Genome-Wide Analysis of the Serine Carboxypeptidase-like (SCPL) Protein Family of Bitter Gourd and Functional Validation of McSCPL22 in Fusarium oxysporum f. sp. Momordicae (FOM) Resistance
Abstract
1. Introduction
2. Results
2.1. Identification of the Bitter Gourd SCPL Gene Family
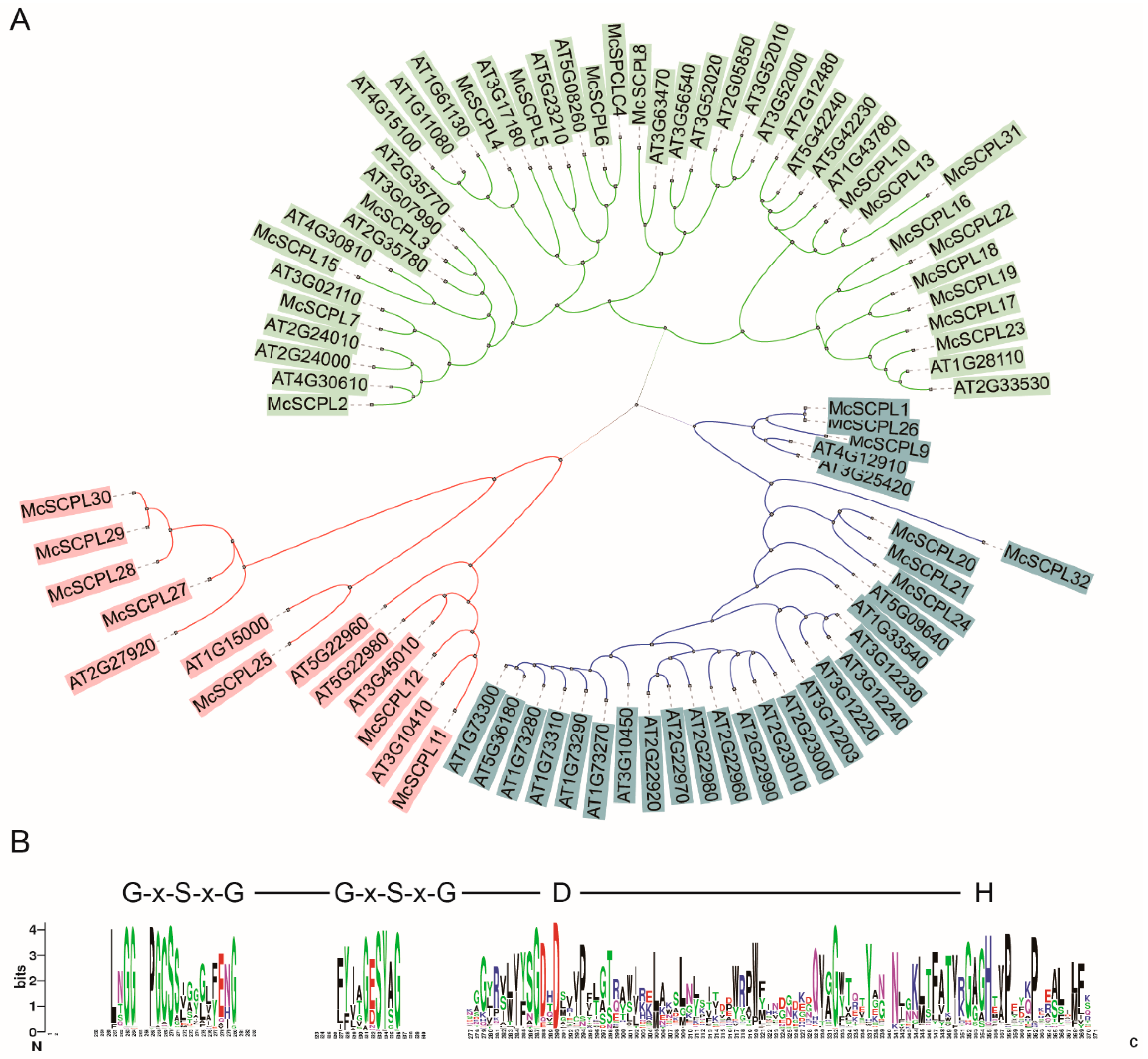
2.2. Evolutionary Relationship of SCPL Genes
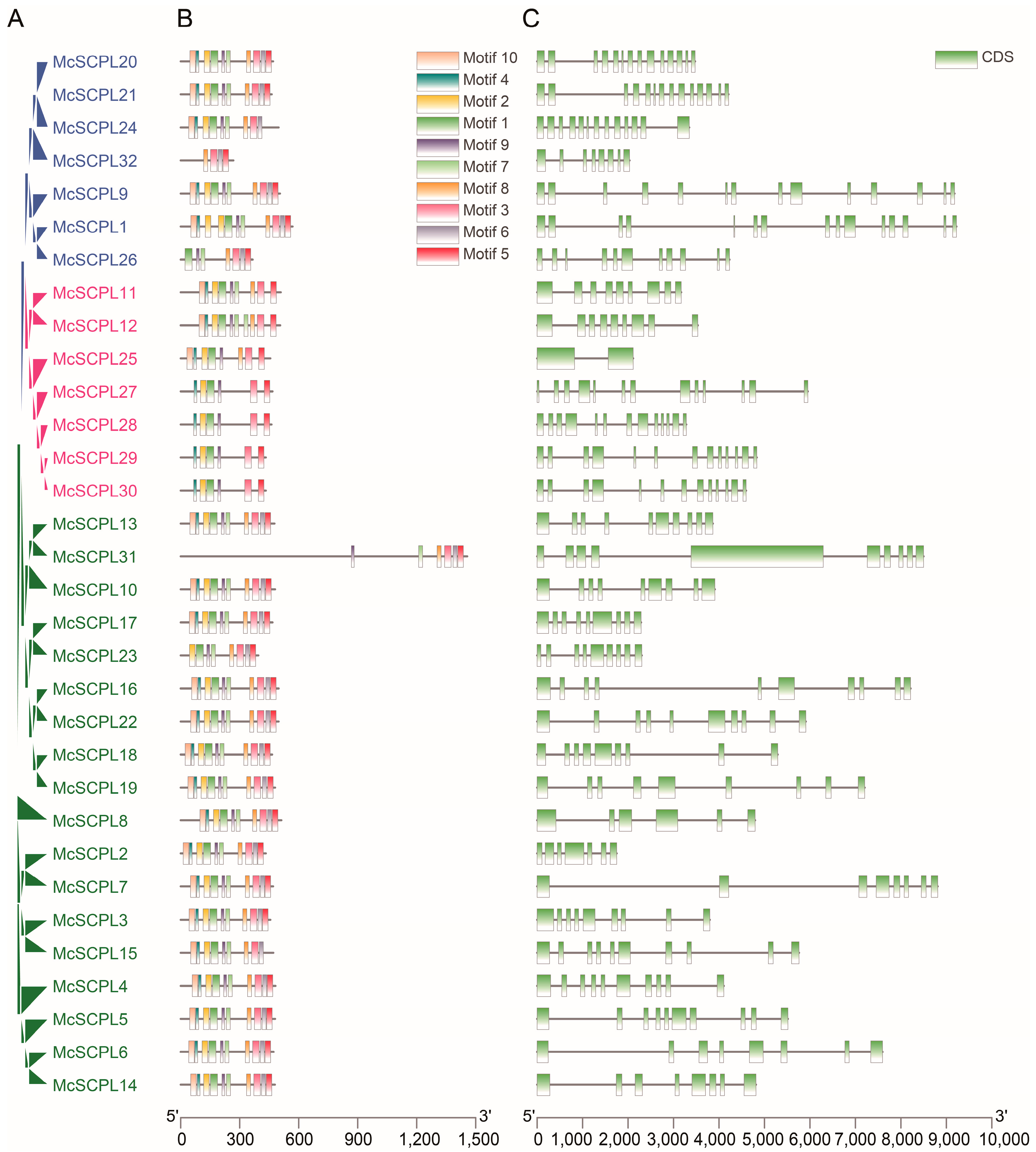
2.3. Evolutionary Tree, Sequence