Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium
Abstract
1. Introduction
2. Hedgehog Protein Maturation
3. Receptors That Bind Hh and Modulate Its Signals
4. Intracellular Hh Signalling
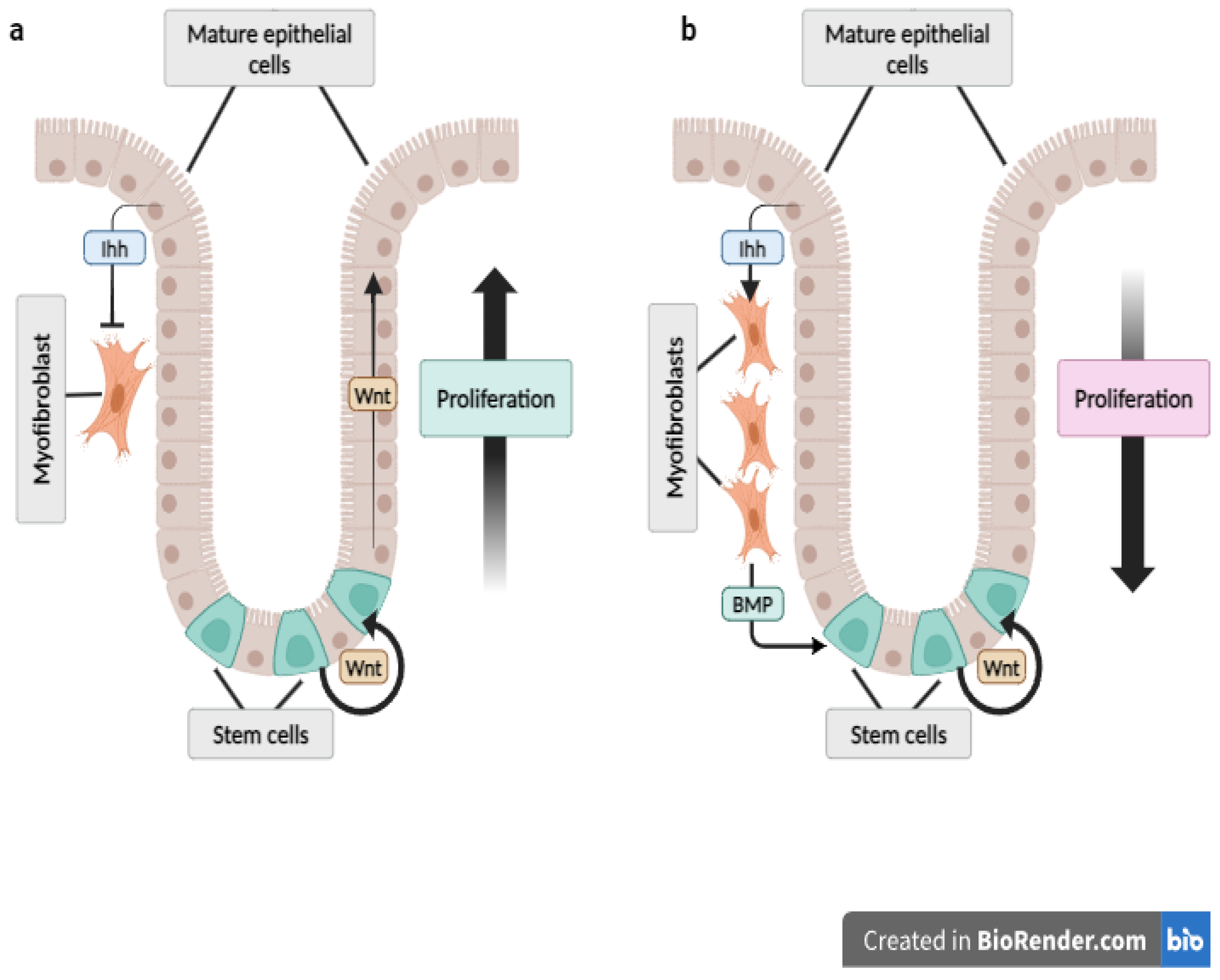
5. The Hh Pathway in the Digestive Tract
6. Nutrition and Hh Signalling
6.1. Fat
6.2. Vitamin A
6.3. Berberine
6.4. Ovotransferrin
6.5. Haem
6.6. Intestinal Microbiota
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Barszcz, M.; Skomiał, J. The development of the small intestine of piglets-chosen aspects. J. Anim. Feed Sci. 2011, 20, 3–15. [Google Scholar] [CrossRef]
- Halas, D.; Hansen, C.F.; Hampson, D.J.; Mullan, B.P.; Kim, J.C.; Wilson, R.H.; Pluske, J.R. Dietary supplementation with benzoic acid improves apparent ileal digestibility of total nitrogen and increases villous height and caecal microbial diversity in weaner pigs. Anim. Feed Sci. Technol. 2010, 160, 137–147. [Google Scholar] [CrossRef]
- Liu, P.; Pieper, R.; Rieger, J.; Vahjen, W.; Davin, R.; Plendl, J.; Meyer, W.; Zentek, J. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS ONE 2014, 9, e91091. [Google Scholar] [CrossRef]
- Święch, E.; Barszcz, M.; Tuśnio, A.; Taciak, M. Gut morphology of young pigs fed diets differing in standardized ileal digestible threonine and wheat gluten used as a source of non-essential amino acids. J. Anim. Feed Sci. 2016, 25, 226–234. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Święch, E.; Skomiał, J. Dose-dependent effects of two inulin types differing in chain length on the small intestinal morphology, contractility and proinflammatory cytokine gene expression in piglets. Arch. Anim. Nutr. 2020, 74, 107–120. [Google Scholar] [CrossRef]
- Tuśnio, A.; Barszcz, M.; Święch, E.; Skomiał, J.; Taciak, M. Large intestine morphology and microflora activity in piglets fed diets with two levels of raw or micronized blue sweet lupin seeds. Livest. Sci. 2020, 240, 104137. [Google Scholar] [CrossRef]
- Barszcz, M.; Gawin, K.; Tuśnio, A.; Konopka, A.; Święch, E.; Taciak, M.; Skomiał, J.; Tokarčiková, K.; Čobanová, K.; Grešáková, L. Comparison between organic and inorganic zinc forms and their combinations with various dietary fibers in respect of the effects on electrolyte concentrations and mucosa in the large intestine of pigs. Int. J. Mol. Sci. 2023, 24, 16743. [Google Scholar] [CrossRef]
- Cho, S.; Cai, L.; Kiarie, E.; Kim, I.H. Effect of multi-enzyme supplementation on growth performance, digestibility, blood profile, intestinal villus height, and faecal gas emission in weaning pigs. J. Anim. Feed Sci. 2024, 33, 211–216. [Google Scholar] [CrossRef]
- Czerwiński, J.; Słupecka-Ziemilska, M.; Woliński, J.; Barszcz, M.; Konieczka, P.; Smulikowska, S. The use of genetically modified Roundup Ready soyabean meal and genetically modified MON 810 maize in broiler chicken diets. Part 2. Functional status of the small intestine. J. Anim. Feed Sci. 2015, 24, 144–152. [Google Scholar] [CrossRef]
