Plasma Glutaminyl-Peptide Cyclotransferase Mediates Glucosamine-Metabolism-Driven Protection Against Hypertension: A Mendelian Randomization Study
Abstract
1. Introduction
2. Results
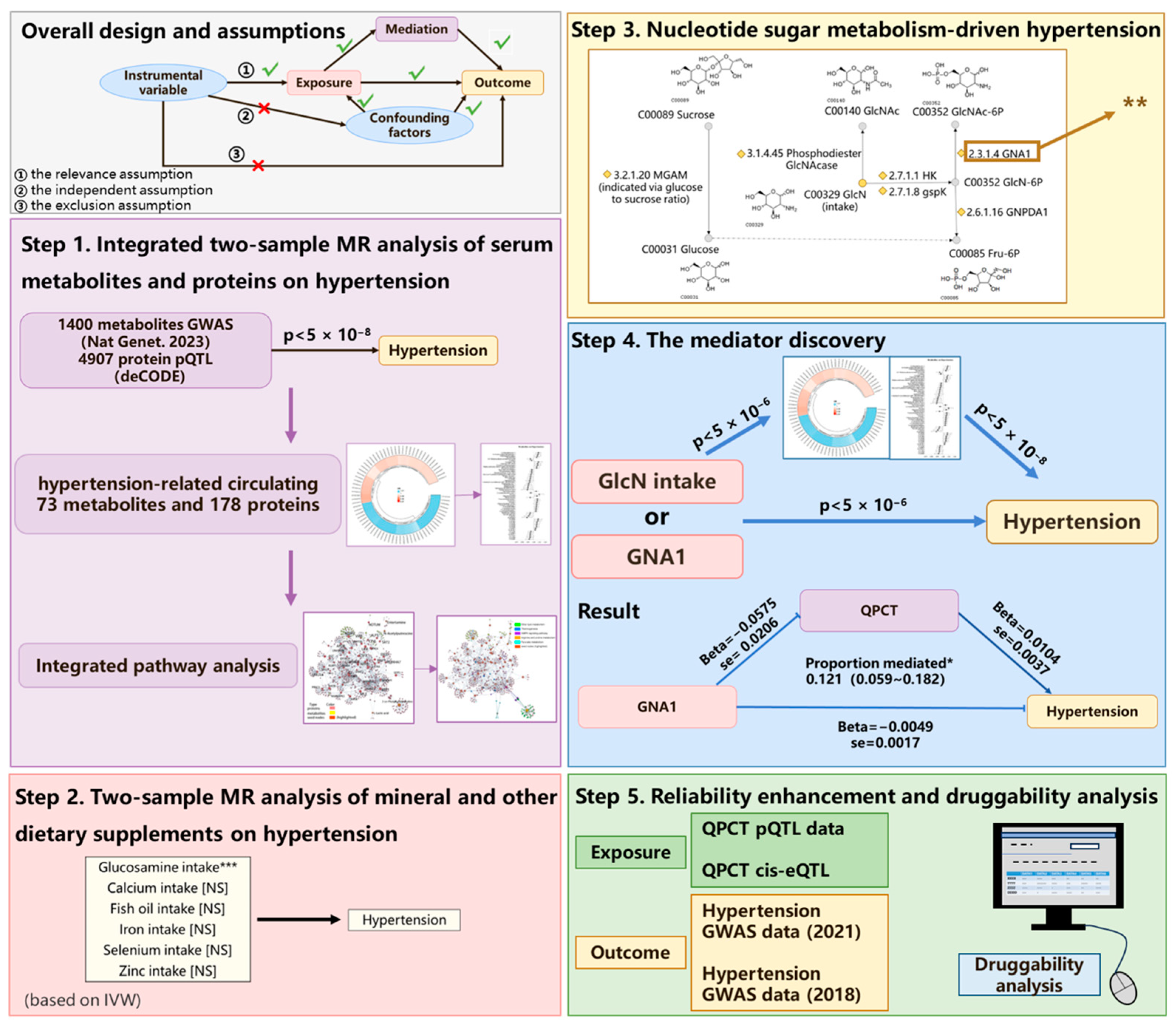
2.1. Study Design
2.2. Effect of Biomolecule Levels on Hypertension and Integrated Pathway Analysis
2.3. Impact of Dietary Supplements and Related Exposures on Hypertension
2.4. Mediation MR Analysis Revealed the Mediative Role of QPCT on Glucosamine-MetaboLlsm-Driven Protection Against Hypertension
2.5. Druggability Analysis of QPCT
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Instrumental Variable Selection
4.3. MR Analysis and Sensitivity Analysis
4.4. Metabolite–Protein Interaction Pathway Enrichment and Druggability Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Full Term |
| BP | Biological process |
| CC | Cellular component |
| CIs | Confidence intervals |
| cis-eQTL | Cis-expression quantitative trait locus |
| CLSA | Canadian Longitudinal Study on Aging |
| CRDL2 | Cancer-associated plasma membrane protein L2 |
| CVDs | Cardiovascular diseases |
| DASH | Dietary Approaches to Stop Hypertension |
| EBI | European Bioinformatics Institute |
| FDR | False discovery rate |
| GlcN | D-glucosamine |
| GlcN-6P | D-glucosamine 6-phosphate |
| GlcNAc | N-acetyl-D-glucosamine |
| GlcNAc-6P | N-acetyl-D-glucosamine 6-phosphate |
| GNA1 | Glucosamine 6-phosphate N-acetyltransferase |
| GNPDA1 | Glucosamine-6-phosphate isomerase1 |
| GO | Gene ontology |
| GspK | N-acetyl-D-glucosamine kinase |
| GWASs | Genome-wide association studies |
| HK | Hexokinase |
| HS3ST3A1 | Heparan sulfate glucosamine 3-O-sulfotransferase 3A1 |
| HS3ST3B1 | Heparan sulfate glucosamine 3-O-sulfotransferase 3B1 |
| HS3ST4 | Heparan sulfate glucosamine 3-O-sulfotransferase 4 |
| ING4 | Inhibitor of growth 4 |
| IVW | Inverse-variance weighted |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAF | Minor allele frequency |
| MedDiet | Mediterranean diet |
| MF | Molecular function |
| MR | Mendelian randomization |
| MR-PRESSO | Mendelian Randomization Pleiotropy Residual Sum and Outlier |
| NO | Nitric oxide |
| NPPB | Natriuretic peptide B |
| pQTL | Protein quantitative trait loci |
| QPCT | Glutaminyl-peptide cyclotransferase |
| SNPs | Single-nucleotide polymorphisms |
References
- Valenzuela, P.L.; Carrera-Bastos, P.; Gálvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2020, 18, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T.R.; Jaffe, M.G. Saving 100 million lives by improving global treatment of hypertension and reducing cardiovascular disease risk factors. J. Clin. Hypertens. 2018, 20, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Singh, G.M.; Paciorek, C.J.; Lin, J.K.; Cowan, M.J.; Finucane, M.M.; Farzadfar, F.; Stevens, G.A.; Riley, L.M.; Lu, Y.; et al. The Global Cardiovascular Risk Transition. Circulation 2013, 127, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Ozemek, C.; Laddu, D.R.; Arena, R.; Lavie, C.J. The role of diet for prevention and management of hypertension. Curr. Opin. Cardiol. 2018, 33, 388–393. [Google Scholar] [CrossRef]