Structure Analysis of SCPL Genes
2.4. The Cis-Acting Elements of the SCPL Genes Promoter
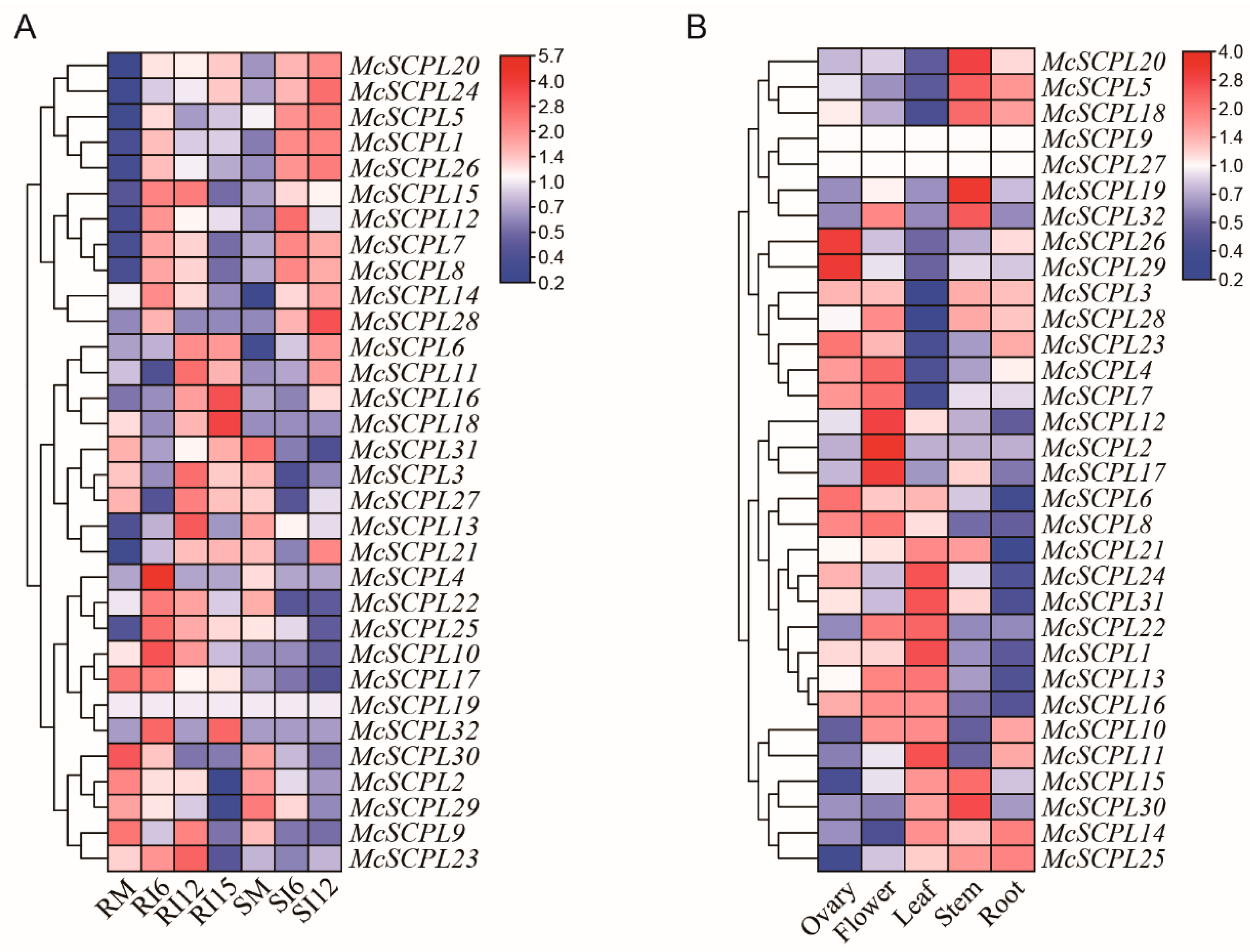