- Konieczka, P.; Barszcz, M.; Choct, M.; Smulikowska, S. The interactive effect of dietary n-6: N-3 fatty acid ratio and vitamin E level on tissue lipid peroxidation, DNA damage in intestinal epithelial cells, and gut morphology in chickens of different ages. Poult. Sci. 2018, 97, 149–158. [Google Scholar] [CrossRef]
- Wan, X.L.; Zheng, X.C.; Liang, J.R.; Xiao, X.; Yang, H.M.; Wang, Z.Y. Dietary vitamin A supplementation improves intestinal morphology and immune performance of goslings. J. Anim. Feed Sci. 2022, 31, 217–223. [Google Scholar] [CrossRef]
- Hanim, C.; Dono, N.D.; Ariyadi, B.; Habibi, M.F.; Al Anas, M.; Hanif, M.F. Effect of protected sodium butyrate on growth performance, carcass traits, relative weight of digestive organs and intestinal histomorphology of broilers. J. Anim. Feed Sci. 2023, 32, 413–419. [Google Scholar] [CrossRef]
- Ju, Y.; Huang, L.L.; Li, L.Y.; Zhao, C.G.; Huang, X.H.; Ye, J.Q. Agaricus blazei Murrill stipe promotes growth by improving anti-inflammatory activity and gut function in broilers. J. Anim. Feed Sci. 2024, 33, 64–75. [Google Scholar] [CrossRef]
- McCullough, J.; Ratcliffe, B.; Mandir, N.; Carr, K.; Goodlad, R. Dietary fibre and intestinal microflora: Effects on intestinal morphometry and crypt branching. Gut 1998, 42, 799–806. [Google Scholar] [CrossRef]
- de Vogel, J.; Jonker-Termont, D.S.M.L.; van Lieshout, E.M.M.; Katan, M.B.; van der Meer, R. Green vegetables, red meat and colon cancer: Chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis 2005, 26, 387–393. [Google Scholar] [CrossRef]
- Murakoshi, S.; Fukatsu, K.; Omata, J.; Moriya, T.; Noguchi, M.; Saitoh, D.; Koyama, I. Effects of adding butyric acid to PN on gut-associated lymphoid tissue and mucosal immunoglobulin. J. Parenter. Enteral. Nutr. 2011, 35, 465–472. [Google Scholar] [CrossRef]
- Taciak, M.; Barszcz, M.; Tuśnio, A.; Pastuszewska, B. Interactive effects of indigestible carbohydrates, protein type, and protein level on biomarkers of large intestine health in rats. PLoS ONE 2015, 10, e0142176. [Google Scholar] [CrossRef]
- Todorov, H.; Kollar, B.; Bayer, F.; Brandão, I.; Mann, A.; Mohr, J.; Pontarollo, G.; Formes, H.; Stauber, R.; Nockher, W.A.; et al. α-Linolenic acid-rich diet influences microbiota composition and villus morphology of the mouse small intestine. Nutrients 2020, 12, 732. [Google Scholar] [CrossRef]
- Barszcz, M.; Tuśnio, A.; Bachanek-Matusiewicz, I.; Gawin, K.; Skomiał, J.; Taciak, M. Growth performance, biochemical blood indices, and large intestine physiology of rats fed diets with alfalfa protein-xanthophyll concentrate. Animals 2021, 12, 2069. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Kamaszewski, M.; Grochowski, P.; Verri, T.; Rzepkowska, M.; Wolnicki, J. The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 15, 158–169. [Google Scholar] [CrossRef]
- Wiszniewski, G.; Jarmołowicz, S.; Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Łuczyńska, J.; Tońska, E.; Terech-Majewska, E.; Ostaszewska, T.; Kamaszewski, M.; et al. The use of bromelain as a feed additive in fish diets: Growth performance, intestinal morphology, digestive enzyme and immune response of juvenile Sterlet (Acipenser ruthenus). Aquac. Nutr. 2019, 25, 1289–1299. [Google Scholar] [CrossRef]
