Abstract
Confining protons into an enclosed carbon cage is expected to give rise to unique electronic properties for both the inner proton and the outer cage. In this work, we systematically investigated the geometric and electronic structures of cationic X+@C60 (X+ = H+, H3O+, and NH4+), and their corresponding neutral species (X = H2O, NH3), by quantum chemical density functional theory calculations. We show that C60 can trap H2O, NH3, H3O+ and NH4+ at the cage center and only slightly influence their geometries. The single proton clings to the inner wall of C60, forming a C-H chemical bond. The encapsulated neutral species almost do not change the electronic structure of the C60, while the internal cations have obvious effects. The charge transfer effect from the inner species to the C60 cage was found for all X@C60 (X = H2O, NH3) (about 0.0 e), X+@C60 (X+ = H3O+, NH4+) (about 0.5 e) and H+@C60 (about 1.0 e) systems. Encapsulating different forms of protons also regulates the fundamental physico-chemical properties of the hollow C60, such as the HOMO-LUMO gaps, infrared spectra, and electrostatic potential, etc., which are discussed in detail. These findings provide a theoretical insight into protons’ applications, especially in energy.
1. Introduction
Protons in aqueous systems play a crucial role in various physical, chemical and biological processes [1,2]. For instance, they are involved in fuel cell membranes [3], ATP synthesis [4], enzymatic reactions in proteins [5], and more. When protons are in confined systems, they exhibit unique electronic, magnetic and optical properties [6,7]. Fullerenes, with hollow inner spaces, can encapsulate a wide variety of species, including atoms, molecules or ions, forming endohedral fullerenes [8,9,10]. They offer a potential platform for studying the behavior of protons in a confined setting. The unique structure of fullerenes not only provides a confined space but also may interact with the protons in ways that could further enhance our understanding of protons’ behavior and their associated properties [11,12,13,14,15]. The inner space of C60, with a diameter of 3.53 Å, is suitable for entrapping a guest species [16,17]. It provides a remarkably simple model system through which to study the properties of species in confinement. As of now, a large number of experimental and theoretical methods have been used to research molecule endohedral fullerenes, such as H2O@C60 [17,18,19,20,21,22,23,24,25,26,27,28,29,30], HF@C60 [26,31,32], H2@C60 [33,34,35,36], etc. These endohedral fullerenes have been synthesized by means of the arc discharge method [37] and molecular surgical method [38,39]. Some theoretical research has been carried out on H+ and NH3 embedded into C60 [40,41,42]. The studies focused on the geometry parameter, interaction energy, charge transfer, and spectra.
The proton, as an elementary particle, differs from all other ions that are stable in aqueous solutions. When introduced into water, a proton does not exist independently but rapidly combines with neighboring water molecules to form a hydronium cation (H3O+) [43]. Protons show the same behavior in ammonia, forming ammonium ion (NH4+) [44]. Moreover, the hydration of H3O+ and NH4+ plays an important role in the physical and natural sciences [45,46]. This is due to hydrogen bonding network formed in aqueous acid–base chemistry. Meanwhile, H3O+ batteries are considered one of the most promising energy technologies as a next-generation power source, thanks to their cost-effectiveness and sustainability [47]. NH4+ and other species react to form the ammonium salt complex species in the natural environment [48]. However, little is known about encapsulation of H3O+ and NH4+ inside C60. Trapping H3O+ and NH4+ in a confined environment provides a unique opportunity to understand their novel properties. Hence, an in-depth study of the geometric and electronic structures of the cationic X+@C60 (X+ = H+, H3O+, and NH4+), as well as their corresponding neutral species (X = H2O, NH3), would certainly be a worthwhile exercise.
In this work, we present the results of first-principles computations to investigate the geometric and electronic structures of cationic X+@C60 (X+ = H+, H3O+, NH4+), as well as their corresponding neutral species (X = H2O, NH3). This paper is organized as follows. First, the computational methodology is described. Then, we present results for the systems, including optimized geometry parameters, interaction energy, charge populations, electron density difference, HOMO-LUMO gaps, infrared spectra, electrostatic potentials, and hydration free energies. Finally, important results are summarized in the conclusions section, which may be useful for furthering sustainable and efficient energy sources.
2. Results
Figure 1 presents the optimized geometries of C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3) at B3LYP-D3(BJ)/6-31G(d, p) at the theoretical level (for coordinates for optimized molecular structure, see Supplementary Materials). The optimized geometry parameters of isolated and confined species are compared to investigate the interaction between the C60 cage and the confined species. The optimized geometry parameters are shown in Table 1. For the neutral species X encapsulated inside C60, species X is located at the center of the cage. The O-H and N-H bond lengths decrease by 0.001 Å and 0.004 Å, respectively, compared to the free molecule. Similar negligible changes have been reported in the literature [7,41,49], indicating that the computational level used in this work is reliable. Additionally, our calculations also show that the interaction between X and C60 is minimal, with almost no perturbation of the C60 geometry. It should be noted that in H+@C60, the H+ is attached to C atom, indicating that the H+ directly interacts with the C atom of C60. The C-H bond length in H+@C60 is 1.130 Å, which is 3.5% longer than that (1.092) of free CH4 due to C-H bond weakening. The H3O+ encapsulated inside fullerene cage shows a slight deviation from the center. The comparison of the H3O+ encapsulated inside C60 and free H3O+ in the gas phase reveals that the H3O+ encapsulated inside C60 has a particular conformation with a longer O-H (0.994 Å) than that of a free H3O+ (0.982 Å), being about 1.2% larger than the O-H bond length, which is consistent with the results of reference [22,40]. The small increase in bond length shows that the attraction of H toward the cage causes the slight stretch. However, for NH4+ encapsulated inside the fullerene cage, NH4+ is located at the center of the cage. The computed N-H bond length of 1.027 Å for the NH4+@C60 is consistent with that of the free NH4+ molecule. This result indicates that the interaction between H3O+ and C60 is stronger than that between NH4+ and C60.