- Belardo, D.; Michos, E.D.; Blankstein, R.; Blumenthal, R.S.; Ferdinand, K.C.; Hall, K.; Klatt, K.; Natajaran, P.; Ostfeld, R.J.; Reddy, K.; et al. Practical, Evidence-Based Approaches to Nutritional Modifications to Reduce Atherosclerotic Cardiovascular Disease: An American Society for Preventive Cardiology Clinical Practice Statement. Am. J. Prev. Cardiol. 2022, 10, 100323. [Google Scholar] [CrossRef]
- Zheng, J.; Ni, C.; Zhang, Y.; Huang, J.; Hukportie, D.N.; Liang, B.; Tang, S. Association of regular glucosamine use with incident dementia: Evidence from a longitudinal cohort and Mendelian randomization study. BMC Med. 2023, 21, 1–13. [Google Scholar] [CrossRef]
- Chiu, H.-W.; Li, L.-H.; Hsieh, C.-Y.; Rao, Y.K.; Chen, F.-H.; Chen, A.; Ka, S.-M.; Hua, K.-F. Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kirkham, S.; Samarasinghe, R. Review Article: Glucosamine. J. Orthop. Surg. 2009, 17, 72–76. [Google Scholar] [CrossRef]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States. Natl. Health Stat. Rep. 2008, 10, 1–23. [Google Scholar]
- Sibbritt, D.; Adams, J.; Lui, C.-W.; Broom, A.; Wardle, J. Who Uses Glucosamine and Why? A Study of 266,848 Australians Aged 45 Years and Older. PLoS ONE 2012, 7, e41540. [Google Scholar] [CrossRef] [PubMed]
- Galvin, R.; Cousins, G.; Boland, F.; Motterlini, N.; Bennett, K.; Fahey, T. Prescribing patterns of glucosamine in an older population: A national cohort study. BMC Complement. Altern. Med. 2013, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Lin, A.-H.; Chen, C.-H.; Huang, W.-C.; Wang, H.-Y.; Liu, M.-H.; Lee, T.-S.; Kou, Y.R. Glucosamine attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Free. Radic. Biol. Med. 2014, 69, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Yang, S.; Champattanachai, V.; Hu, S.; Chaudry, I.H.; Marchase, R.B.; Chatham, J.C. Glucosamine improves cardiac function following trauma-hemorrhage by increased proteinO-GlcNAcylation and attenuation of NF-κB signaling. Am. J. Physiol. Circ. Physiol. 2009, 296, H515–H523. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Smith, G.D.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2023, 4, 186. [Google Scholar] [CrossRef]
- Kantor, E.D.; Newton, C.C.; Giovannucci, E.L.; McCullough, M.L.; Campbell, P.T.; Jacobs, E.J. Glucosamine use and risk of colorectal cancer: Results from the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control. 2018, 29, 389–397. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Liu, Y.; Zhang, J.; Li, L.; Huang, X.; Thabane, L.; Lip, G.Y. Relationship between glucosamine use and the risk of lung cancer: Data from a nationwide prospective cohort study. Eur. Respir. J. 2021, 59, 2101399. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zhou, T.; Sun, D.; Liang, Z.; Li, Y.; Heianza, Y.; Qi, L. Glucosamine Use, Inflammation, and Genetic Susceptibility, and Incidence of Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2020, 43, 719–725. [Google Scholar] [CrossRef]
