The Emerging Role of IGF2BP2 in Cancer Therapy Resistance: From Molecular Mechanism to Future Potential
Abstract
1. Introduction
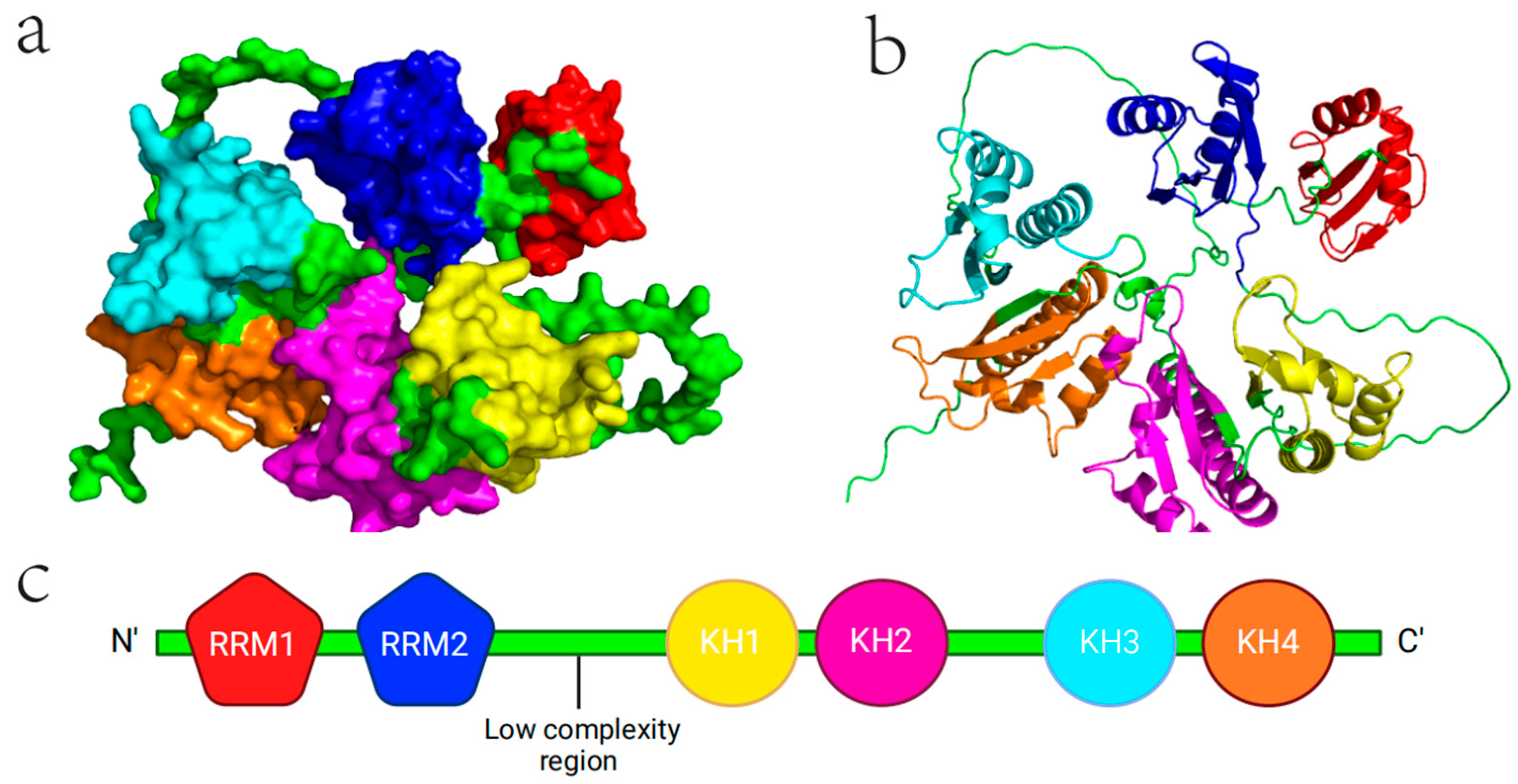
2. Structure and Physiological Function of IGF2BP2
2.1. Molecular Structure of IGF2BP2
2.2. Physiological Role of IGF2BP2
3. Pathological Function of IGF2BP2 in Tumors
3.1. IGF2BP2 Expression in Pan-Cancer
3.2. IGF2BP2 Function in Tumor RNA
3.3. Role of IGF2BP2 in Tumor Development
4. Impact of IGF2BP2 on the Cancer Treatment
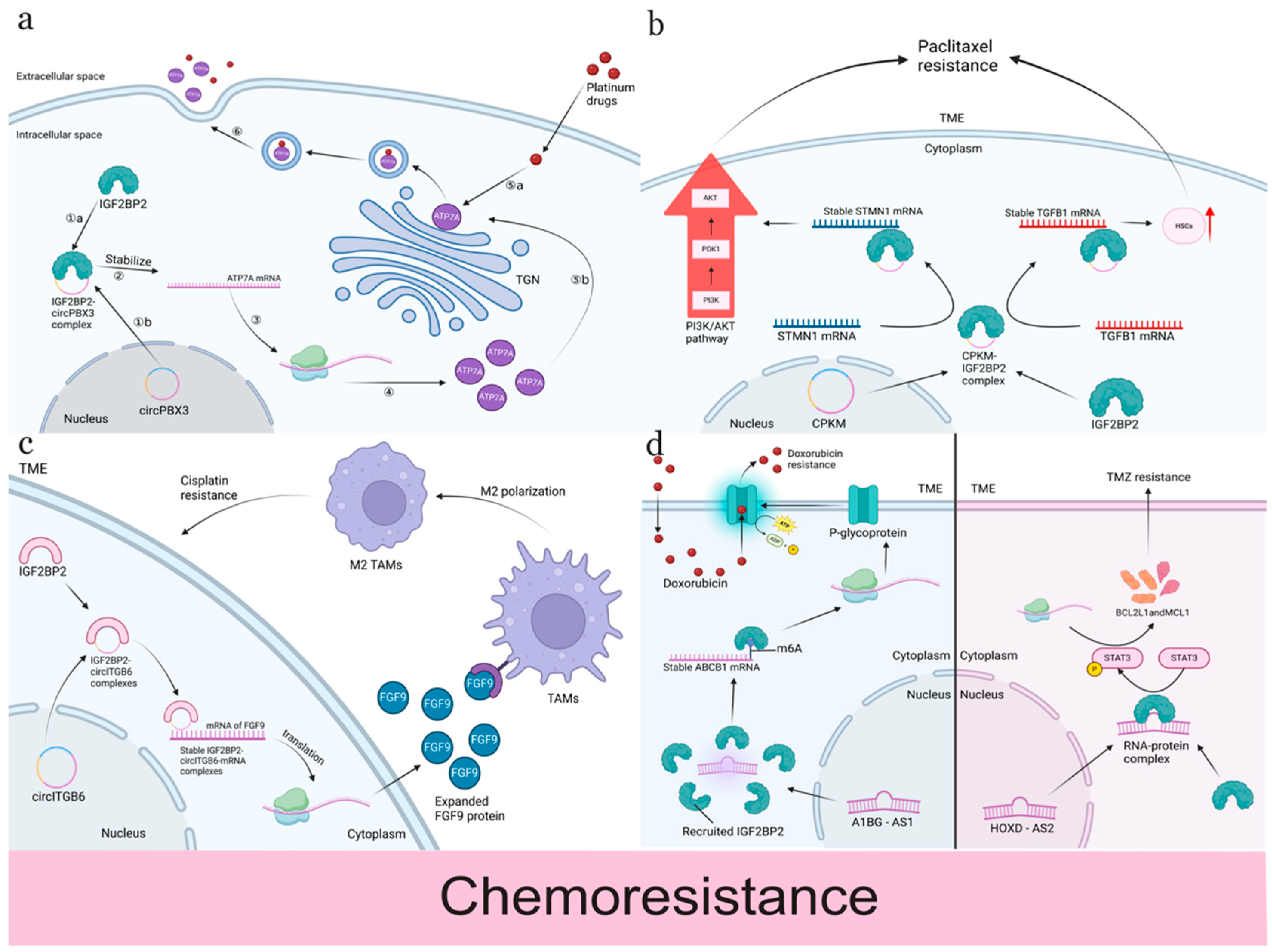
4.1. Chemotherapy Resistance
4.2. Targeted Chemotherapy Resistance
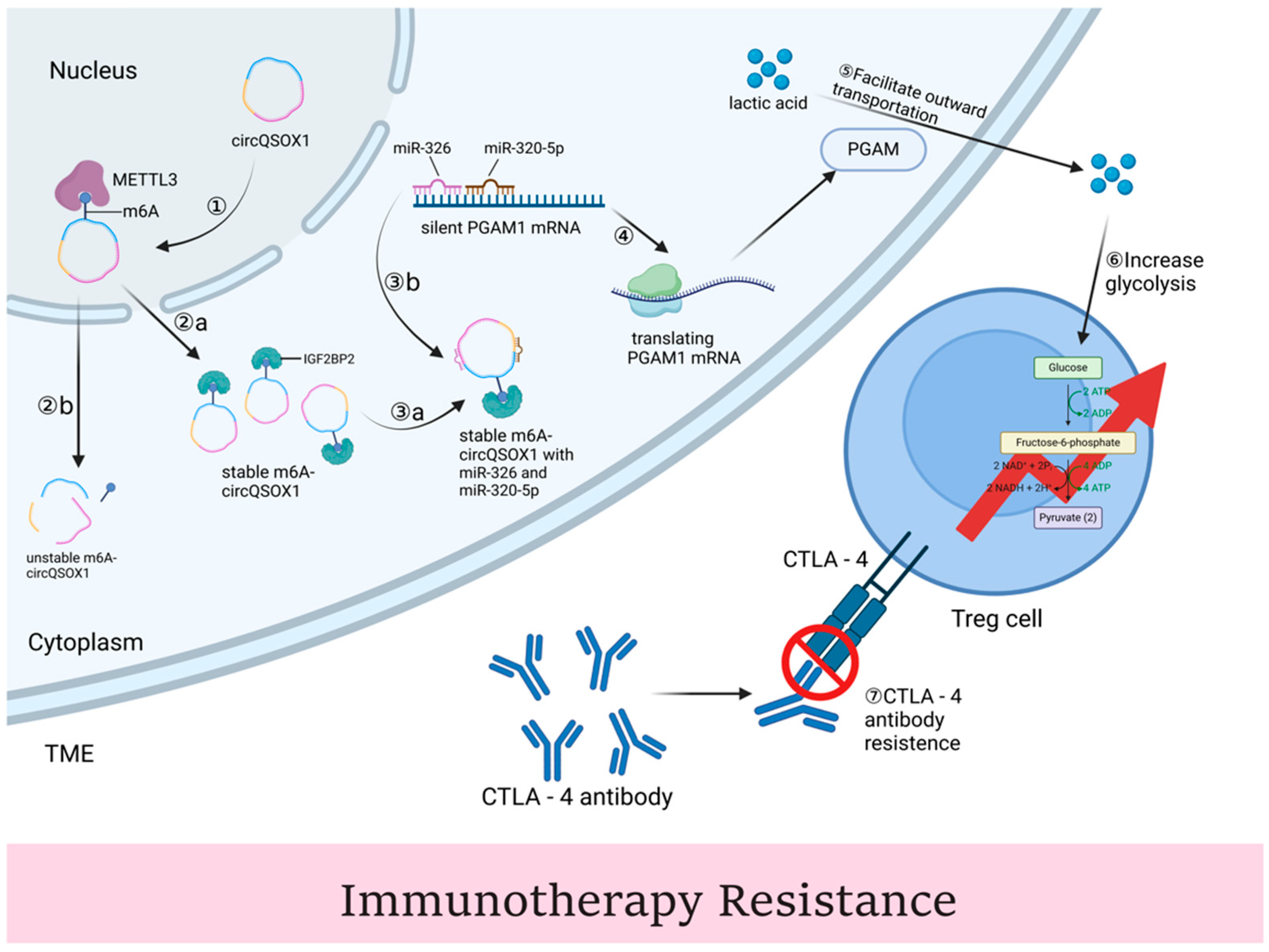
4.3. Immunotherapy
4.4. Radiation Therapy Resistance
5. Targeting the IGF2BP2 to Improve the Effect on Cancer Treatment
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Azzwi, Z.H.N.; Nazarov, A.N. Brain Tumor Classification based on Improved Stacked Ensemble Deep Learning Methods. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Xi, X.; Fan, X.; Ma, M.; Zhang, Y.; Yang, Y. Overexpression of METTL3 attenuates high-glucose induced RPE cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade through DGCR8. Aging 2020, 12, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, S.H.; Yang, E.; Kang, M.; Joo, J.Y. Molecular insights into regulatory RNAs in the cellular machinery. Exp. Mol. Med. 2024, 56, 1235–1249. [Google Scholar] [CrossRef]
- Wang, H.; Huang, T.; Wang, D.; Zeng, W.; Sun, Y.; Zhang, L. MSCAN: Multi-scale self- and cross-attention network for RNA methylation site prediction. BMC Bioinform. 2024, 25, 32. [Google Scholar] [CrossRef]
- Wang, R.; Chung, C.R.; Lee, T.Y. Interpretable Multi-Scale Deep Learning for RNA Methylation Analysis across Multiple Species. Int. J. Mol. Sci. 2024, 25, 2869. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Li, H.; Liu, L.; Lin, Y.; Zhang, L.; Zu, X.; Zhu, Y.; Shu, Y.; Shen, D.; et al. METTL14 regulates CD8(+)T-cell activation and immune responses to anti-PD-1 therapy in lung cancer. World J. Surg. Oncol. 2024, 22, 128. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.; Chen, L. Antimicrobial resistance and mechanisms of epigenetic regulation. Front. Cell. Infect. Microbiol. 2023, 13, 1199646. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, N.; Kim, S.; Jin, M.S.; Shen, H.; Kim, Y.C. Eltrombopag as an Allosteric Inhibitor of the METTL3-14 Complex Affecting the m(6)A Methylation of RNA in Acute Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 440. [Google Scholar] [CrossRef]
- Duan, M.; Liu, H.; Xu, S.; Yang, Z.; Zhang, F.; Wang, G.; Wang, Y.; Zhao, S.; Jiang, X. IGF2BPs as novel m(6)A readers: Diverse roles in regulating cancer cell biological functions, hypoxia adaptation, metabolism, and immunosuppressive tumor microenvironment. Genes Dis. 2024, 11, 890–920. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, X. Epigenetic regulation of N6-methyladenosine modifications in obesity. J. Diabetes Investig. 2021, 12, 1306–1315. [Google Scholar] [CrossRef]
- Müeller-Pillasch, F.; Lacher, U.; Wallrapp, C.; Micha, A.; Zimmerhackl, F.; Hameister, H.; Varga, G.; Friess, H.; Büchler, M.; Beger, H.G.; et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997, 14, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Gut, H.; Chao, J.A. Structural basis of IMP3 RRM12 recognition of RNA. RNA 2018, 24, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.C.; Nielsen, J.; Kristensen, M.A.; Koch, G.; Christiansen, J. Cytoplasmic trafficking of IGF-II mRNA-binding protein by conserved KH domains. J. Cell Sci. 2002, 115, 2087–2097. [Google Scholar] [CrossRef]
- Biswas, J.; Patel, V.L.; Bhaskar, V.; Chao, J.A.; Singer, R.H.; Eliscovich, C. The structural basis for RNA selectivity by the IMP family of RNA-binding proteins. Nat. Commun. 2019, 10, 4440. [Google Scholar] [CrossRef]
- Dagil, R.; Ball, N.J.; Ogrodowicz, R.W.; Hobor, F.; Purkiss, A.G.; Kelly, G.; Martin, S.R.; Taylor, I.A.; Ramos, A. IMP1 KH1 and KH2 domains create a structural platform with unique RNA recognition and re-modelling properties. Nucleic Acids Res. 2019, 47, 4334–4348. [Google Scholar] [CrossRef]
- Zorc, S.; Munoz-Tello, P.; O’Leary, T.; Yu, X.; Giridhar, M.N.K.; Hansel-Harris, A.; Forli, S.; Griffin, P.R.; Kojetin, D.J.; Roy, R.N.; et al. Structural insights into IMP2 dimerization and RNA binding. bioRxiv 2024, 580656. [Google Scholar]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Author Correction: Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- hen, R.-X.; Chen, X.; Xia, L.-P.; Zhang, J.-X.; Pan, Z.-Z.; Ma, X.-D.; Han, K.; Chen, J.-W.; Judde, J.-G.; Deas, O.; et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 2019, 10, 4695. [Google Scholar]