2.5. Tissue-Specific Expression Profiles of SCPL Genes
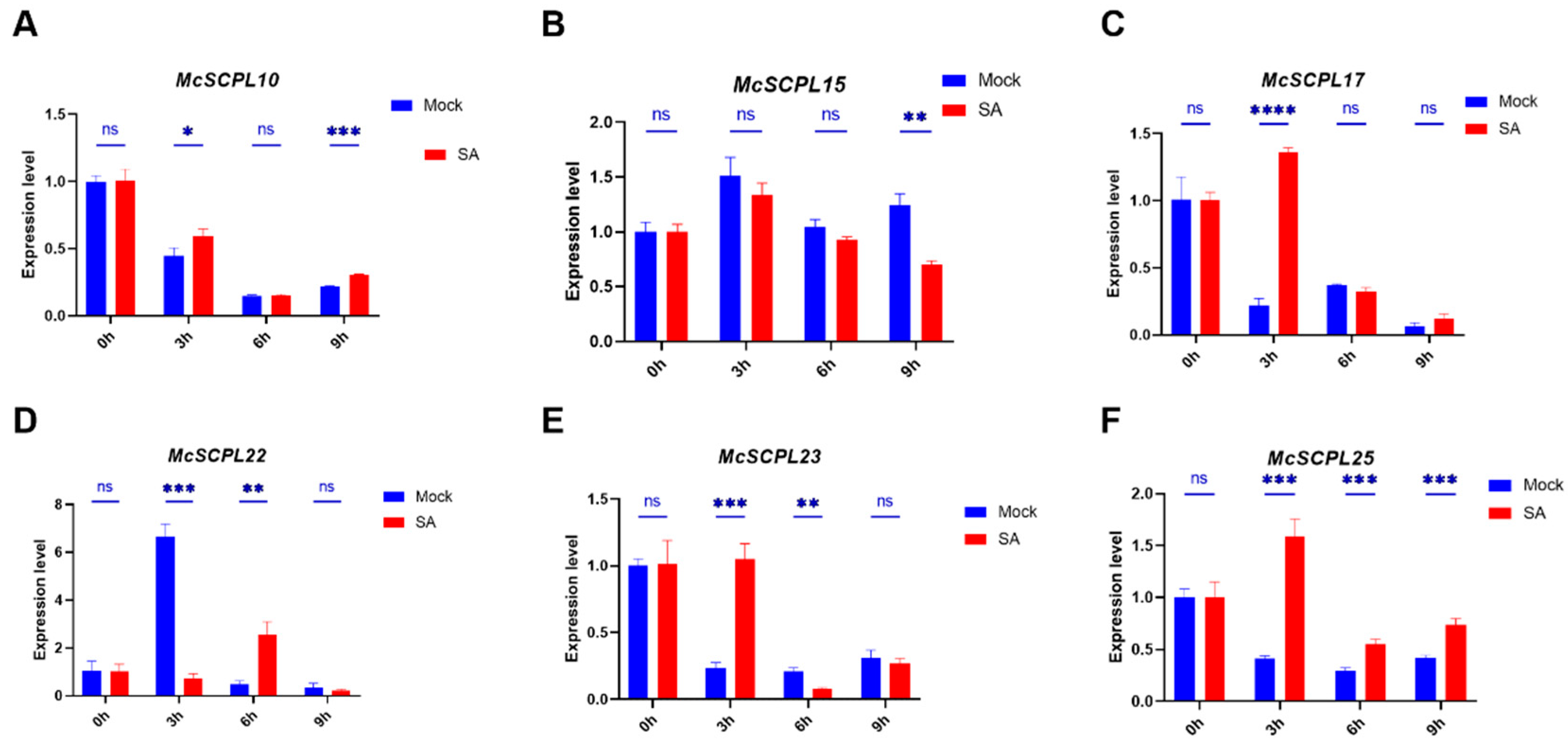
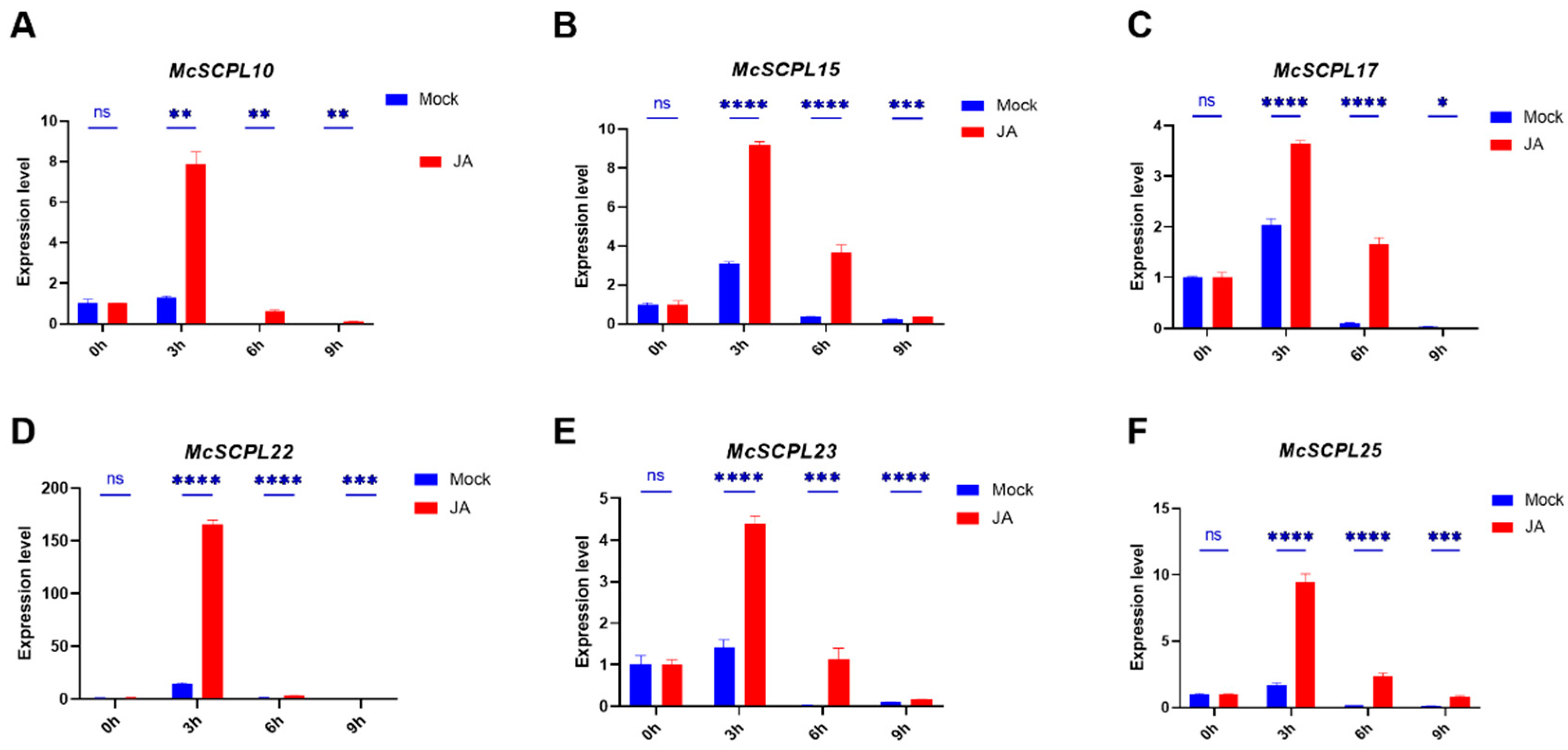
2.6. Expression Analysis of