- King, D.E.; Xiang, J. Glucosamine/Chondroitin and Mortality in a US NHANES Cohort. J. Am. Board Fam. Med. 2020, 33, 842–847. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Sun, D.; Zhou, T.; Ley, S.H.; Gustat, J.; Heianza, Y.; Qi, L. Association of habitual glucosamine use with risk of cardiovascular disease: Prospective study in UK Biobank. BMJ 2019, 365, l1628. [Google Scholar] [CrossRef]
- Suissa, K.; Hudson, M.; Suissa, S. Glucosamine and lower mortality and cancer incidence: Selection bias in the observational studies. Pharmacoepidemiol. Drug Saf. 2022, 31, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Gao, X.; Chung, V.C.; Zhong, W.-F.; Fu, Q.; Lv, Y.-B.; Wang, Z.-H.; Shen, D.; Zhang, X.-R.; Zhang, P.-D.; et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: A large prospective cohort study. Ann. Rheum. Dis. 2020, 79, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Feng, W.; Nöt, L.G.; Miller, A.P.; Zhang, Y.; Chen, Y.-F.; Majid-Hassan, E.; Chatham, J.C.; Oparil, S. Increased proteinO-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am. J. Physiol. Circ. Physiol. 2008, 295, H335–H342. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Xu, L.; Xu, O.; Li, R.; Chen, M.; Shen, H.; Zhu, H.; Zhang, F.; Yao, D.; Chen, Y.-F.; et al. O-Linked β-N-Acetylglucosamine Modification of A20 Enhances the Inhibition of NF-κB (Nuclear Factor-κB) Activation and Elicits Vascular Protection After Acute Endoluminal Arterial Injury. Arter. Thromb. Vasc. Biol. 2018, 38, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Yazdani, A.; Mendez-Giraldez, R.; Samiei, A.; Kosorok, M.R.; Schaid, D.J. From classical mendelian randomization to causal networks for systematic integration of multi-omics. Front. Genet. 2022, 13, 990486. [Google Scholar] [CrossRef]
- Li, Y.; Xie, D.; Li, L.; Jiang, P. Comprehensive analysis of metabolic changes in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2023, 45, 2190529. [Google Scholar] [CrossRef]
- Osborn, J.W.; Fink, G.D. Region-specific changes in sympathetic nerve activity in angiotensin II–salt hypertension in the rat. Exp. Physiol. 2009, 95, 61–68. [Google Scholar] [CrossRef]
- Hilser, J.R.; Lusis, A.J.; Allayee, H. Genetics unravels protein–metabolite relationships. Trends Endocrinol. Metab. 2024, 35, 183–184. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Hear. J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef]
- Evans, D.M.; Smith, G.D. Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu. Rev. Genom. Hum. Genet. 2015, 16, 327–350. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Hear. J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.; Stevenson, J.; Stahl, K.; Basu, R.; Coffman, A.; Kiwala, S.; McMichael, J.F.; Kuzma, K.; Morrissey, D.; Cotto, K.; et al. DGIdb 5.0: Rebuilding the drug–gene interaction database for precision medicine and drug discovery platforms. Nucleic Acids Res. 2023, 52, D1227–D1235. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zhao, D.; Yu, X.; Shen, X.; Zhou, Y.; Wang, S.; Qiu, Y.; Chen, Y.; Zhu, F. TTD: Therapeutic Target Database describing target druggability information. Nucleic Acids Res. 2023, 52, D1465–D1477. [Google Scholar] [CrossRef]