- Nielsen, J.; Kristensen, M.A.; Willemoës, M.; Nielsen, F.C.; Christiansen, J. Sequential dimerization of human zipcode-binding protein IMP1 on RNA: A cooperative mechanism providing RNP stability. Nucleic Acids Res. 2004, 32, 4368–4376. [Google Scholar] [CrossRef]
- Bastian, F.B.; Roux, J.; Niknejad, A.; Comte, A.; Costa, S.S.F.; de Farias, T.M.; Moretti, S.; Parmentier, G.; de Laval, V.R.; Rosikiewicz, M.; et al. The Bgee suite: Integrated curated expression atlas and comparative transcriptomics in animals. Nucleic Acids Res. 2021, 49, D831–D847. [Google Scholar] [CrossRef] [PubMed]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.-J.; Chen, Q.; et al. Reversible methylation of m(6)A(m) in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, S.; Jaffrey, S.R. A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 2020, 181, 1582–1595.e18. [Google Scholar] [CrossRef] [PubMed]
- Viegas, I.J.; de Macedo, J.P.; Serra, L.; De Niz, M.; Temporão, A.; Pereira, S.S.; Mirza, A.H.; Bergstrom, E.; Rodrigues, J.A.; Aresta-Branco, F.; et al. N(6)-methyladenosine in poly(A) tails stabilize VSG transcripts. Nature 2022, 604, 362–370. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Bai, L.; Xiang, Y.; Tang, M.; Liu, S.; Chen, Q.; Chen, Q.; Zhang, M.; Wan, S.; Sang, Y.; Li, Q.; et al. ALKBH5 controls the meiosis-coupled mRNA clearance in oocytes by removing the N (6)-methyladenosine methylation. Nat. Commun. 2023, 14, 6532. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, P.; Cao, Y.; Wang, T.; Huang, G.; Li, Y.; Zhou, S.; Yang, W.; Yang, L.; Liu, G.; et al. HOXD-AS2-STAT3 feedback loop attenuates sensitivity to temozolomide in glioblastoma. CNS Neurosci. Ther. 2023, 29, 3430–3445. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Duan, J.-L.; Chen, W.; Xie, J.-J.; Zhang, M.-L.; Nie, R.-C.; Liang, H.; Mei, J.; Han, K.; Xiang, Z.-C.; Wang, F.-W.; et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol. Cancer 2022, 21, 93. [Google Scholar] [CrossRef]
- Schaeffer, V.; Hansen, K.M.; Morris, D.R.; LeBoeuf, R.C.; Abrass, C.K. RNA-binding protein IGF2BP2/IMP2 is required for laminin-β2 mRNA translation and is modulated by glucose concentration. Am. J. Physiol. Ren. Physiol. 2012, 303, F75–F82. [Google Scholar] [CrossRef] [PubMed]
- Regué, L.; Wang, W.; Ji, F.; Avruch, J.; Wang, H.; Dai, N. Human T2D-Associated Gene IMP2/IGF2BP2 Promotes the Commitment of Mesenchymal Stem Cells Into Adipogenic Lineage. Diabetes 2023, 72, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, T.; Ping, X.; Wang, D.; Chen, Y.; Yu, J.; Liu, C.; Liu, Z.; Zheng, Y.; et al. Rna M(6) a Methylation Regulates Glycolysis of Beige Fat and Contributes to Systemic Metabolic Homeostasis. Adv. Sci. 2023, 10, e2300436. [Google Scholar] [CrossRef] [PubMed]
- Regué, L.; Zhao, L.; Ji, F.; Wang, H.; Avruch, J.; Dai, N. RNA m6A reader IMP2/IGF2BP2 promotes pancreatic β-cell proliferation and insulin secretion by enhancing PDX1 expression. Mol. Metab. 2021, 48, 101209. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Mo, K.; Huang, H.; Yu, Y.; Huang, Y.; Liu, J.; Li, M.; Tan, J.; Lin, Z.; et al. IGF2BP2 Maintains Retinal Pigment Epithelium Homeostasis by Stabilizing PAX6 and OTX2. Investig. Ophthalmol. Vis. Sci. 2024, 65, 17. [Google Scholar] [CrossRef]
- Okamura, T.; Okada, H.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Hamaguchi, M.; Fukui, M. Let-7e-5p Regulates IGF2BP2, and Induces Muscle Atrophy. Front. Endocrinol. 2021, 12, 791363. [Google Scholar] [CrossRef]
- Suo, M.; Rommelfanger, M.K.; Chen, Y.; Amro, E.M.; Han, B.; Chen, Z.; Szafranski, K.; Chakkarappan, S.R.; Boehm, B.O.; MacLean, A.L.; et al. Age-dependent effects of Igf2bp2 on gene regulation, function, and aging of hematopoietic stem cells in mice. Blood 2022, 139, 2653–2665. [Google Scholar] [CrossRef]
- Fu, D.; Shi, X.; Yi, X.; Wu, D.; He, H.; Zhou, W.; Cheng, W. m6A reader IGF2BP2 promotes M2 macrophage polarization and malignant biological behavior of bladder cancer by stabilizing NRP1 mRNA expression. BMC Urol. 2024, 24, 147. [Google Scholar] [CrossRef]