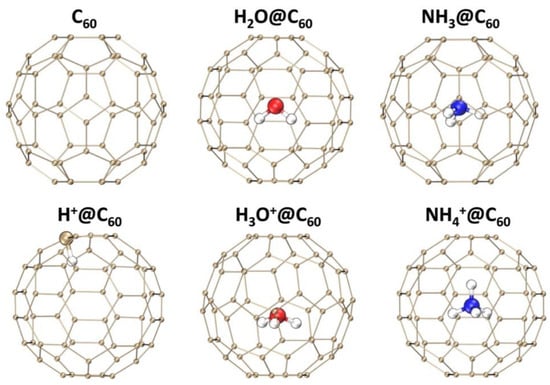
Figure 1.
Structures of C60, X+@C60 (X+ = H+, H3O+, and NH4+) and X@C60 (X = H2O, NH3) at the B3LYP-D3(BJ)/6-31G(d, p) level of theory. C, H, O, and N atoms are indicated by orange, white, red, and blue spheres, respectively.

Table 1.
Parameters optimized at the B3LYP-D3(BJ)/6-31G(d, p) level of theory (unit is Å).
3. Discussion
To generate the stable structure of H+ adsorbs, we previously considered proposed adsorption sites, atoms, bridges, and hollow sites. The optimization procedure of the C60 geometrical structure yielded the 5-6 bonds at the junctions between a five- and a six-membered ring and the 6-6 bonds at the junctions between a six- and a six-membered ring. The lowest-energy adsorption site is the atom site, where a C-H bond is formed. Figure 2 displays the calculated energy profile along pathway from the atom site to the bridge site. In the transition state, the H+ is located on the 5-6 bond and 6-6 bond. We carried out intrinsic reaction coordinate (IRC) calculations in two parts. The corresponding energy barriers are 5.82 kcal/mol and 1.93 kcal/mol, respectively. Our results show that the transition from the atom to the 6-6 bond is much easier than that to the 5-6 bond due to the low energy barrier, suggesting that the pathway proposed here is reasonable.
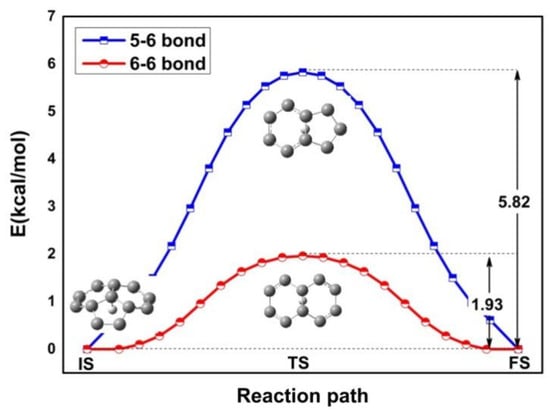
Figure 2.
Energy profile for H diffusion from the atom to the 5-6 bond and 6-6 bond. The atomic geometries of the initial (IS), transition (TS), and final (FS) states are also given. The blue boxes and red circles indicate the 5-6 bond and 6-6 bond of the intrinsic reaction coordinate (IRC), respectively. The diffusion barrier is denoted by an arrow.
Energy decomposition analysis is a powerful method of assessing qualitative and quantitative interaction. Table 2 presents the contributions of electrostatic (∆Eelstat), Pauli (∆EPauli), orbital (∆Eorb), and dispersion (∆Edis) interaction to the total interaction energy (∆Eint). Clearly, for X@C60 (X = H2O, NH3), the terms ∆Eelstat, ∆Eorb and ∆Edisp act as the stabilizing components, while the ∆EPauli term constitutes the destabilization factor for X@C60. Decomposition of this term indicates that the total attractive interaction between the X and C60 is dominated by the ∆Eelstat and ∆Edisp, the covalent term introduced by ∆Eorb. However, for cationic X+@C60 (X+ = H+, H3O+, NH4+), the terms ∆Eorb and ∆Edisp act as the stabilizing components, while the ∆EPauli and ∆Eelstat terms constitute the destabilization factor. In general, the ∆Eelstat term is an attractive interaction. The results in Table 2 reinforce the observation that ∆Eelstat plays the most significant role in connection with the unfavorable nature of X+@C60, as the ∆EPauli is of comparable magnitude to ∆Edisp, but the electrostatic term has a much more destabilizing effect. The ∆Eint results suggest that the interaction between X+ and C60 is strong but significantly weaker in X@C60. The ∆Eorb component of ∆Eint is rather stronger in X+@C60 than in X@C60, and its magnitude is sufficient to compensate for the destabilizing influence of ∆Eelstat and ∆EPauli, which is ultimately responsible for the stability of X+@C60.

Table 2.
Energy decomposition analysis of X@C60 (X = H2O, NH3) and X+@C60 (X+ = H+, H3O+, NH4+) at B3LYP-D3BJ/TZP in kcal/mol.
The electron density difference was calculated to explain the orbital interactions between X+ and C60. Figure 3 displays the electron density difference, with green and blue parts corresponding to regions where the electron density is increased and decreased, respectively. Generally, significant electron polarization between the host and guest leads to a stronger electrostatic interaction. As shown in Figure 3, in X@C60, the C60 cage has almost no polarization of electron density. This result indicates that the interaction between the carbon cage and X is weak. In the X+@C60 system, except for H+, there is electron accumulation localized in the C60 cage, while there is electron depletion on X+. This corresponds to the electron transfer resulting from the interaction between X+ and C60. For the H+@C60 system, it is noteworthy that the H+ shows a very high density of polarized electrons, which verifies the strong binding fore of the C-H bond. Meanwhile, it can be found that there is obvious electron density electron polarization in the H atom of H3O+@C60. The relatively strong electron polarization between the H3O+ and C60 cage suggests the stronger electrostatic interaction between them. However, although the NH4+ is confined in the C60 cage, the cage shows only slight polarization. Our calculation result also indicates that the interaction between NH4+ and C60 molecules is weak. These findings are thoroughly in accordance with previous studies based on energy decomposition analysis.