- Nephale, L.E.; Moyo, N.A.G.; Rapatsa-Malatji, M.M. Partial replacement of fish meal with soldier termite in juvenile Mozambique tilapia: Effects on growth performance, blood serum chemistry and histomorphology. J. Anim. Feed Sci. 2024, 33, 243–252. [Google Scholar] [CrossRef]
- Porter, E.M.; Bevins, C.L.; Ghosh, D.; Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life. Sci. 2002, 59, 156–170. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef]
- Heretsch, P.; Tzagkaroulaki, L.; Giannis, A. Modulators of the hedgehog signaling pathway. Bioorg. Med. Chem. 2010, 15, 6613–6624. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Cross, S.S.; Bury, J.P. The Hedgehog signalling pathways in human pathology. Curr. Diagn. Pathol. 2004, 10, 157–168. [Google Scholar] [CrossRef]
- Wolpert, L. One hundred years of positional information. Trends Genet. 1996, 12, 359–364. [Google Scholar] [CrossRef]
- Lee, J.J.; Ekker, S.C.; Von Kessler, D.P.; Porter, J.A.; Sun, B.I.; Beach, P.A. Autoproteolysis in hedgehog protein biogenesis. Science 1994, 266, 1528–1537. [Google Scholar] [CrossRef]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol modification of hedgehog signaling proteins in animal development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef]
- Mann, R.K.; Beachy, P.A. Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 2004, 73, 891–923. [Google Scholar] [CrossRef]
- Burke, R.; Nellen, D.; Bellotto, M.; Hafen, E.; Senti, K.A.; Dickson, B.J.; Basler, K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified Hedgehog from signaling cells. Cell 1999, 99, 803–815. [Google Scholar] [CrossRef]
- Goodrich, L.V.; Johnson, R.L.; Milenkovic, L.; McMahon, J.A.; Scott, M.P. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by hedgehog. Genes Dev. 1996, 10, 301–312. [Google Scholar] [CrossRef]
- Motoyama, J.; Takabatake, T.; Takeshima, K.; Hui, C. Ptch2, a second mouse Patched gene is co-expressed with Sonic hedgehog. Nat. Genet. 1998, 18, 104–106. [Google Scholar] [CrossRef]
- Taipale, J.; Cooper, M.K.; Maiti, T.; Beachy, P.A. Patched acts catalytically to suppress the activity of Smoothened. Nature 2002, 418, 892–896. [Google Scholar] [CrossRef]
- Denef, N.; Neubüser, D.; Perez, L.; Cohen, S.M. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 2000, 102, 521–531. [Google Scholar] [CrossRef]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018–1021. [Google Scholar] [CrossRef]
- Chuang, P.T.; McMahon, A.P. Vertebrate Hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature 1999, 397, 617–621. [Google Scholar] [CrossRef]
- Yao, S.; Lum, L.; Beachy, P. The ihog cell-surface proteins bind hedgehog and mediate pathway activation. Cell 2006, 125, 343–357. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, J.S.; Cole, F.; Yi, M.J.; Krauss, R.S. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell 2006, 10, 657–665. [Google Scholar] [CrossRef]
- Kang, J.S.; Mulieri, P.J.; Hu, Y.; Taliana, L.; Krauss, R.S. BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 2002, 21, 114–124. [Google Scholar] [CrossRef]
