–262. [Google Scholar] [CrossRef]
- Falsafi, T.; Ehsani, A.; Attaran, B.; Niknam, V. Association of hp1181 and hp1184 Genes with the Active Efflux Phenotype in Multidrug-Resistant Isolates of Helicobacter pylori. Jundishapur J. Microbiol. 2016, 9, e30726. [Google Scholar] [CrossRef]
- Reyes, C.L.; Ward, A.; Yu, J.; Chang, G. The Structures of MsbA: Insight into ABC Transporter-Mediated Multidrug Efflux. FEBS Lett. 2006, 580, 1042–1048. [Google Scholar] [CrossRef]
- Chiu, H.C.; Lin, T.L.; Yang, J.C.; Wang, J.T. Synergistic Effect of Imp/OstA and MsbA in Hydrophobic Drug Resistance of Helicobacter pylori. BMC Microbiol. 2009, 9, 136. [Google Scholar] [CrossRef][Green Version]
- Mi, Y.; Zheng, P.-Y.; Zhang, B.-Y.; Liu, Z.-Q.; Song, C.-H.; Yang, P.-C. Role of ABC Transporter Genes MsbA and SpaB in Multidrug Resistance of Helicobacter pylori. World Chin. J. Dig. 2011, 19, 1500–1505. [Google Scholar]
- McKinlay, J.B. Are Bacteria Leaky? Mechanisms of Metabolite Externalization in Bacterial Cross-Feeding. Annu. Rev. Microbiol. 2023, 77, 277–297. [Google Scholar] [CrossRef]
- Gude, S.; Pherribo, G.J.; Taga, M.E. Emergence of Metabolite Provisioning as a By-Product of Evolved Biological Functions. mSystems 2020, 5, e00259-20. [Google Scholar] [CrossRef]
- Douglas, A.E. The Microbial Exometabolome: Ecological Resource and Architect of Microbial Communities. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2020, 375, 20190250. [Google Scholar] [CrossRef]
- Geng, X.; Li, W.; Chen, Z.; Gao, S.; Hong, W.; Ge, X.; Hou, G.; Hu, Z.; Zhou, Y.; Zeng, B.; et al. The Bifunctional Enzyme SpoT Is Involved in the Clarithromycin Tolerance of Helicobacter pylori by Upregulating the Transporters HP0939, HP1017, HP0497, and HP0471. Antimicrob. Agents Chemother. 2017, 61, e02011-16. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, C.; Chen, Z.; Xu, Z.; Li, H.; Li, W.; Sun, Y. Transporters HP0939, HP0497, and HP0471 Participate in Intrinsic Multidrug Resistance and Biofilm Formation in Helicobacter pylori by Enhancing Drug Efflux. Helicobacter 2020, 25, e12715. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial Persisters: Molecular Mechanisms and Therapeutic Development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Zhang, H.; Jia, Y.; Wang, Z. Combating Antibiotic Tolerance Through Activating Bacterial Metabolism. Front. Microbiol. 2020, 11, 577564. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm Formation, and Related Impacts on Healthcare, Food Processing and Packaging, Industrial Manufacturing, Marine Industries, and Sanitation–A Review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID* Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The Biofilm Matrix: Multitasking in a Shared Space. Nat. Rev. Microbiol. 2022, 21, 70–86. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Hojo, F.; Kamiya, S. Effect of Helicobacter pylori Biofilm Formation on Susceptibility to Amoxicillin, Metronidazole and Clarithromycin. Microb. Pathog. 2019, 132, 100–108. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Clarke, A.; Ottemann, K.M. Helicobacter pylori Biofilm Confers Antibiotic Tolerance in Part via a Protein-Dependent Mechanism. Antibiotics 2020, 9, 355. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Tusiewicz, K.; Zawadzki, M.; Szpot, P. Subinhibitory Concentrations of Antibiotics Affect Development and Parameters of Helicobacter pylori Biofilm. Front. Pharmacol. 2024, 15, 1477317. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Miftahussurur, M.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Rezkitha, Y.A.A.; Matsumoto, T.; Tuan, V.P.; Akada, J.; Yonezawa, H.; et al. Biofilm Formation and Antibiotic Resistance Phenotype of Helicobacter pylori Clinical Isolates. Toxins 2020, 12, 473. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Grande, R.; Gościniak, G. Biofilm Formation of Helicobacter pylori in Both Static and Microfluidic Conditions Is Associated With Resistance to Clarithromycin. Front. Cell. Infect. Microbiol. 2022, 12, 868905. [Google Scholar] [CrossRef]
- Wu, X.; Wu, D.; Cui, G.; Lee, K.H.; Yang, T.; Zhang, Z.; Liu, Q.; Zhang, J.; Chua, E.G.; Chen, Z. Association Between Biofilm Formation and Structure and Antibiotic Resistance in H. pylori. Infect. Drug Resist. 2024, 17, 2512. [Google Scholar] [CrossRef]
- Attaran, B.; Falsafi, T.; Ghorbanmehr, N. Effect of Biofilm Formation by Clinical Isolates of Helicobacter pylori on the Efflux-Mediated Resistance to Commonly Used Antibiotics. World J. Gastroenterol. 2017, 23, 1163–1170. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Hanawa, T.; Kurata, S.; Ochiai, K.; Kamiya, S. Impact of Helicobacter pylori Biofilm Formation on Clarithromycin Susceptibility and Generation of Resistance Mutations. PLoS ONE 2013, 8, e73301. [Google Scholar] [CrossRef]