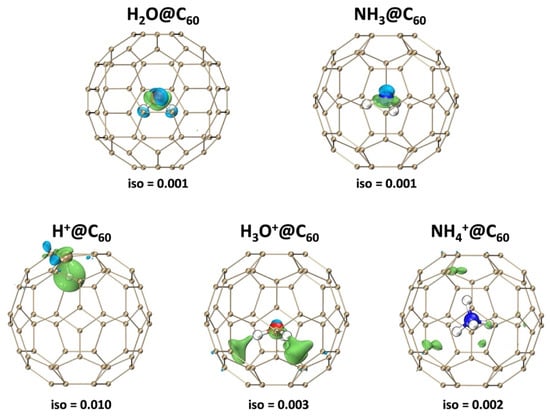
Figure 3.
Electron density difference maps of X/X+@C60 (X+ = H+, H3O+, NH4+, X = H2O, NH3) and C60 cage at the B3LYP-D3(BJ)/6-31G(d, p) level of theory. The green and blue indicate the accumulation and the depletion of the electron density, respectively.
In order to quantitatively estimate the interaction between X/X+ and C60, we performed atomic dipole moment corrected Hirshfeld charge fitting (ADCH), as shown in Table 3. Generally, the ADCH charge is a good charge population analysis method for confined systems [14]. The ADCH charge on X/X+@C60 is higher than that on free X/X+ because the electronegativity of C is greater than that of X/X+. Here, it can be seen that the H2O (O: −0.714 e) and NH3 (N: −0.968 e) possess larger negative charges than H2O@C60 (O: −0.538 e) and NH3@C60 (N: −0.538 e), and H2O (H: 0.357 e) and NH3 (H: 0.323 e) possess larger positive charges than H2O@C60 (H: 0.266 e) and NH3@C60 (H: 0.233 e), indicating the interaction between H2O, NH3 and C60. However, the ADCH charges of H+@C60 (H: 0.053 e) are noticeably smaller than that of free H+(H: 1 e), indicating stronger interaction between H+ and C60. The H3O+ (O: −0.480 e) has a relatively larger negative charge than H3O+@C60 (O: −0.165 e). The charges are less accumulated in the H3O+@ C60, which implies a charge transfer effect from H3O+ to the C60 cage. In addition, the ADCH charges of H (0.493 e) are the same in the H3O+, while the ADCH charges of H are not equal in H3O+@C60. This may be because the H3O+ encapsulated inside the fullerene cage shows a slight deviation from the center. The ADCH charges of NH4+ (N: −0.249 e) possess a larger negative charge than NH4+@C60 (N: −0.134 e). However, The ADCH charges of NH4+ (H: 0.312 e) are the same, and the charges of H are also not equal in NH4+@C60.

Table 3.
The ADCH charge populations of the neutral species X = H2O ad NH3 and the cationic species X+ = H+, H3O+, NH4+, and X/X+@C60 (unit is e).
To gain a deeper insight into the effect of confined species X/X+ on the electronic structure of C60 cage, the density of states (DOS) of C60 and X/X+@C60 are calculated (as shown in Figure 4). In Figure 4, the red and blue lines represent the contributions of two fragments of C60 cage and X/X+ to the DOS. H and L represent the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (HOMO), respectively. The HOMO-LUMO gaps (i.e., the energy difference between HOMO and LUMO levels) of C60 and X/X+@C60 are marked in Figure 4. Compared with the total density of state (TDOS) distributions of C60, the TDOS distributions of X@C60 are basically unchanged. This implies that there is only a slight interaction between X and C60. Moreover, the TDOS is mostly attributed to the contribution from C60, indicating that there are few electrons occupied by embedded species in the molecular orbital. The energy gaps of C60, H2O@C60 and NH3@C60 are 2.77 kcal/mol, 2.75 kcal/mol and 2.74 kcal/mol, respectively. The results reveal that the energy gap of X@C60 is slightly smaller than that of the C60 cage. Therefore, this indicates a weak interaction between X and the C60 cage. This is consistent with the results of previous encapsulated species studies [27,50,51,52]. However, the DOS curves of X+@C60 change significantly, especially for H+@C60 and H3O+@C60 in the TDOS curves. This is attributed to the H atom adsorbed on the C60 cage in H3O+@C60, which is different from other systems. For the H3O+@C60 system, it indicates a strong interaction between the H3O+ and C60. In contrast, the DOS curves of NH4+@C60 show only slight variations, indicating the weak interaction between NH4+ and C60. Meanwhile, the calculated energy gap of H+@C60 is 2.24 kcal/mol, which is the lowest energy gap of H+@C60 among all the systems. This is because H+ is attached to the C atom. In the H+@C60 system, the LUMO orbitals split into two small peaks. This state is a hybrid of C-2p and H-1s orbitals, causing novel peaks in DOS. The energy gaps of H3O+@C60 and NH4+@C60 are 2.75 kcal/mol and 2.79 kcal/mol, respectively. The H3O+@C60 and H2O@C60 have the same energy gap. In contrast, the energy gap of H3O+@C60 is reduced by 0.02 kcal/mol compared to that of NH4+@C60.
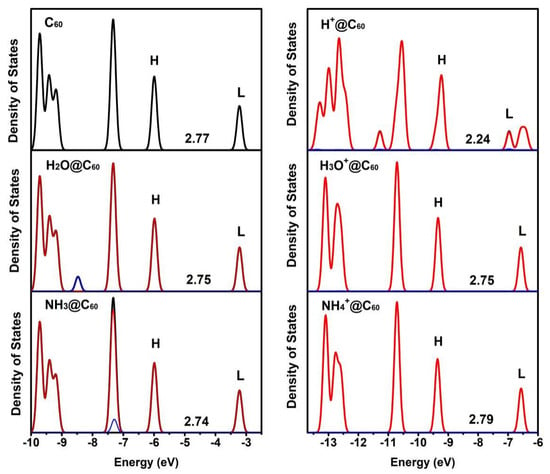
Figure 4.
Total density of states (TDOS) and the partial density of states (PDOS) for C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3) at the B3LYP-D3(BJ)/6-31G(d, p) level of theory. Black represents the TDOS of X/X+@C60. Red and blue lines represent the contribution of two fragments of the C60 cage and X/X+ to the DOS, respectively. H and L represent the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), respectively. The energy gap is marked in the figure (unit is kcal/mol).