- Alam, S.; Giri, P.K. Emerging role of m6A modification in ovarian cancer: Progression, drug resistance, and therapeutic prospects. Front. Oncol. 2024, 14, 1366223. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, H.; Xu, H.; Dong, S.; Ma, H. Critical roles of m(6)A methylation in cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1187514. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Q.; Shi, C.; Shao, Y.; Ni, J.; Lou, J.; Wei, S. RNA N6-Methyladenosine Patterns in Hepatocellular Carcinoma Reveal a Distinct Immune Infiltration Landscape and Clinical Significance. Med. Sci. Monit. 2021, 27, e930994. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Deng, L.; Huang, N.; Sun, F. The Biological Roles of lncRNAs and Future Prospects in Clinical Application. Diseases 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Tahir, M.; Zhang, F.; Ran, Y.; Liu, Z.; Wang, J. Current insights into the implications of m6A RNA methylation and autophagy interaction in human diseases. Cell Biosci. 2021, 11, 147. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Zhang, Z.; Lai, Y.; Lin, X.; Lin, B.; Ma, M.; Liang, X.; Li, X.; Lv, W.; et al. CircCD44 plays oncogenic roles in triple-negative breast cancer by modulating the miR-502-5p/KRAS and IGF2BP2/Myc axes. Mol. Cancer 2021, 20, 138. [Google Scholar] [CrossRef]
- Xie, Z.; Luo, H.; Wang, T.; Wang, L.; Zhang, J.; Dong, W.; Liu, G.; Li, F.; Kang, Q.; Zhu, X.; et al. METTL3 inhibits BMSC apoptosis and facilitates osteonecrosis repair via an m6A-IGF2BP2-dependent mechanism. Heliyon 2024, 10, e30195. [Google Scholar] [CrossRef]
- ang, J.; Zhu, M.; Zhu, J.; Li, J.; Zhu, X.; Wang, K.; Shen, K.; Yang, K.; Ni, X.; Liu, X.; et al. HES1 promotes aerobic glycolysis and cancer progression of colorectal cancer via IGF2BP2-mediated GLUT1 m6A modification. Cell Death Discov. 2023, 9, 411. [Google Scholar]
- Ye, M.; Chen, J.; Lu, F.; Zhao, M.; Wu, S.; Hu, C.; Yu, P.; Kan, J.; Bai, J.; Tian, Y.; et al. Down-regulated FTO and ALKBH5 co-operatively activates FOXO signaling through m6A methylation modification in HK2 mRNA mediated by IGF2BP2 to enhance glycolysis in colorectal cancer. Cell Biosci. 2023, 13, 148. [Google Scholar] [CrossRef]
- Li, T.; Hu, P.-S.; Zuo, Z.; Lin, J.-F.; Li, X.; Wu, Q.-N.; Chen, Z.-H.; Zeng, Z.-L.; Wang, F.; Zheng, J.; et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer 2019, 18, 112. [Google Scholar] [CrossRef]
- Hu, X.; Peng, W.-X.; Zhou, H.; Jiang, J.; Zhou, X.; Huang, D.; Mo, Y.-Y.; Yang, L. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020, 27, 1782–1794. [Google Scholar] [CrossRef]
- Zhao, R.; Li, T.; Zhao, X.; Yang, Z.; Ma, L.; Wang, X. The m6A reader IGF2BP2 promotes the progression of esophageal squamous cell carcinoma cells by increasing the stability of OCT4 mRNA. Biochem. Cell Biol. 2024, 102, 169–178. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zheng, J. A1BG-AS1 promotes adriamycin resistance of breast cancer by recruiting IGF2BP2 to upregulate ABCB1 in an m6A-dependent manner. Sci. Rep. 2023, 13, 20730. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhao, H.; Zhang, S.; Ding, Q.; Guo, Y.; Hou, K.; Kan, Y.; Deng, F.; Xu, Q. A pan-cancer landscape of IGF2BPs and their association with prognosis, stemness and tumor immune microenvironment. Front. Oncol. 2022, 12, 1049183. [Google Scholar] [CrossRef] [PubMed]
- Kendzia, S.; Franke, S.; Kröhler, T.; Golob-Schwarzl, N.; Schweiger, C.; Toeglhofer, A.M.; Skofler, C.; Uranitsch, S.; El-Heliebi, A.; Fuchs, J.; et al. A combined computational and functional approach identifies IGF2BP2 as a driver of chemoresistance in a wide array of pre-clinical models of colorectal cancer. Mol. Cancer 2023, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, L.; Chen, W.; Zhang, Q.; Wang, F.; Wu, Y.; Cui, B.; Qi, J.; Zhang, X.; Liu, C.; et al. N6-methyladenine-mediated aberrant activation of the lncRNA SOX2OT-GLI1 loop promotes non-small-cell lung cancer stemness. Cell Death Discov. 2023, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, D.; Yi, N.; Cao, Y.; Wei, Y.; Wang, W.; Li, L. Circular RNA circPBX3 promotes cisplatin resistance of ovarian cancer cells via interacting with IGF2BP2 to stabilize ATP7A mRNA expression. Hum. Cell 2022, 35, 1560–1576. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhou, Y.; Huang, X.; Jiang, X. Long non-coding RNA OIP5-AS1 inhibition upregulates microRNA-129-5p to repress resistance to temozolomide in glioblastoma cells via downregulating IGF2BP2. Cell Biol. Toxicol. 2022, 38, 963–977. [Google Scholar] [CrossRef]
- eng, B.; Cheng, S.; Wang, H.; Liu, T.; Gu, Y.; Duan, L.; Cheng, T.; Wang, X.; Wang, X.; Zhang, Q.; et al. N(6)-methyladenosine enhances the expression of TGF-β-SMAD signaling family to inhibit cell growth and promote cell metastasis. Cancer Lett. 2024, 603, 217195. [Google Scholar]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef]