- Vijverberg, E.G.B.; Axelsen, T.M.; Bihlet, A.R.; Henriksen, K.; Weber, F.; Fuchs, K.; Harrison, J.E.; Kühn-Wache, K.; Alexandersen, P.; Prins, N.D.; et al. Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD—VIVIAD. Alzheimer’s Res. Ther. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Buchholz, M.; Heiser, U.; Schilling, S.; Niestroj, A.J.; Zunkel, K.; Demuth, H.-U. The First Potent Inhibitors for Human Glutaminyl Cyclase: Synthesis and Structure−Activity Relationship. J. Med. Chem. 2005, 49, 664–677. [Google Scholar] [CrossRef]
- Tuttle, R.S.; Garcia-Minor, C.; Simon, M. Cardiovascular effects of 1-benzylimidazole. J. Pharmacol. Exp. Ther. 1975, 194, 624–632. [Google Scholar]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2023, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Dönertaş, H.M.; Fabian, D.K.; Fuentealba, M.; Partridge, L.; Thornton, J.M. Common genetic associations between age-related diseases. Nat. Aging 2021, 1, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, D.; Hercules, A.; Carmona, M.; Suveges, D.; Gonzalez-Uriarte, A.; Malangone, C.; Miranda, A.; Fumis, L.; Carvalho-Silva, D.; Spitzer, M.; et al. Open Targets Platform: Supporting systematic drug–target identification and prioritisation. Nucleic Acids Res. 2020, 49, D1302–D1310. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhao, P.; Sun, Y.; Zheng, Z.; Du, W.; Zhang, L.; Li, Y.; Xie, L.; Xu, S.; Wang, P. Development of a potent benzonitrile-based inhibitor of glutaminyl-peptide cyclotransferase-like protein (QPCTL) with antitumor efficacy. Signal Transduct. Target. Ther. 2023, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-F.; Liaw, S.-S.; Huang, W.-L.; Chia, C.-Y.; Lo, Y.-C.; Chen, Y.-L.; Wang, A.H.-J. Structures of Human Golgi-resident Glutaminyl Cyclase and Its Complexes with Inhibitors Reveal a Large Loop Movement upon Inhibitor Binding. J. Biol. Chem. 2011, 286, 12439–12449. [Google Scholar] [CrossRef]
- Bell, G.A.; Kantor, E.D.; Lampe, J.W.; Shen, D.D.; White, E. Use of glucosamine and chondroitin in relation to mortality. Eur. J. Epidemiol. 2012, 27, 593–603. [Google Scholar] [CrossRef]
- Setnikar, I.; Cereda, R.; A Pacini, M.; Revel, L. Antireactive properties of glucosamine sulfate. Arzneimittel-Forschung 1991, 41, 157–161. [Google Scholar]
- Dong, W.; Imdad, L.; Xu, S.; Wang, Y.; Liu, C.; Song, S.; Li, Z.; Kong, Y.; Kong, L.; Ren, X. O-GlcNAc Modification Is a Promising Therapeutic Target for Diabetic Retinopathy. Int. J. Mol. Sci. 2024, 25, 6286. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Kim, K.-H.; Park, J.; Kim, S.-M.; Cho, H.; Lee, Y.; Han, I.-O. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J. Biol. Chem. 2019, 294, 608–622. [Google Scholar] [CrossRef]