To understand the electronic properties, we displayed the frontline molecular orbital diagrams of C60 and X/X+@C60. The HOMO and LUMO orbitals for C60 and X/X+@C60 are plotted in Figure 5. The molecular orbitals involved for the first time by confined species are also depicted in Figure 5. The corresponding frontier molecular orbitals are similar in all these cases. The HOMO iso-surface of C60 is symmetrically distributed on its surface, formed by contribution of π-π bonds and some contribution of σ bonds located on C atoms. The LUMO iso-surface is located on C atoms due to contribution of s electrons, and some π-π bonds are displayed on the rest of its surface as well. For X/X+@C60 systems, the HOMO orbitals are also mainly located on the C60 cage, whereas the LUMO orbitals of X/X+@C60 are located on both confined species and the C60 cage, similar to those reported for other endohedral fullerenes [53,54]. For the H2O@C60 system, the HOMO electronic distributed is on the C60 cage, and the π bonds remain in both parts; the LUMO iso-surface is uniformly distributed on the surface with π-π bonds. The HOMO-32 orbital is mainly constructed by the O-2p orbital, with a very small contribution from the C-2p orbital. For the HOMO frontier orbital associated with the NH3@C60 system, it is concentered on the C60 cage displaying π-π bonds, whereas the LUMO electronic distribution is exhibited on C atoms that come from π-π bonds around the surface, alongside the contribution of p electrons. Whereas for the HOMO-12 orbital, the contribution comes from N-2p and C-2p orbitals in NH3@C60, for the H+@C60 system, the HOMO iso-surface is concentrated on the C60 cage displaying π-π bonds. The electronic distribution of LUMO is located on the C-H bond, whereas π-π bonds are displayed on the surface of the C60 cage. It can be seen that the main contribution in HOMO-13 comes from H-1s and C-2p orbitals. For the H3O+@C60 system, the HOMO iso-surface is concentered on the C60 cage displaying π-π bonds, the electronic distribution of LUMO is located on the bond of O-H and C60 cage, and the HOMO-37 orbital involved in confined species is mainly constructed by the C-2p orbitals and a few H-s orbitals. For NH4+@C60 systems, similar features are shown on the HOMO iso-surface; the electronic distribution of LUMO is located on the C60 cage. The HOMO-37 orbital is involved in confined species and is mainly constructed by the C-2p orbital displaying π-π bonds.
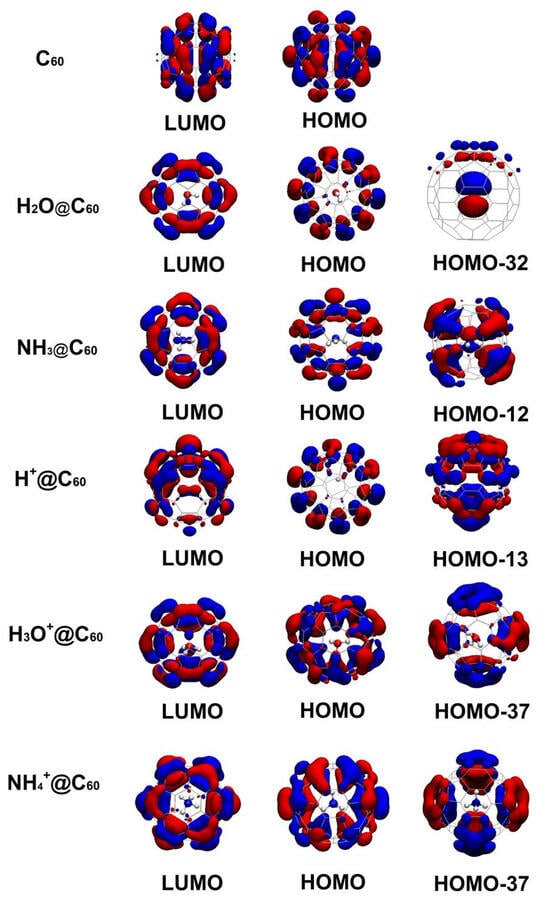
Figure 5.
Frontier molecular orbitals (HOMO and LUMO) for C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3) at the B3LYP-D3(BJ)/6-31G(d, p) level of theory. The molecular orbitals involved for the first time in confined species are also depicted. Molecular orbitals are denoted by blue and red.
The IR spectra of X = H2O, NH3, X+ = H+, H3O+, NH4+, and X/X+@C60 are presented in Figure 6. The vibrational spectra of H2O@C60, H3O+@C60, and NH4+@C60 share many characteristics with spectrum of C60. Figure 6 shows that the spectrum of H2O@C60 closely resembles the spectrum of the pristine C60. In addition, the vibrational features of H2O are very weak in the IR spectrum of H2O@C60, indicating the potential screening effect of the fullerene cage [17]. However, most of the bands in the spectrum of the NH3@C60 look quite similar to that of C60, except for one weak peak observed (marked with a circle in Figure 6). This peak is mainly derived from the N-H wagging mode and is slightly blue-shifted compared to NH3. The IR spectrum of H+@C60 is much more complicated than that of the other embedded systems. This is mainly due to the formation of the C-H bond between the H and C60 cage. The IR spectrum of the H3O+@C60 looks quite similar to that of C60, except for two weak peaks and a strong peak (marked with a square in Figure 6). These peaks are mainly derived from the O-H stretching mode. For the H3O+@C60 system, except for the O-H free stretch mode, all the normal modes of confined H3O+ are slightly red-shifted compared to free H3O+. Interestingly, in the O-H stretching mode, a small peak is observed inside H3O+ (marked with arrows in Figure 6). The peak change due to encapsulation can be attributed to the interaction effect between H3O+ and the C60 cage. Moreover, the NH4+@C60 and C60 spectra show similarity in the whole spectral region, except for the N-H free stretch mode. This result indicates a weak interaction between NH4+ and the C60 cage.