- Sun, L.; Fazal, F.M.; Li, P.; Broughton, J.P.; Lee, B.; Tang, L.; Huang, W.; Kool, E.T.; Chang, H.Y.; Zhang, Q.C. RNA structure maps across mammalian cellular compartments. Nat. Struct. Mol. Biol. 2019, 26, 322–330. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Q.; Zheng, G.; He, C.; Parisien, M.; Pan, T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef]
- Wu, B.; Su, S.; Patil, D.P.; Liu, H.; Gan, J.; Jaffrey, S.R.; Ma, J. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat. Commun. 2018, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liang, W.; Shi, Z.; Li, X.; Zhou, S.; Hu, W.; Yang, Z.; Wang, X. Systematic characterization and biological functions of non-coding RNAs in glioblastoma. Cell Prolif. 2023, 56, e13375. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Zou, Y.-X.; Zhu, W.-J.; Liu, S.; Zhang, G.-H.; Ma, R.-R.; Guo, X.-Y.; Gao, P. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 2022, 29, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ding, X.; Hu, X.; Zhao, Q.; Chen, Q.; Sun, T.; Li, Y.; Guo, H.; Li, M.; Gao, Z.; et al. LINC01021 maintains tumorigenicity by enhancing N6-methyladenosine reader IMP2 dependent stabilization of MSX1 and JARID2: Implication in colorectal cancer. Oncogene 2022, 41, 1959–1973. [Google Scholar] [CrossRef]
- Ruan, D.-Y.; Li, T.; Wang, Y.-N.; Meng, Q.; Li, Y.; Yu, K.; Wang, M.; Lin, J.-F.; Luo, L.-Z.; Wang, D.-S.; et al. FTO downregulation mediated by hypoxia facilitates colorectal cancer metastasis. Oncogene 2021, 40, 5168–5181. [Google Scholar] [CrossRef]
- Sa, R.; Liang, R.; Qiu, X.; He, Z.; Liu, Z.; Chen, L. IGF2BP2-dependent activation of ERBB2 signaling contributes to acquired resistance to tyrosine kinase inhibitor in differentiation therapy of radioiodine-refractory papillary thyroid cancer. Cancer Lett. 2022, 527, 10–23. [Google Scholar] [CrossRef]
- Li, H.-B.; Huang, G.; Tu, J.; Lv, D.-M.; Jin, Q.-L.; Chen, J.-K.; Zou, Y.-T.; Lee, D.-F.; Shen, J.-N.; Xie, X.-B. METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine 2022, 82, 104142. [Google Scholar] [CrossRef]
- Dai, N.; Rapley, J.; Angel, M.; Yanik, M.F.; Blower, M.D.; Avruch, J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011, 25, 1159–1172. [Google Scholar] [CrossRef]
- Janiszewska, M.; Suvà, M.L.; Riggi, N.; Houtkooper, R.H.; Auwerx, J.; Clément-Schatlo, V.; Radovanovic, I.; Rheinbay, E.; Provero, P.; Stamenkovic, I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012, 26, 1926–1944. [Google Scholar] [CrossRef]
- PPeng, F.; Xu, J.; Cui, B.; Liang, Q.; Zeng, S.; He, B.; Zou, H.; Li, M.; Zhao, H.; Meng, Y.; et al. Oncogenic AURKA-enhanced N(6)-methyladenosine modification increases DROSHA mRNA stability to transactivate STC1 in breast cancer stem-like cells. Cell Res. 2021, 31, 345–361. [Google Scholar] [CrossRef]
- Feng, M.; Xie, X.; Han, G.; Zhang, T.; Li, Y.; Li, Y.; Yin, R.; Wang, Q.; Zhang, T.; Wang, P.; et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood 2021, 138, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Xu, Z.; Xie, J.; Zhang, J.; Wang, X.; Peng, C.; Li, H.; Chen, H.; Shen, B.; Deng, X. Epigenetic silencing of LncRNA LINC00261 promotes c-myc-mediated aerobic glycolysis by regulating miR-222-3p/HIPK2/ERK axis and sequestering IGF2BP1. Oncogene 2021, 40, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Z.; Lv, D.-J.; Wang, C.; Song, X.-L.; Xie, T.; Wang, T.; Li, Z.-M.; Guo, J.-D.; Fu, D.-J.; Li, K.-J.; et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol. Cancer 2022, 21, 12. [Google Scholar] [CrossRef]
- Lang, C.; Yin, C.; Lin, K.; Li, Y.; Yang, Q.; Wu, Z.; Du, H.; Ren, D.; Dai, Y.; Peng, X. m(6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin. Transl. Med. 2021, 11, e426. [Google Scholar] [CrossRef]
- Lu, S.; Han, L.; Hu, X.; Sun, T.; Xu, D.; Li, Y.; Chen, Q.; Yao, W.; He, M.; Wang, Z.; et al. N6-methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: Implication in colorectal cancer. J. Hematol. Oncol. 2021, 14, 188. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Nie, H.; Huang, Y.; Du, J.; Xi, Y.; Guo, C.; Mu, M.; Li, X.; Zheng, X.; et al. The IGF2BP2-lncRNA TRPC7-AS1 axis promotes hepatocellular carcinoma cell proliferation and invasion. Cell. Signal. 2024, 117, 111078. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Jønson, L.; Christiansen, J.; Hansen, T.V.O.; Vikeså, J.; Yamamoto, Y.; Nielsen, F.C. IMP3 RNP safe houses prevent miRNA-directed HMGA2 mRNA decay in cancer and development. Cell Rep. 2014, 7, 539–551. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, D.; Han, C.; Zhao, Z.; Wang, X.; Jiang, T.; Li, Q.; Liu, S.; Chen, L.; Chen, Y.; et al. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine 2019, 41, 357–369. [Google Scholar] [CrossRef]