- Zheng, J.; Hukportie, D.N.; Zhang, Y.; Huang, J.; Ni, C.; Lip, G.Y.; Tang, S. Association Between Glucosamine Use and the Risk of Incident Heart Failure. Mayo Clin. Proc. 2023, 98, 1177–1191. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Chen, H.; Wang, M.; Wang, S.; Yang, R.; Zhan, S.; Qin, X.; Wu, T.; Wu, Y.; et al. Glucosamine Use Is Associated with a Higher Risk of Cardiovascular Diseases in Patients with Osteoarthritis: Results from a Large Study in 685,778 Subjects. Nutrients 2022, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-M.; Koh, J.-H.; Kim, S.-G.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.-C.; Han, S.-S.; Lee, H.; et al. Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study. Nutrients 2023, 15, 3318. [Google Scholar] [CrossRef] [PubMed]
- Tapadinhas, M.J.; Rivera, I.C.; Bignamini, A.A. Oral glucosamine sulphate in the management of arthrosis: Report on a multi-centre open investigation in Portugal. Pharmatherapeutica 1982, 3, 157–168. [Google Scholar] [PubMed]
- Cerda, C. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J. Gastroenterol. 2013, 19, 5381–5384. [Google Scholar] [CrossRef]
- Vo, N.X.; Le, N.N.H.; Chu, T.D.P.; Pham, H.L.; Dinh, K.X.A.; Che, U.T.T.; Ngo, T.T.T.; Bui, T.T. Effectiveness and Safety of Glucosamine in Osteoarthritis: A Systematic Review. Pharmacy 2023, 11, 117. [Google Scholar] [CrossRef]
- Knudsen, J.F.; Sokol, G.H. Potential Glucosamine-Warfarin Interaction Resulting in Increased International Normalized Ratio: Case Report and Review of the Literature and MedWatch Database. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 540–548. [Google Scholar] [CrossRef]
- Dahmer, S.; Schiller, R.M. Glucosamine. Am. Fam. Physician 2008, 78, 471–476. [Google Scholar]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Saravia, J.; Raynor, J.L.; Chapman, N.M.; Lim, S.A.; Chi, H. Signaling networks in immunometabolism. Cell Res. 2020, 30, 328–342. [Google Scholar] [CrossRef]
- Li, Z.; Fu, B.; Wei, A.; Wu, Y.; Huang, M.; Zhang, E.; Cui, B.; Wang, B.; Peng, H. d-Glucosamine induces circadian phase delay by promoting BMAL1 degradation through AMPK/mTOR pathway. Life Sci. 2023, 325, 121765. [Google Scholar] [CrossRef]
- Coimbra, J.R.; Moreira, P.I.; Santos, A.E.; Salvador, J.A. Therapeutic potential of glutaminyl cyclases: Current status and emerging trends. Drug Discov. Today 2023, 28, 103644. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.H.; Spiess, J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc. Natl. Acad. Sci. 1987, 84, 3628–3632. [Google Scholar] [CrossRef] [PubMed]
- Seifert, F.; Schulz, K.; Koch, B.; Manhart, S.; Demuth, H.-U.; Schilling, S. Glutaminyl Cyclases Display Significant Catalytic Proficiency for Glutamyl Substrates. Biochemistry 2009, 48, 11831–11833. [Google Scholar] [CrossRef] [PubMed]
- Cynis, H.; Scheel, E.; Saido, T.C.; Schilling, S.; Demuth, H.-U. Amyloidogenic Processing of Amyloid Precursor Protein: Evidence of a Pivotal Role of Glutaminyl Cyclase in Generation of Pyroglutamate-Modified Amyloid-β. Biochemistry 2008, 47, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Sun, Y.; Wang, F.; Yu, S.; Chen, X.; Wu, L.-L.; Yang, H.; Shi, Y.; Zhao, K. Deciphering the role of QPCTL in glioma progression and cancer immunotherapy. Front. Immunol. 2023, 14, 1166377. [Google Scholar] [CrossRef]