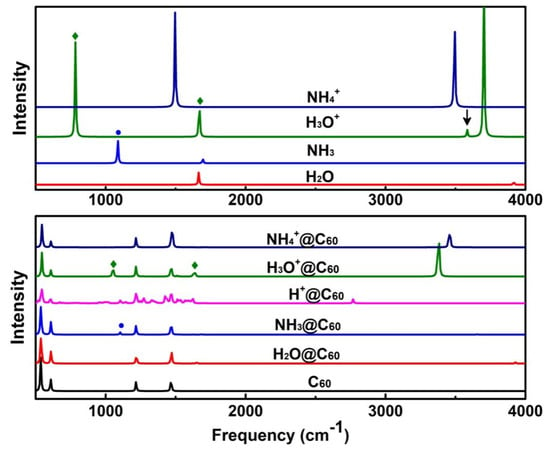
Figure 6.
IR spectra for X = H2O, NH3, X+ = H+, H3O+, NH4+, and X/X+@C60 at the B3LYP-D3(BJ)/6-31G(d, p) level of theory.
Electrostatic potential has been widely used in the study of interactions and has become one of the most common means of analyzing the interaction between molecules. Moreover, the electrostatic potential explains the charge distribution on a molecule and indicates electrophilic positively charged or nucleophilic negatively charged properties. Therefore, we calculated the electrostatic potential of C60 and X/X+@C60. Blue and red colors represent negative potential and positive potential, respectively. As shown in Figure 7, for the electrostatic potential of the C60 cage, the positive electrostatic potential region is mainly distributed in center of the cage, and the surface of the cage is close to neutral. For the electrostatic potential of X@C60, there is no obvious change in the electrostatic potential due to encapsulation of neutral species inside the cage. Encapsulation of X+ induces positive electrostatic potential both inside and outside the C60 cage; it can be seen from the figure that the electrostatic potential distribution of H+@C60 in C60 cage is different from that of H3O+@C60 and NH4+@C60. This is mainly due to the H+ adsorbing on the C atom. For H3O+@C60, the positive electrostatic potential is more distributed on the O atom. This result indicates that there is electron polarization between H3O+ and C60, which is consistent with our previous result. For NH4+@C60, NH4+ is at the center of the cage, and there is a uniform distribution of the electrostatic potential. However, when the guest species is in an off-centered position, the electrostatic potential is more localized inside the cage. This shows the electron polarization of the X+ system compared to the X species located at the center of the cage.
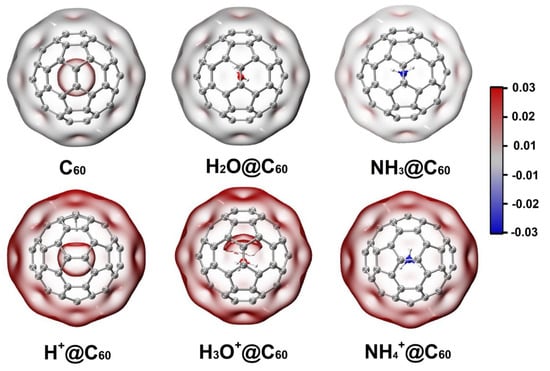
Figure 7.
The electrostatic potential of C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3) at the B3LYP-D3(BJ)/6-31G(d, p) level of theory. Blue and red colors represent negative potential and positive potential, respectively (unit is a.u.).
To explore the effects of H2O on the adsorption behavior, we calculated the structure of H2O adsorbed on the surface of C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3). The lowest-energy structures are shown in Figure 8 (for coordinates of the optimized molecular structure, see Supplementary Materials). For H2O adsorption on neutral species X@C60, the H atoms of H2O point towards the C atoms of the C60 cage. However, when H2O adsorbs on ionic species X+@C60, the O atom of H2O is most likely to point towards the six-membered ring of the C60 cage. This result indicates that O atoms prefer to be located in the region of positive electrostatic potential outside the C60 cage. For H2O adsorbed on H+@C60, the O atom of H2O is close to the C atoms of C60. This is probably due to the H atom being absorbed on the C atom and having the lowest adsorption energy (−9.23 kcal/mol). Furthermore, from Figure 8, we can also learn that the interaction between H2O and X+@C60 is much stronger than that with X@C60, indicating the presence of positive electrostatic potential.
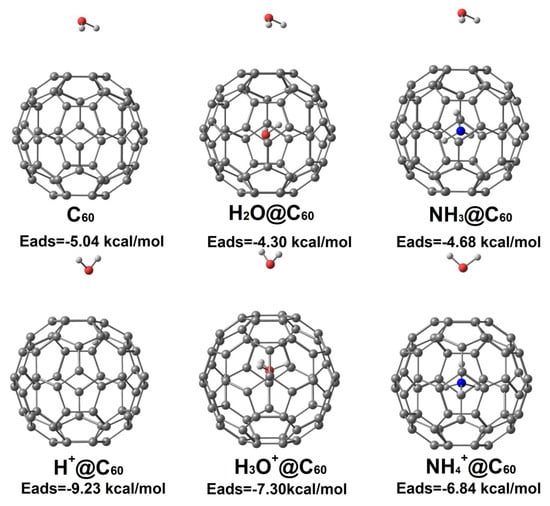
Figure 8.
Optimized water adsorption structures on C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3). In these structures, red, white, cyan, and blue denote O, H, C, and N atoms, respectively.
To explicitly assess the effect of solvent on X/X+@C60 interactions, the IEFPCM (integral equation formalism–PCM model) was calculated (see Figure 9). All the negative solvation energies indicate spontaneous solvation. It can be inferred from the above data that a smaller value of solvation energy leads to improved solubility of the complex in the aqueous phase. Solvation studies also reveal that the solvent medium, i.e., water, stabilizes the complex by decreasing its solvation energy. The solvation energies for C60, H2O@C60 and NH3@C60 are −8.08 kcal/mol, −14.26 kcal/mol, and −8.51 kcal/mol, respectively. It is observed that the solvation energy of C60 is slightly higher than the calculated solvation energy of NH3@C60. Among the neutral species, the solvation energy of H2O@C60 is the lowest, indicating that H2O@C60 is stable in the aqueous phase. However, the solvation energies for H+@C60, H3O+@C60 and NH4+@C60 are −51.42 kcal/mol, −63.94 kcal/mol, and −58.02 kcal/mol, respectively. H3O+@C60 has the lowest solvation energy, followed by NH4+@C60. H+@C60 has a relatively lower solvation energy, suggesting that H+@C60 is stable in the aqueous phase. This is consistent with the results from the adsorption energy analysis.