- Baptiste, C.K.; Gurtan, A.M.; Thai, K.K.; Lu, V.; Bhutkar, A.; Su, M.J.; Rotem, A.; Jacks, T.; Sharp, P.A. Dicer loss and recovery induce an oncogenic switch driven by transcriptional activation of the oncofetal Imp1-3 family. Genes Dev. 2017, 31, 674–687. [Google Scholar] [CrossRef]
- Degrauwe, N.; Schlumpf, T.B.; Janiszewska, M.; Martin, P.; Cauderay, A.; Provero, P.; Riggi, N.; Suvà, M.-L.; Paro, R.; Stamenkovic, I. The RNA Binding Protein IMP2 Preserves Glioblastoma Stem Cells by Preventing let-7 Target Gene Silencing. Cell Rep. 2016, 15, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Lu, Y.; Chen, S.; Yu, Y.; Lin, X.; Zhu, Y.; Luo, X. IGF2BP2-modified circular RNA circARHGAP12 promotes cervical cancer progression by interacting m(6)A/FOXM1 manner. Cell Death Discov. 2021, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.B.; Wang, M.C.; Yu, J.; Huang, G.; Sun, D.P.; Liu, L.; Zhang, J.N.; Yang, Y.; Liu, H.; Zhou, W.P.; et al. HBV/Pregenomic RNA Increases the Stemness and Promotes the Development of HBV-Related HCC Through Reciprocal Regulation With Insulin-Like Growth Factor 2 mRNA-Binding Protein 3. Hepatology 2021, 74, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gao, Z.; Zhang, T.; Wang, Y.; Xie, X.; Han, G.; Li, Y.; Yin, R.; Chen, Y.; Wang, P.; et al. Decoding m(6)A RNA methylome identifies PRMT6-regulated lipid transport promoting AML stem cell maintenance. Cell Stem Cell 2023, 30, 69–85.e7. [Google Scholar] [CrossRef]
- Xia, T.; Dai, X.; Sang, M.; Zhang, X.; Xu, F.; Wu, J.; Shi, L.; Wei, J.; Ding, Q. IGF2BP2 Drives Cell Cycle Progression in Triple-Negative Breast Cancer by Recruiting EIF4A1 to Promote the m6A-Modified CDK6 Translation Initiation Process. Adv. Sci. 2024, 11, e2305142. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Jiang, Y.; Wang, Z.; Li, H.; Wang, W.; Wang, C.; Hou, J. KLF7 regulates super-enhancer-driven IGF2BP2 overexpression to promote the progression of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. CR 2024, 43, 69. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Qian, Y.; Wei, W.; Lin, X.; Mao, S.; Sun, J.; Jin, J. m(6)A-dependent upregulation of DDX21 by super-enhancer-driven IGF2BP2 and IGF2BP3 facilitates progression of acute myeloid leukaemia. Clin. Transl. Med. 2024, 14, e1628. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Zhang, F.; Hu, X.; Bi, F.; Li, K.; Yu, H.; Zhao, Y.; Teng, X.; Li, J.; et al. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death Dis. 2022, 13, 483. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yi, B.; Cai, H.; Wang, Y.; Lou, X.; Xi, Z.; Li, Z. SUMOylation of IGF2BP2 promotes vasculogenic mimicry of glioma via regulating OIP5-AS1/miR-495-3p axis. Int. J. Biol. Sci. 2021, 17, 2912–2930. [Google Scholar] [CrossRef]
- Fang, H.; Sun, Q.; Zhou, J.; Zhang, H.; Song, Q.; Zhang, H.; Yu, G.; Guo, Y.; Huang, C.; Mou, Y.; et al. m(6)A methylation reader IGF2BP2 activates endothelial cells to promote angiogenesis and metastasis of lung adenocarcinoma. Mol. Cancer 2023, 22, 99. [Google Scholar] [CrossRef]
- He, Z.; Zhong, Y.; Regmi, P.; Lv, T.; Ma, W.; Wang, J.; Liu, F.; Yang, S.; Zhong, Y.; Zhou, R.; et al. Exosomal long non-coding RNA TRPM2-AS promotes angiogenesis in gallbladder cancer through interacting with PABPC1 to activate NOTCH1 signaling pathway. Mol. Cancer 2024, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guo, S.; Wang, S.; Zhang, Y.; Chen, H.; Wang, Y.; Liu, R.; Niu, Y.; Xu, Y. EIF4A3-Induced circARHGAP29 Promotes Aerobic Glycolysis in Docetaxel-Resistant Prostate Cancer through IGF2BP2/c-Myc/LDHA Signaling. Cancer Res. 2022, 82, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, X.; Jiang, X.; Dong, Z.; Hu, S.; Xiao, M. RNA demethylase ALKBH5 regulates hypopharyngeal squamous cell carcinoma ferroptosis by posttranscriptionally activating NFE2L2/NRF2 in an m(6) A-IGF2BP2-dependent manner. J. Clin. Lab. Anal. 2022, 36, e24514. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Wu, X.; Yin, D.; Jia, X.-H.; Chen, X.; Gu, Z.-Y.; Zhu, X.-M. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol. Ther. 2023, 249, 108485. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Li, L.; Yang, W.; Zhang, Z.; Zhang, K.; Ma, K.; Xie, H.; Zhang, Z.; Cai, L.; et al. FTO-mediated autophagy promotes progression of clear cell renal cell carcinoma via regulating SIK2 mRNA stability. Int. J. Biol. Sci. 2022, 18, 5943–5962. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, H.; Bian, Y.; Cui, Z.; Wang, N.; Wang, Z.; Yang, Y.; Liu, Z.; He, Z.; et al. N(6)-methyladenosine-modified USP13 induces pro-survival autophagy and imatinib resistance via regulating the stabilization of autophagy-related protein 5 in gastrointestinal stromal tumors. Cell Death Differ. 2023, 30, 544–559. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Wang, Z.; Lv, C.; Zhang, T.; Zhang, D.; Dong, W.; Shao, L.; He, L.; Ji, X.; et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J. Exp. Clin. Cancer Res. CR 2022, 41, 42. [Google Scholar] [CrossRef]
- Shen, C.; Xuan, B.; Yan, T.; Ma, Y.; Xu, P.; Tian, X.; Zhang, X.; Cao, Y.; Ma, D.; Zhu, X.; et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer 2020, 19, 72. [Google Scholar] [CrossRef]
- Weng, H.; Huang, F.; Yu, Z.; Chen, Z.; Prince, E.; Kang, Y.; Zhou, K.; Li, W.; Hu, J.; Fu, C.; et al. The m(6)A reader IGF2BP2 regulates glutamine metabolism and represents a therapeutic target in acute myeloid leukemia. Cancer Cell 2022, 40, 1566–1582.e10. [Google Scholar] [CrossRef]
- Li, H.; Luo, F.; Jiang, X.; Zhang, W.; Xiang, T.; Pan, Q.; Cai, L.; Zhao, J.; Weng, D.; Li, Y.; et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J. Immunother. Cancer 2022, 10, e004029. [Google Scholar] [CrossRef]
- Xie, J.; Huang, Z.; Jiang, P.; Wu, R.; Jiang, H.; Luo, C.; Hong, H.; Yin, H. Elevated N6-Methyladenosine RNA Levels in Peripheral Blood Immune Cells: A Novel Predictive Biomarker and Therapeutic Target for Colorectal Cancer. Front. Immunol. 2021, 12, 760747. [Google Scholar] [CrossRef] [PubMed]
- MMineo, M.; Ricklefs, F.; Rooj, A.K.; Lyons, S.M.; Ivanov, P.; Ansari, K.I.; Nakano, I.; Chiocca, E.A.; Godlewski, J.; Bronisz, A. The Long Non-coding RNA HIF1A-AS2 Facilitates the Maintenance of Mesenchymal Glioblastoma Stem-like Cells in Hypoxic Niches. Cell Rep. 2016, 15, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.D.; Zhang, Z.; Zhao, J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Tong, J.; Zhu, S.; Batista, P.J.; Duffy, E.E.; Zhao, J.; Bailis, W.; Cao, G.; Kroehling, L.; Chen, Y.; et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 2017, 548, 338–342. [Google Scholar] [CrossRef]
- Tong, J.; Cao, G.; Zhang, T.; Sefik, E.; Vesely, M.C.A.; Broughton, J.P.; Zhu, S.; Li, H.; Li, B.; Chen, L.; et al. m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res. 2018, 28, 253–256. [Google Scholar] [CrossRef]
- Chen, M.; Wong, C.M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer 2020, 19, 44. [Google Scholar] [CrossRef]
- Wang, C.X.; Cui, G.S.; Liu, X.; Xu, K.; Wang, M.; Zhang, X.X.; Jiang, L.Y.; Li, A.; Yang, Y.; Lai, W.Y.; et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018, 16, e2004880. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, M.; He, X.; Cao, Y.; Liu, P.; Li, F.; Zou, S.; Wen, C.; Zhan, Q.; Xu, Z.; et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2022, 15, 52. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhou, Y.; Qu, M.; Wang, Y.; Guo, K.; Shen, R.; Sun, Z.; Cata, J.P.; Yang, S.; et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int. J. Biol. Sci. 2022, 18, 3337–3357. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.Y.; Huang, L.-P.; Bao, J.-H. miR-96-5p regulates cervical cancer cell resistance to cisplatin by inhibiting lncRNA TRIM52-AS1 and promoting IGF2BP2. Kaohsiung J. Med. Sci. 2022, 38, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huang, Z.; Qiu, J.; Shi, Y.; Zuo, D.; Qiu, Z.; He, W.; Niu, Y.; Yuan, Y.; Li, B. m(6)A-mediated lnc-OXAR promotes oxaliplatin resistance by enhancing Ku70 stability in non-alcoholic steatohepatitis-related hepatocellular carcinoma. J. Exp. Clin. Cancer Res. CR 2024, 43, 206. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, L.; Yu, F.; Gao, H.; Lei, T.; Li, P.; Liu, P.; Zheng, X.; Hu, X.; Chen, Y.; et al. Imp2 regulates GBM progression by activating IGF2/PI3K/Akt pathway. Cancer Biol. Ther. 2015, 16, 623–633. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Yuan, Y.; Jin, Z.; Zhai, H.; Liu, B.; Li, Y.; Zhang, C.; Chen, M.; Shi, Y.; et al. LncRNA GSCAR promotes glioma stem cell maintenance via stabilizing SOX2 expression. Int. J. Biol. Sci. 2023, 19, 1681–1697. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Kang, F.-P.; Xie, C.-K.; Liao, C.-Y.; Li, G.; Wu, Y.