- Ren, J.; Wang, M.; Zhou, W.; Liu, Z. Efflux Pumps as Potential Targets for Biofilm Inhibition. Front. Microbiol. 2024, 15, 1315238. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 2019, 149, 145. [Google Scholar] [CrossRef]
- Compagne, N.; Vieira Da Cruz, A.; Müller, R.T.; Hartkoorn, R.C.; Flipo, M.; Pos, K.M. Update on the Discovery of Efflux Pump Inhibitors against Critical Priority Gram-Negative Bacteria. Antibiotics 2023, 12, 180. [Google Scholar] [CrossRef]
- Hirata, K.; Suzuki, H.; Nishizawa, T.; Tsugawa, H.; Muraoka, H.; Saito, Y.; Matsuzaki, J.; Hibi, T. Contribution of Efflux Pumps to Clarithromycin Resistance in Helicobacter pylori. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. S1), 79. [Google Scholar] [CrossRef]
- Anoushiravani, M.; Falsafi, T.; Niknam, V. Proton Motive Force-Dependent Efflux of Tetracycline in Clinical Isolates of Helicobacter pylori. J. Med. Microbiol. 2009, 58, 1313. [Google Scholar] [CrossRef]
- Falsafi, T.; Ehsani, A.; Niknam, V. The Role of Active Efflux in Antibiotic—Resistance of Clinical Isolates of Helicobacter pylori. Indian J. Med. Microbiol. 2009, 27, 335–340. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, L.; Deng, Q.; Jing, L.; Zhang, J.; Zhang, Y.; Yu, F.; Ou, Y.; Guo, S.; Huang, B.; et al. Unraveling the Novel Effect of Patchouli Alcohol Against the Antibiotic Resistance of Helicobacter pylori. Front. Microbiol. 2021, 12, 674560. [Google Scholar] [CrossRef]
- Jia, X.; Huang, Q.; Lin, M.; Chu, Y.; Shi, Z.; Zhang, X.; Ye, H. Revealing the Novel Effect of Jinghua Weikang Capsule against the Antibiotic Resistance of Helicobacter pylori. Front. Microbiol. 2022, 13, 962354. [Google Scholar] [CrossRef]
- Lin, M.M.; Yang, S.S.; Huang, Q.Y.; Cui, G.H.; Jia, X.F.; Yang, Y.; Shi, Z.M.; Ye, H.; Zhang, X.Z. Effect and Mechanism of Qingre Huashi Decoction on Drug-Resistant Helicobacter pylori. World J. Gastroenterol. 2024, 30, 3086–3105. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Y.; Ye, H.; Wang, X.; Zhang, X.; Yu, J. Transcending Antibiotic Resistance: The Potential of Mass Galla Chinensis et Camelliae Fermentata to Dismantle Helicobacter pylori Biofilms and Enhance Anti-Biotic Activity. J. Ethnopharmacol. 2024, 334, 118594. [Google Scholar] [CrossRef]
- Gadar, K.; McCarthy, R.R. Using Next Generation Antimicrobials to Target the Mechanisms of Infection. Npj Antimicrob. Resist. 2023, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Teelucksingh, T.; Thompson, L.K.; Cox, G. The Evolutionary Conservation of Escherichia coli Drug Efflux Pumps Supports Physiological Functions. J. Bacteriol. 2020, 202, e00367-20. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | Antibiotic Class | Molecular Target | Typical Mechanism of Resistance |
|---|---|---|---|
| Amoxicillin | Beta-lactams | Cell wall synthesis | Mutations in pbp1a, pbp2 or pbp3 |
| Clarithromycin | Macrolides | Protein synthesis | Mutations in 23S rRNA |
| Tetracycline | Tetracyclines | Protein synthesis | Mutations in 16S rRNA |
| Rifabutin | Ansamycins | DNA transcription | Mutations in rpoB |
| Levofloxacin | Fluoroquinolones | DNA replication | Mutations in gyrA or gyrB |
| Metronidazole | Nitroimidazoles | DNA structure | Inactivation of rdxA and/or frxA |
| Metabolic Transporter | Metabolism-Related Substrates | Antibiotic Substrates | Association with MDR | References |
|---|---|---|---|---|
| HP0471 (KefB) | K+ ions | penicillin G, ampicillin, amoxicillin, tetracycline, clarithromycin, ciprofloxacin, metronidazole, furazolidone | + | [63,64] |
| HP0497 | Na+ and Cl− ions | |||
| HP0939 (YckJ) | Amino acids | |||
| HP1017 (RocE) | Amino acids | clarithromycin | ND | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżek, P. Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. Int. J. Mol. Sci. 2024, 25, 12222. https://doi.org/10.3390/ijms252212222
Krzyżek P. Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. International Journal of Molecular Sciences. 2024; 25(22):12222. https://doi.org/10.3390/ijms252212222
Chicago/Turabian StyleKrzyżek, Paweł. 2024. "Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation" International Journal of Molecular Sciences 25, no. 22: 12222. https://doi.org/10.3390/ijms252212222
APA StyleKrzyżek, P. (2024). Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. International Journal of Molecular Sciences, 25(22), 12222. https://doi.org/10.3390/ijms252212222