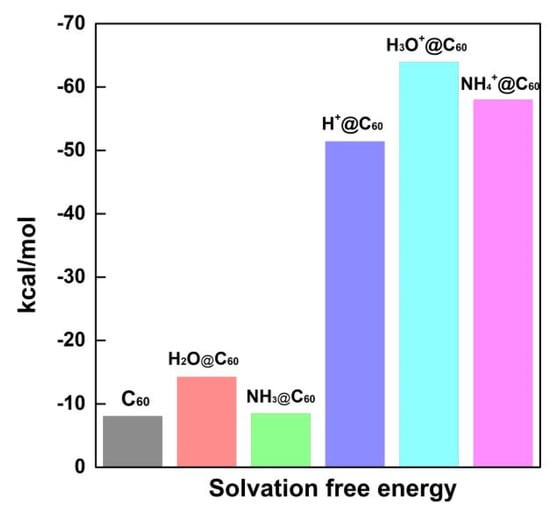
Figure 9.
The solvation afree energy of C60, X+@C60 (X+ = H+, H3O+, NH4+), and X@C60 (X = H2O, NH3) (unit is kcal/mol).
4. Materials and Methods
In this work, the cationic X+@C60 (X+ = H+, H3O+, and NH4+), as well as their corresponding neutral species (X = H2O, NH3), have been studied using the hybrid generalized gradient approximation (hybrid GGA) B3LYP [55,56] function. Grimme’s empirical dispersion correction with Beck-Johnson damping (D3BJ) was used to consider weak interactions. The 6-31G(d, p) was used to describe all the atoms. In this case, all atoms were allowed to relax. To ensure we obtained the minimum energy structures, vibrational frequency verifications were also performed at the same level of theory. All the structures were assessed with the Gaussian 09 package [57]. The energy decomposition analysis was performed using the B3LYP-D3BJ and TZP basis sets in ADF 2016 [58]. Multiwfn 3.8 [59] programs were used to obtain the electronic structure data.
The interaction energy can be decomposed into electroatatic interaction , repulsive exchange (Pauli) interaction , orbital interaction and dispersion interaction .
where is the conventional electrostatic interaction between two molecules. is the repulsive Pauli interaction between the occupied orbitals of two different molecules. stands for the stabilizing interactions between the occupied molecular orbitals in one molecule and the unoccupied molecular orbitals in the other molecule along with the interaction of occupied and virtual orbitals within the same molecule in the final geometry. represents the dispersion forces.
To obtain a deep insight into the nature of the charge transformation, we calculated the electron density difference between X/X+ and the C60 cage. This can be exactly calculated as
where , , and represent the electron density of X/X+@C60, C60, and X/X+, respectively.
The adsorption energy () is an important factor in measuring the adsorption capacity of a structure. When molecule A is adsorbed on surface B, the calculation formula of adsorption energy is as follows:
where is the total energy after H2O adsorption, and and are the total energy of adsorbate and H2O, respectively. A negative value indicates a favorable exothermic adsorption.
The integral equation formalism-PCM model (IEFPCM) was applied [60]. Being an essential part of the living system, water was chosen as a solvent with which to study the solubility and stability of the complex. The stability and solubility of X/X+@C60 can be calculated using the following expression:
where represents system’s total solvation energy, while and stand for the energy in the solvent phase and gas phase, respectively. All the negative solvation energies indicate a spontaneous solvation.
5. Conclusions
In this study, we performed a systematic theoretical investigation on the geometric and electronic structures of cationic X+@C60 (X+ = H+, H3O+, and NH4+) as well as their corresponding neutral species (X = H2O, NH3). A deeper understanding of cationic X+@C60 was obtained. Our results revealed that the confined species almost did not change the geometric structure of the C60 cage, except for H+. For the H+@C60 system, the H+ was attached to C atom in H+@C60, forming a C-H chemical bond. Meanwhile, we found that orbital interaction plays a significant role between the cationic X+ species and the C60 cage. Electronic structure analysis showed that the electron polarization of X+@C60 was stronger than that of X@C60. This has important implications for energy applications. For instance, the unique electronic properties of these systems could potentially be harnessed in the development of advanced energy storage materials. The strong electron polarization in X+@C60 could lead to more efficient charge transfer and storage capabilities. Additionally, understanding the interactions between protons and the C60 cage could contribute to the design of novel fuel cells or batteries that utilize proton conduction. The encapsulated protons could offer a stable and controlled environment for energy conversion processes. We hope that this work will pave the way for further development of proton applications in the future, especially in the field of energy, where innovative solutions are desperately needed to meet the growing demand for sustainable and efficient energy sources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms252212014/s1.