- Hui, C.C.; Slusarski, D.; Platt, K.A.; Holmgren, R.; Joyner, A.L. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 1994, 162, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.; Stone, D.M.; Dowd, M.; Pitts-Meek, S.; Goddard, A.; Gurney, A.; Rosenthal, A. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron 1997, 19, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Regl, G.; Neill, G.W.; Eichberger, T.; Kasper, M.; Ikram, M.S.; Koller, J.; Hintner, H.; Quinn, A.G.; Frischaufv, A.M.; Aberger, F. Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene 2002, 21, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Bai, C.B.; Joyner, A.L.; Wang, B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol. Cell. Biol. 2006, 26, 3365–3377. [Google Scholar] [CrossRef]
- Wang, B.; Fallon, J.F.; Beachy, P.A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 2000, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, O.V.; Golemis, E.A.; Pugacheva, E.N. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008, 68, 2058–2061. [Google Scholar] [CrossRef]
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signaling center during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Gordon, J.I.; Hermiston, M.L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr. Opin. Cell Biol. 1994, 6, 795–803. [Google Scholar] [CrossRef]
- Madison, B.B.; Braunstein, K.; Kuizon, E.; Portman, K.; Qiao, X.T.; Gumucio, D.L. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 2005, 132, 279–289. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, G.R.; Hardwick, J.C.; Nielsen, C.; Xu, C.; ten Kate, F.J.; Glickman, J.; van Deventer, S.J.H.; Roberts, D.J.; Peppelenbosch, M.P. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 2002, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, G.R. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol. Rev. 2007, 87, 1343–1375. [Google Scholar] [CrossRef]
- Gagné-Sansfaçon, J.; Allaire, J.M.; Jones, C.; Boudreau, F.; Perreault, N. Loss of Sonic Hedgehog leads to alterations in intestinal secretory cell maturation and autophagy. PLoS ONE 2014, 9, 98751. [Google Scholar] [CrossRef]
- Vanat, F.; Bordier-Ten Heggeler, B.; Grisel, P.; Boucard, N.; Corthésy-Theulaz, I.; Wahli, W.; Desvergne, B. PPARβ/δ regulates Paneth cell differentiation via controlling the hedgehog signalling pathway. Gastroenterology 2006, 131, 538–553. [Google Scholar] [CrossRef]
- van den Brink, G.R.; Hardwick, J.C.; Tytgat, G.N.; Brink, M.A.; Ten Kate, F.J.; van Deventer, S.J.; Peppelenbosch, M.P. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology 2001, 121, 317–328. [Google Scholar] [CrossRef]
- Chen, J.K.; Taipale, J.; Cooper, M.K.; Beachy, P.A. Inhibition of hedgehog signaling by direct binding of cyclopamine to smoothened. Genes Dev. 2002, 16, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Nassir, F.; Liu, Z.Y.; Ling, L.; Kuo, F.; Crowell, T.; Olson, D.; Davidson, N.O.; Burkly, L. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology 2002, 122, 469–482. [Google Scholar] [CrossRef]