- da Silva, R.B.; Leitao, R.M.; Pechuan-Jorge, X.; Werneke, S.; Oeh, J.; Javinal, V.; Wang, Y.; Phung, W.; Everett, C.; Nonomiya, J.; et al. Loss of the intracellular enzyme QPCTL limits chemokine function and reshapes myeloid infiltration to augment tumor immunity. Nat. Immunol. 2022, 23, 568–580. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Solmi, M.; Smith, T.O.; Noale, M.; Schofield, P.; Maggi, S. Knee Osteoarthritis and Risk of Hypertension: A Longitudinal Cohort Study. Rejuvenation Res. 2018, 21, 15–21. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Liu, Y.; Liu, Y.-L.; Qi, B.; Yuan, X.; Shi, W.-X.; Miao, L. Osteoarthritis and hypertension: Observational and Mendelian randomization analyses. Arthritis Res. Ther. 2024, 26, 1–11. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Martire, P.; Garofoli, M.; Gentile, G.; Reboldi, G. Treatment strategies for osteoarthritis patients with pain and hypertension. Ther. Adv. Musculoskelet. Dis. 2010, 2, 229–240. [Google Scholar] [CrossRef]
- Singh, G.; Miller, J.D.; Huse, D.M.; Pettitt, D.; D’Agostino, R.B.; Russell, M.W. Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J. Rheumatol. 2003, 30, 714–719. [Google Scholar]
- Ching, K.; Houard, X.; Berenbaum, F.; Wen, C. Hypertension meets osteoarthritis — revisiting the vascular aetiology hypothesis. Nat. Rev. Rheumatol. 2021, 17, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Coca, A.; Force, O.B.O.T.T. Hypertension and cardiac arrhythmias. Eur. Hear. J. 2017, 38, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Au, M.; Wang, X.; Chan, P.-M.B.; Lai, P.; Sun, L.; Zheng, Y.; Rong, L.; Wen, C. Photoacoustic imaging of synovial tissue hypoxia in experimental post-traumatic osteoarthritis. Prog. Biophys. Mol. Biol. 2018, 148, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kiær, T.; Dahl, B.; Lausten, G.S. The relationship between inert gas wash-out and radioactive tracer microspheres in measurement of bone blood flow: Effect of decreased arterial supply and venous congestion on bone blood flow in an animal model. J. Orthop. Res. 1993, 11, 28–35. [Google Scholar] [CrossRef]
- Ferkingstad, E.; Sulem, P.; Atlason, B.A.; Sveinbjornsson, G.; Styrmisdottir, E.L.; Gunnarsdottir, K.; Helgason, A.; Oddsson, A.; Halldorsson, B.V.; Jensson, B.O.; et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 2021, 53, 1712–1721. [Google Scholar] [CrossRef]
- Võsa, U.; Claringbould, A.; Westra, H.-J.; Bonder, M.J.; Deelen, P.; Zeng, B.; Kirsten, H.; Saha, A.; Kreuzhuber, R.; Yazar, S.; et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 2021, 53, 1300–1310. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Hou, Y.; Xiao, Z.; Zhu, Y.; Li, Y.; Liu, Q.; Wang, Z. Blood metabolites and chronic kidney disease: A Mendelian randomization study. BMC Med. Genom. 2024, 17, 1–14. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Smith, G.D.