Author Contributions
Conceptualization, L.Z. and B.W.; methodology, B.W.; software, L.Z.; validation, L.Z. and B.W.; formal analysis, L.Z.; investigation, L.Z.; resources, B.W.; writing—original draft preparation, L.Z.; writing—review and editing, B.W.; visualization, L.Z.; supervision, B.W.; project administration, B.W.; funding acquisition, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (11747151, 11847153), the Outstanding Young Talents Fund Project of Jilin Province (20180520172JH), and the Project of Education Department of Jilin Province (JJKH20180426KJ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.M.J.; Maupin, C.M.; Chen, H.N.; Petersen, M.K.; Xu, J.C.; Wu, Y.J.; Voth, G.A. Proton solvation and transport in aqueous and biomolecular systems: Insights from computer simulations. J. Phys. Chem. B 2007, 111, 4300–4314. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed]
- Stowell, M.H.B.; McPhillips, T.M.; Rees, D.C.; Soltis, S.M.; Abresch, E.; Feher, G. Light-induced structural changes in photosynthetic reaction center: Implications for mechanism of electron-proton transfer. Science 1997, 276, 812–816. [Google Scholar] [CrossRef]
- Sham, Y.Y.; Muegge, I.; Warshel, A. Simulating proton translocations in proteins: Probing proton transfer pathways in the Rhodobacter sphaeroides reaction center. Proteins Struct. Funct. Bioinform. 1999, 36, 484–500. [Google Scholar] [CrossRef]
- Turro, N.J.; Chen, J.Y.C.; Sartori, E.; Ruzzi, M.; Marti, A.; Lawler, R.; Jockusch, S.; López-Gejo, J.; Komatsu, K.; Murata, Y. The spin chemistry and magnetic resonance of H2@C60. From the pauli principle to trapping a long lived nuclear excited spin state inside a buckyball. Acc. Chem. Res. 2010, 43, 335–345. [Google Scholar] [CrossRef]
- Ramachandran, C.N.; Sathyamurthy, N. Water clusters in a confined nonpolar environment. Chem. Phys. Lett. 2005, 410, 348–351. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Sokolov, V.I. Activation energies and information entropies of helium penetration through fullerene walls. Insights into the formation of endofullerenes nX@C60/70 (n= 1 and 2) from the information entropy approach. RSC Adv. 2016, 6, 72230–72237. [Google Scholar] [CrossRef]
- Dolgonos, G.A.; Peslherbe, G.H. Encapsulation of diatomic molecules in fullerene C60: Implications for their main properties. Phys. Chem. Chem. Phys. 2014, 16, 26294–26305. [Google Scholar] [CrossRef]
- Equbal, A.; Srinivasan, S.; Ramachandran, C.N.; Sathyamurthy, N. Encapsulation of paramagnetic diatomic molecules B2, O2 and Ge2 inside C60. Chem. Phys. Lett. 2014, 610, 251–255. [Google Scholar] [CrossRef]
- Umeta, Y.; Suga, H.; Takeuchi, M.; Zheng, S.; Wakahara, T.; Wang, Y.C.; Naitoh, Y.; Lu, X.; Kumatani, A.; Tsukagoshi, K. Stable resistance switching in Lu3N@C80 nanowires promoted by the endohedral effect: Implications for eingle-fullerene motion resistance switching. ACS Appl. Nano Mater. 2021, 4, 7935–7942. [Google Scholar] [CrossRef]
- Kawashima, Y.; Ohkubo, K.; Fukuzumi, S. Enhanced photoinduced electron-transfer reduction of Li+@C60 in comparison with C60. J. Phys. Chem. A 2012, 116, 8942–8948. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, A.; Hedin, N. Local energy decomposition analysis and molecular properties of encapsulated methane in fullerene (CH4@C60). Phys. Chem. Chem. Phys. 2021, 23, 21554–21567. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Dai, X.; Zhou, H.; Yang, Z.; Zhou, R. Stabilization of open-shell single bonds within endohedral metallofullerene. Inorg. Chem. 2020, 59, 3606–3618. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Molecular structure, electronic properties and stability of carbon-coated M13@C60 (M= Cu, Ag, Pt) nanoclusters. Comput. Theor. Chem. 2021, 1205, 113434. [Google Scholar] [CrossRef]
- Mallick, S.; Mishra, B.K.; Kumar, P.; Sathyamurthy, N. Effect of confinement on ammonia inversion. Eue. Phys. J. D 2021, 75, 1–10. [Google Scholar] [CrossRef]
- Kurotobi, K.; Murata, Y. A single molecule of water encapsulated in fullerene C60. Science 2011, 333, 613–616. [Google Scholar] [CrossRef]
- Ramachandran, C.N.; Roy, D.; Sathyamurthy, N. Host–guest interaction in endohedral fullerenes. Chem. Phys. Lett. 2008, 46, 87–92. [Google Scholar] [CrossRef]
- Thilgen, C. A single water molecule trapped inside hydrophobic C60. Angew. Chem. Int. Ed. 2012, 51, 587–589. [Google Scholar] [CrossRef]
- Ensing, B.; Costanzo, F.; Silvestrelli, P.L. On the polarity of buckminsterfullerene with a water molecule inside. J. Phys. Chem. A 2012, 116, 12184–12188. [Google Scholar] [CrossRef]
- Sabirov, D.S. From endohedral complexes to endohedral fullerene covalent derivatives: A density functional theory prognosis of chemical transformation of water endofullerene H2O@C60 upon its compression. J. Phys. Chem. C 2013, 117, 1178–1182. [Google Scholar] [CrossRef]
- Farimani, A.B.; Wu, Y.; Aluru, N.R. Rotational motion of a single water molecule in a buckyball. Phys. Chem. Chem. Phys. 2013, 15, 17993–18000. [Google Scholar] [CrossRef]
- Aoyagi, S.; Hoshino, N.; Akutagawa, T.; Sado, Y.; Kitaura, R.; Shinohara, H.; Sugimoto, K.; Zhang, R.; Murata, Y. A cubic dipole lattice of water molecules trapped inside carbon cages. Chem. Commun. 2013, 50, 524–526. [Google Scholar] [CrossRef]
- Kaneko, S.; Hashikawa, Y.; Fujii, S.; Murata, Y.; Kiguchi, M. Single molecular junction study on H2O@C60: H2O is “electrostatically isolated”. Chem. Phys. Chem. 2017, 18, 1229–1233. [Google Scholar] [CrossRef]
- Rashed, E.; Dunn, J.L. Interactions between a water molecule and C60 in the endohedral fullerene H2O@C60. Phys. Chem. Chem. Phys. 2019, 21, 3347–3359. [Google Scholar] [CrossRef]
- Biskupek, J.; Skowron, S.T.; Stoppiello, C.T.; Rance, G.A.; Alom, S.; Fung, K.L.; Fung, Y.; Whitby, R.J.; Levitt, M.H.; Ramasse, Q.M.; et al. Bond dissociation and reactivity of HF and H2O in a nano test tube. ACS Nano 2020, 14, 11178–11189. [Google Scholar] [CrossRef]