- van Dop, W.A.; Heijmans, J.; Büller, N.V.J.A.; Snoek, S.A.; Rosekrans, S.L.; Wassenberg, E.A.; van den Bergh Weerman, M.A.; Lanske, B.; Clarke, A.R.; Winton, D.J.; et al. Loss of Indian hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology 2010, 139, 1665–1676. [Google Scholar] [CrossRef]
- Ramalho-Santos, M.; Melton, D.A.; McMahon, A.P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 2000, 127, 2763–2772. [Google Scholar] [CrossRef]
- Sukegawa, A.; Narita, T.; Kameda, T.; Saitoh, K.; Nohno, T.; Iba, H.; Yasugi, S.; Fukuda, K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 2000, 127, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Lui, V.C.; Sham, M.H.; Pachnis, V.; Tam, P.K. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J. Cell Biol. 2004, 166, 673–684. [Google Scholar] [CrossRef]
- Zorn, A.M.; Wells, J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 221–251. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, G.R.; Bleuming, S.A.; Hardwick, J.C.; Schepman, B.L.; Offerhaus, G.J.; Keller, J.J.; Nielsen, C.; Gaffield, W.; van Deventer, S.J.H.; Roberts, D.J.; et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004, 36, 277–282. [Google Scholar] [CrossRef]
- van Dop, W.A.; Uhmann, A.; Wijgerde, M.; Sleddens-Linkels, E.; Heijmans, J.; Offerhaus, G.J. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology 2009, 136, 2195–2203. [Google Scholar] [CrossRef]
- Rodenfels, J.; Lavrynenko, O.; Ayciriex, S.; Sampaio, J.L.; Carvalho, M.; Shevchenko, A.; Eaton, S. Production of systematically circulating hedgehog by the intestine couples nutrition to growth and development. Genes Dev. 2014, 28, 2636–2651. [Google Scholar] [CrossRef] [PubMed]
- Buhman, K.K.; Wang, L.C.; Tang, Y.; Swietlicki, E.A.; Kennedy, S.; Xie, Y.; Liu, Z.Y.; Burkly, L.C.; Levin, M.S.; Rubin, D.C.; et al. Inhibition of hedgehog signaling protects adult mice from diet-induced weight gain. J. Nutr. 2004, 134, 2979–2984. [Google Scholar] [CrossRef]
- Beena, T.B.; Jesil, M.A.; Harikumar, K.B. Cross-talk between AMP-activated protein kinase and the sonic hedgehog pathway in the high-fat diet triggered colorectal cancer. Arch. Biochem. Biophys. 2023, 735, 109500. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Rubin, D.C.; Levin, M.S. Chronically administered retinoic acid has trophic effects in the rat small intestine and promotes adaptation in a resection model of short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1559–G1569. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Yang, H.; Liu, M.; Ren, S.; Zhao, H.; Ming, T.; Tang, S.; Tao, Q.; Chen, L.; Zeng, S.; et al. Berberine suppresses colorectal cancer by regulation of hedgehog signaling pathway activity and gut microbiota. Phytomedicine 2022, 103, 154227. [Google Scholar] [CrossRef]
- Dehau, T.; Cherlet, M.; Croubels, S.; van Immerseel, F.; Goossens, E. A high dose of dietary berberine improves gut wall morphology, despite an expansion of Enterobacteriaceae and a reduction in beneficial microbiota in broiler chickens. mSystems 2023, 8, e01239-22. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yu, Z.; Li, Z.; Liu, H.; Li, W.; Zhao, J.J.; Ren, Y.; Ma, L. Dietary berberine and ellagic acid supplementation improve growth performance and intestinal damage by regulating the structural function of gut microbiota and SCFAs in weaned piglets. Microorganisms 2023, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Bazrafkan, M.; Ismail, P.; Ling, K.H. Molecular modelling of berberine derivatives as inhibitors of human smoothened receptor and hedgehog signalling pathway using a newly developed algorithm on anti-cancer drugs. Anticancer Drug Discov. 2017, 12, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta 2012, 1820, 218–225. [Google Scholar] [CrossRef]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, W.; Yang, L.; Lu, M.; Dong, S.; Liu, X.; Duan, X. Multi-omics analysis of the effects of egg ovotransferrin on the gut environment in mice: Mucosal gene expression, microbiota composition, and intestinal structural homeostasis. Mol. Nutr. Food Res. 2020, 64, 1901024. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Rijnierse, A.; de Wit, N.; Jonker-Termont, D.; Dekker, J.; Müller, M.; van der Meer, R. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut 2012, 61, 1041–1049. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.; Smidt, H.; Verstegen, M.W.A.; Mosenthin, E. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intestinal. Microbiol. 2006, 7, 35–52. [Google Scholar]
- Pontarollo, G.; Kollar, B.; Mann, A.; Khuu, M.P.; Kiouptsi, K.; Bayer, F.; Brandão, I.; Zinina, V.V.; Hahlbrock, J.; Malinarich, F.; et al. Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling. Nat. Metab. 2023, 5, 1174–1187. [Google Scholar] [CrossRef]
- Rapozo, D.C.M.; Bernardazzi, C.; Pereira de Souza, H.S. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124–2140. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; Savoy de Giori, G.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.H.; Schiapparelli, P.; Afzal, M.; Sinha, S.; Rey, J.A.; Castresana, J.S.l. Expression and epigenetic modulation of sonic hedgehog—GLI1 pathway genes in neuroblastoma cell lines and tumors. Tumor Biol. 2011, 32, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Deng, H.; Zhao, L.; Li, J.; Zhou, Y.; Zhang, Y. Distinct expression patterns of hedgehog ligands between cultured and primary colorectal cancers are associated with aberrant methylation of their promoters. Mol. Cell Biochem. 2010, 337, 185–192. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, A.; Gawin, K.; Barszcz, M. Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium. Int. J. Mol. Sci. 2024, 25, 12007. https://doi.org/10.3390/ijms252212007
Konopka A, Gawin K, Barszcz M. Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium. International Journal of Molecular Sciences. 2024; 25(22):12007. https://doi.org/10.3390/ijms252212007
Chicago/Turabian StyleKonopka, Adrianna, Kamil Gawin, and Marcin Barszcz. 2024. "Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium" International Journal of Molecular Sciences 25, no. 22: 12007. https://doi.org/10.3390/ijms252212007
APA StyleKonopka, A., Gawin, K., & Barszcz, M. (2024). Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium. International Journal of Molecular Sciences, 25(22), 12007. https://doi.org/10.3390/ijms252212007