-D.; Lin, H.-Y.; Zhu, S.-C.; Hu, J.-F.; Lin, C.-F.; et al. A Novel Trojan Horse Nanotherapy Strategy Targeting the cPKM-STMN1/TGFB1 Axis for Effective Treatment of Intrahepatic Cholangiocarcinoma. Adv. Sci. 2023, 10, e2303814. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, N.; Li, J.; Li, C.; Zheng, D.; Jiang, X.; Ge, X.; Liu, M.; Liu, L.; Song, Z.; et al. N6-methyladenosine-modified circular RNA QSOX1 promotes colorectal cancer resistance to anti-CTLA-4 therapy through induction of intratumoral regulatory T cells. Drug Resist. Updates 2022, 65, 100886. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Deng, Y.; Deng, S.; Li, J.; Wei, W.; Wang, Y.; Wang, J.; Feng, Z.; Che, M.; et al. FBW7/GSK3β mediated degradation of IGF2BP2 inhibits IGF2BP2-SLC7A5 positive feedback loop and radioresistance in lung cancer. J. Exp. Clin. Cancer Res. CR 2024, 43, 34. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Du, Q.-Y.; Zhu, Z.-M.; Pei, D.-S. The biological function of IGF2BPs and their role in tumorigenesis. Investig. New Drugs 2021, 39, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Andrini, E.; Ricco, G.; Zappi, A.; Aloi, S.; Giordano, M.; Altimari, A.; Gruppioni, E.; Maloberti, T.; de Biase, D.; Campana, D.; et al. Challenges and future perspectives for the use of temozolomide in the treatment of SCLC. Cancer Treat. Rev. 2024, 129, 102798. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, L.; Zhang, Y.; Tang, X.; Lei, B.; Zhang, Q. IGF2BP2 modulates autophagy and serves as a prognostic marker in glioma. Ibrain 2024, 10, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, X.; Wang, F.; Liu, H.; Guan, W.; Xu, G. Insulin-like growth factor 2 mRNA-binding protein 2 is a therapeutic target in ovarian cancer. Exp. Biol. Med. 2023, 248, 2198–2209. [Google Scholar] [CrossRef]
- Bruserud, O.; Tsykunova, G.; Hernandez-Valladares, M.; Reikvam, H.; Tvedt, T.H.A. Therapeutic Use of Valproic Acid and All-Trans Retinoic Acid in Acute Myeloid Leukemia-Literature Review and Discussion of Possible Use in Relapse after Allogeneic Stem Cell Transplantation. Pharmaceuticals 2021, 14, 423. [Google Scholar] [CrossRef]
- Heuser, M.; Beutel, G.; Krauter, J.; Döhner, K.; von Neuhoff, N.; Schlegelberger, B.; Ganser, A. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood 2006, 108, 3898–3905. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER 2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Ebrahimnezhad, M.; Natami, M.; Bakhtiari, G.H.; Tabnak, P.; Ebrahimnezhad, N.; Yousefi, B.; Majidinia, M. FOXO1, a tiny protein with intricate interactions: Promising therapeutic candidate in lung cancer. Biomed. Pharmacother. 2023, 169, 115900. [Google Scholar] [CrossRef]
- Han, J.; Yu, X.; Wang, S.; Wang, Y.; Liu, Q.; Xu, H.; Wang, X. IGF2BP2 Induces U251 Glioblastoma Cell Chemoresistance by Inhibiting FOXO1-Mediated PID1 Expression Through Stabilizing lncRNA DANCR. Front. Cell Dev. Biol. 2021, 9, 659228. [Google Scholar] [CrossRef]
- Chong, X.; Chen, J.; Zheng, N.; Zhou, Z.; Hai, Y.; Chen, S.; Zhang, Y.; Yu, Q.; Yu, S.; Chen, Z.; et al. PIK3CA mutations-mediated downregulation of circLHFPL2 inhibits colorectal cancer progression via upregulating PTEN. Mol. Cancer 2022, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Lin, C.-W.; Lu, J.-W.; Yang, W.-E.; Lin, Y.-M.; Lu, H.-J.; Yang, S.-F. Cytoplasmic IGF2BP2 Protein Expression in Human Patients with Oral Squamous Cell Carcinoma: Prognostic and Clinical Implications. Int. J. Med. Sci. 2022, 19, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.; Xu, X.; Shu, C.; Cao, C.; Wang, Z.; Fu, Y.; Xu, L.; Xu, K.; Xu, J.; et al. APAF1-Binding Long Noncoding RNA Promotes Tumor Growth and Multidrug Resistance in Gastric Cancer by Blocking Apoptosome Assembly. Adv. Sci. 2022, 9, e2201889. [Google Scholar] [CrossRef]
- Wang, G.; Zhuang, T.; Zhen, F.; Zhang, C.; Wang, Q.; Miao, X.; Qi, N.; Yao, R. IGF2BP2 inhibits invasion and migration of clear cell renal cell carcinoma via targeting Netrin-4 in an m6A-dependent manner. Mol. Carcinog. 2024, 63, 1572–1587. [Google Scholar] [CrossRef]
- Chanda, S.; Lepikhov, K.; Dahlem, C.; Schymik, H.S.; Hoppstädter, J.; Geber, A.-K.; Wagner, K.; Kessler, S.M.; Empting, M.; Kiemer, A. Gene Editing and Small Molecule Inhibitors of the RNA Binding Protein IGF2BP2/IMP2 Show its Potential as an Anti-Cancer Drug Target. Front. Biosci. 2024, 29, 41. [Google Scholar] [CrossRef]