- Fujii, S.; Cho, H.; Hashikawa, Y.; Nishino, T.; Murata, Y.; Kiguchi, M. Tuneable single-molecule electronic conductance of C60 by encapsulation. Phys. Chem. Chem. Phys. 2019, 21, 12606–12610. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Murata, Y. H2O/Olefinic-π interaction inside a carbon nanocage. J. Am. Chem. Soc. 2019, 141, 12928–12938. [Google Scholar] [CrossRef]
- Jarvis, S.P.; Sang, H.; Junqueira, F.; Gordon, O.; Hodgkinson, J.E.; Saywell, A.; Rahe, P.; Mamone, S.; Taylor, S.; Sweetman, A.; et al. Chemical shielding of H2O and HF encapsulated inside a C60 cage. Commun. Chem. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Valdés, Á.; Carrillo-Bohórquez, O.; Prosmiti, R. Fully coupled quantum treatment of nanoconfined systems: A water molecule inside a fullerene C60. J. Chem. Theory Comput. 2018, 14, 6521–6531. [Google Scholar] [CrossRef]
- Krachmalnicoff, A.; Bounds, R.; Mamone, S.; Alom, S.; Concistre, M.; Meier, B.; Kouril, K.; Light, M.E.; Johnson, M.R.; Rols, S.; et al. The dipolar endofullerene HF@ C60. Nat. Chem. 2016, 8, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Izquierdo, M.; Alom, S.; Garcia-Borràs, M.; Filippone, S.; Osuna, S.; Sola, M.; Whitby, R.J.; Martín, N. Effect of incarcerated HF on the exohedral chemical reactivity of HF@C60. Chem. Commun. 2017, 53, 10993–10996. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Murata, M.; Murata, Y. Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 2005, 307, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.B.; McKee, M.L. Endohedral hydrogen exchange reactions in C60 (nH2@C60, n=1−5): Comparison of recent methods in a high-pressure Cooker. J. Am. Chem. Soc. 2008, 130, 17610–17619. [Google Scholar] [CrossRef] [PubMed]
- Korona, T.; Hesselmann, A.; Dodziuk, H. Symmetry-adapted perturbation theory applied to endohedral fullerene complexes: A stability study of H2@C60 and 2H2@C60. J. Chem. Theory Comput. 2009, 5, 1585–1596. [Google Scholar] [CrossRef]
- Zeinalinezhad, A.; Sahnoun, R. Encapsulation of hydrogen molecules in C50 fullerene: An ab initio study of structural, energetic, and electronic properties of H2@C50 and 2H2@C50 complexes. ACS Omega 2020, 5, 12853–12864. [Google Scholar] [CrossRef]
- Kratschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–357. [Google Scholar] [CrossRef]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.O.; Jasty, M.; Harris, W.H. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties: Recipient of the 1999 HAP paul award. J. Arthroplast. 2001, 16, 149–160. [Google Scholar] [CrossRef]
- Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 46, 6083–6094. [Google Scholar] [CrossRef]
- Shameema, O.; Ramachandran, C.N.; Sathyamurthy, N. Blue shift in X−H stretching frequency of molecules due to confinement. J. Phys. Chem. A 2006, 110, 2–4. [Google Scholar] [CrossRef]
- Pizzagalli, L. First principles molecular dynamics calculations of the mechanical properties of endofullerenes containing noble gas atoms or small molecules. Phys. Chem. Chem. Phys. 2022, 24, 9449–9458. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, A.; Nomura, K.I.; Baradwaj, N.; Shimamur, K.; Ma, R.; Fukushima, S.; Shimojo, F.; Kalia, R.K.; Nakano, A.; Vashishta, P. Hydrogen bonding in liquid ammonia. J. Phys. Chem. Lett. 2022, 13, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Brodskaya, E.; Lyubartsev, A.P.; Laaksonen, A. Investigation of water clusters containing OH− and H3O+ ions in atmospheric conditions. A molecular dynamics simulation study. J. Phys. Chem. B 2002, 106, 6479–6487. [Google Scholar] [CrossRef]
- Datka, J.; Góra-Marek, K. IR studies of the formation of ammonia dimers in zeolites TON. Catal. Today 2006, 114, 205–210. [Google Scholar] [CrossRef]
- Tuckerman, M.; Laasonen, K.; Sprik, M.; Parrinello, M. Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxyl ions in water. J. Chem. Phys. 1995, 103, 150–161. [Google Scholar] [CrossRef]
- Spanel, P.; Spesyvyi, A.; Smith, D. Electrostatic switching and selection of H3O+, NO+, and O2+• reagent ions for selected ion flow-drift tube mass spectrometric analyses of air and breath. Anal. Chem. 2019, 91, 5380–5388. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Dong, X.; Xi, Y.; Yan, L.; Wang, Z.; Wang, Y. An organic/inorganic electrode-based hydronium-ion battery. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Ma, F.; Li, Z.R.; Xu, H.L.; Li, Z.J.; Wu, D.; Li, Z.S.; Gu, F.L. Proton Transfer in the complex H3N…HCl catalyzed by encapsulation into a C60 Cage. Chem. Phys. Chem. 2009, 10, 1112–1116. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R. Can a single molecule of water be completely isolated within the subnano-space inside the fullerene C60 cage? A quantum chemical prospective. Chem. Eur. J. 2012, 18, 15345–15360. [Google Scholar] [CrossRef]
- Wang, L.; Ye, J.T.; Wang, H.Q.; Xie, H.M.; Qiu, Y.Q. Third-order nonlinear optical properties of endohedral fullerene (H2)2@C70 and (H2O)2@C70 accompanied by the prospective of novel (HF)2@C70. J. Phys. Chem. C 2018, 122, 6835–6845. [Google Scholar] [CrossRef]
- Schwedhelm, R.; Kipp, L.; Dallmeyer, A.; Skibowski, M. Experimental band gap and core-hole electron interaction in epitaxial C60 films. Phys. Rev. B 1998, 58, 13176–13180. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Long, Z.; Chen, D. Structures, stabilities, and spectral properties of endohedral borospherenes M@B400/–(M=H2, HF, and H2O). ACS Omega 2019, 4, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Liu, A.; Hao, Y.; Feng, L.; Slanina, Z.; Uhlik, F. Ho2O@C84: Crystallographic evidence showing linear metallic oxide cluster encapsulated in IPR fullerene cage of D2d(51591)-C84. Inorg. Chem. 2019, 58, 10905–10911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lei, D.; Gan, L.H.; Zhang, Z.X.; Wang, C.R. Theoretical study on experimentally detected Sc2S@C84. Chem. Phys. Chem. 2014, 15, 2780–2784. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision and C. D.01; Gaussian: Wallingford, CT, USA, 2013. [Google Scholar]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; Van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).