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.D.; Zhou, G.; Lu, Y.; Kolic, J.; Ellis, C.; Johnson, J.D.; Macdonald, P.E.; Xia, J. Web-based multi-omics integration using the Analyst software suite. Nat. Protoc. 2024, 19, 1467–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xia, J. OmicsNet: A web-based tool for creation and visual analysis of biological networks in 3D space. Nucleic Acids Res. 2018, 46, W514–W522. [Google Scholar] [CrossRef] [PubMed]




| Trait | GWAS ID | Year | Sample Size | nSNPs |
|---|---|---|---|---|
| Glucosamine intake | ukb-b-11535 | 2018 | 461,384 | 9,851,867 |
| Calcium intake | ukb-b-7043 | 2018 | 461,384 | 9,851,867 |
| Fish oil intake | ukb-b-11075 | 2018 | 461,384 | 9,851,867 |
| Iron intake | ukb-b-14863 | 2018 | 461,384 | 9,851,867 |
| Selenium intake | ukb-b-19158 | 2018 | 461,384 | 9,851,867 |
| Zinc intake | ukb-b-13891 | 2018 | 461,384 | 9,851,867 |
| Hypertension | ebi-a-GCST90038604 | 2021 | 484,598 | 9,587,836 |
| Vascular/heart problems diagnosed by doctor: high blood pressure | ukb-b-14177 | 2018 | 461,880 | 9,851,867 |
| GNA1 | prot-a-1231 | 2018 | 3301 | 10,534,735 |
| Phosphodiester GlcNAcase | prot-a-1996 | 2018 | 3301 | 10,534,735 |
| HS3ST4 | prot-a-1377 | 2018 | 3301 | 10,534,735 |
| HS3ST3B1 | prot-a-1376 | 2018 | 3301 | 10,534,735 |
| HS3ST3A1 | prot-a-1375 | 2018 | 3301 | 10,534,735 |
| gspK | prot-a-1995 | 2018 | 3301 | 10,534,735 |
| GNS | prot-a-1235 | 2018 | 3301 | 10,534,735 |
| GNPTG | prot-a-1232 | 2018 | 3301 | 10,534,735 |
| GNPDA1 | prot-a-1230 | 2018 | 3301 | 10,534,735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, F.; Sun, Y.; Han, C.-C.; Wei, Z.-L.; Guan, X.; Guo, S.-W.; Quan, S.; Zhou, J.-G.; Pang, R.-P. Plasma Glutaminyl-Peptide Cyclotransferase Mediates Glucosamine-Metabolism-Driven Protection Against Hypertension: A Mendelian Randomization Study. Int. J. Mol. Sci. 2024, 25, 12106. https://doi.org/10.3390/ijms252212106
Ge F, Sun Y, Han C-C, Wei Z-L, Guan X, Guo S-W, Quan S, Zhou J-G, Pang R-P. Plasma Glutaminyl-Peptide Cyclotransferase Mediates Glucosamine-Metabolism-Driven Protection Against Hypertension: A Mendelian Randomization Study. International Journal of Molecular Sciences. 2024; 25(22):12106. https://doi.org/10.3390/ijms252212106
Chicago/Turabian StyleGe, Fei, Yu Sun, Cong-Cong Han, Zi-Liang Wei, Xin Guan, Si-Wan Guo, Shui Quan, Jia-Guo Zhou, and Rui-Ping Pang. 2024. "Plasma Glutaminyl-Peptide Cyclotransferase Mediates Glucosamine-Metabolism-Driven Protection Against Hypertension: A Mendelian Randomization Study" International Journal of Molecular Sciences 25, no. 22: 12106. https://doi.org/10.3390/ijms252212106
APA StyleGe, F., Sun, Y., Han, C.-C., Wei, Z.-L., Guan, X., Guo, S.-W., Quan, S., Zhou, J.-G., & Pang, R.-P. (2024). Plasma Glutaminyl-Peptide Cyclotransferase Mediates Glucosamine-Metabolism-Driven Protection Against Hypertension: A Mendelian Randomization Study. International Journal of Molecular Sciences, 25(22), 12106. https://doi.org/10.3390/ijms252212106