- Feng, P.; Chen, D.; Wang, X.; Li, Y.; Li, Z.; Li, B.; Zhang, Y.; Li, W.; Zhang, J.; Ye, J.; et al. Inhibition of the m6A reader IGF2BP2 as a strategy against T-cell acute lymphoblastic leukemia. Leukemia 2022, 36, 2180–2188. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Zhao, Y.; Li, X. m6A reader IGF2BP2 promotes lymphatic metastasis by stabilizing DPP4 in papillary thyroid carcinoma. Cancer Gene Ther. 2024, 31, 285–299. [Google Scholar] [CrossRef]
- Dahlem, C.; Abuhaliema, A.; Kessler, S.M.; Kröhler, T.; Zoller, B.G.E.; Chanda, S.; Wu, Y.; Both, S.; Müller, F.; Lepikhov, K.; et al. First Small-Molecule Inhibitors Targeting the RNA-Binding Protein IGF2BP2/IMP2 for Cancer Therapy. ACS Chem. Biol. 2022, 17, 361–375. [Google Scholar] [CrossRef]

| Cell Type | Normal Function of m6A | Oncogenic Function of m6A | Reference |
|---|---|---|---|
| Stem Cells | Regulates self-renewal and differentiation. | Promotes stemness and tumorigenesis. | [103] |
| T Cells | Modulates activation and differentiation. | Inhibits anti-tumor immunity by enhancing T cell exhaustion. | [104,105] |
| Cancer Cells | Maintains normal cell growth and apoptosis. | Promotes proliferation and survival by altering metabolism. | [106,107] |
| Macrophages | Regulates inflammatory response and tissue repair. | Enhances M2 polarization, supporting tumor progression. | [108] |
| Endothelial Cells | Facilitates angiogenesis in normal tissue development. | Drives abnormal angiogenesis in tumors. | [109] |
| Cancer Treatment | Drug | Cancer Type | Effect | Mechanism | Ref |
|---|---|---|---|---|---|
| Chemotherapy | Cisplatin | Ovarian cancer | Induce resistance | circRNA interacts with IGF2BP2-FGF9 complex to induce polarization of TAMs toward M2 phenotype to enhance CDDP resistance. | [111] |
| Cisplatin | Ovarian cancer | Induce resistance | circPBX3/IGF2BP2/ATP7A axis to induce efflux of cisplatin. | [111] | |
| Cisplatin | Cervical cancer | Induce resistance | miR-96-5p enhances IGF2BP2 to induce drug resistance. | [112] | |
| Oxaliplatin | Cervical cancer | Induce resistance | WTAP/IGF2BP2/lnc-OXAR axis regulates the recruitment of ku70 and cystatin A to facilitate DNA double-strand break repair, which enhances drug resistance. | [113] | |
| TMZ | Glioblastoma multiforme | Induce resistance | IGF2BP2/IGF2/PI3K/Akt signaling pathway to regulate TMZ resistance. | [114] | |
| TMZ | Glioma cells | Induce resistance | SOX2/IGF2BP2/DHX9 axis to increase TMZ resistance. | [115] | |
| TMZ | Glioblastoma multiforme | Induce resistance | HOXD-AS2/IGF2BP2/STAT3 positive feedback loop to regulate the sensitivity of TMZ. | [28] | |
| Paclitaxel | Ovarian cancer cells | Induce resistance | cPKM-IGF2BP2/STMN1/TGFB1 axis to facilitate proliferation and metastasis and increase the drug resistance. | [116] | |
| ATRA | Acute promyelocytic leukemia | Induce resistance | METTL14/IGF2BP2/MN1 axis leads to ATRA resistance. | [18] | |
| Doxorubicin | Breast cancer cells | Induce resistance | A1BG-AS1/IGF2BP2/ABCB1 axis. | [51] | |
| Targeted chemotherapy | Imatinib | Gastrointestinal stromal tumors | Induce resistance | METTL3/IGF2BP2/USP13/ATG5 axis. | [96] |
| Immunotherapy | Immune checkpoint like CTLA-4 | Colorectal cancer | Induce resistance | miR-326/miR-330-5p/PGAM1 axis AND induce Tregs. | [117] |
| Radiation therapy | Radiation | Lung cancer | Induce resistance | FBW7/GSK3β/IGF2BP2/SLC7A5 axis. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Hu, S.; Ye, J.; Zhai, C.; Liu, J.; Wang, Z.; Zhou, X.; Chen, L.; Zhou, F. The Emerging Role of IGF2BP2 in Cancer Therapy Resistance: From Molecular Mechanism to Future Potential. Int. J. Mol. Sci. 2024, 25, 12150. https://doi.org/10.3390/ijms252212150
Li D, Hu S, Ye J, Zhai C, Liu J, Wang Z, Zhou X, Chen L, Zhou F. The Emerging Role of IGF2BP2 in Cancer Therapy Resistance: From Molecular Mechanism to Future Potential. International Journal of Molecular Sciences. 2024; 25(22):12150. https://doi.org/10.3390/ijms252212150
Chicago/Turabian StyleLi, Die, Shiqi Hu, Jiarong Ye, Chaojie Zhai, Jipeng Liu, Zuao Wang, Xinchi Zhou, Leifeng Chen, and Fan Zhou. 2024. "The Emerging Role of IGF2BP2 in Cancer Therapy Resistance: From Molecular Mechanism to Future Potential" International Journal of Molecular Sciences 25, no. 22: 12150. https://doi.org/10.3390/ijms252212150
APA StyleLi, D., Hu, S., Ye, J., Zhai, C., Liu, J., Wang, Z., Zhou, X., Chen, L., & Zhou, F. (2024). The Emerging Role of IGF2BP2 in Cancer Therapy Resistance: From Molecular Mechanism to Future Potential. International Journal of Molecular Sciences, 25(22), 12150. https://doi.org/10.3390/ijms252212150