McSCPLs Response to SA and JA Treatment
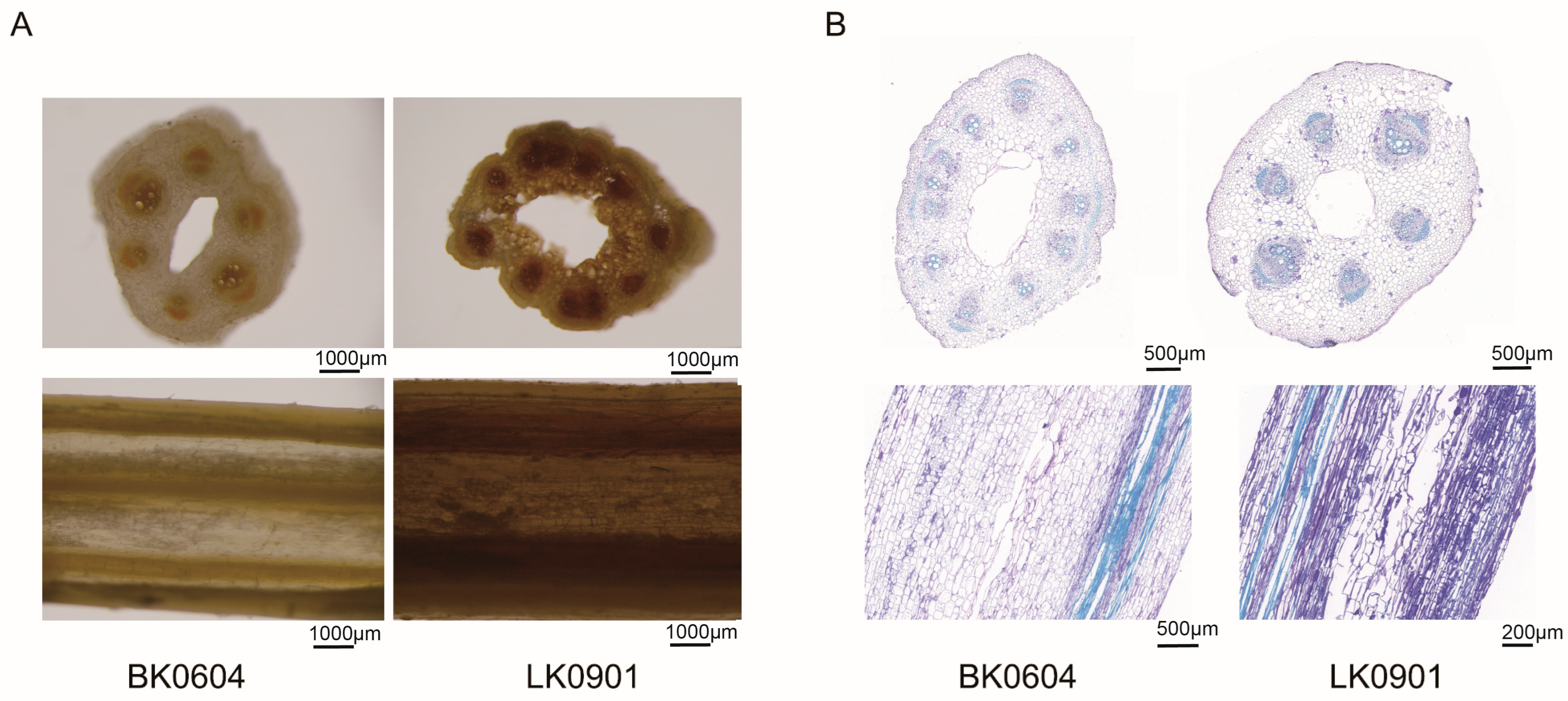
2.7. FOM Infection Promoted the Accumulation of Hydrogen Peroxide
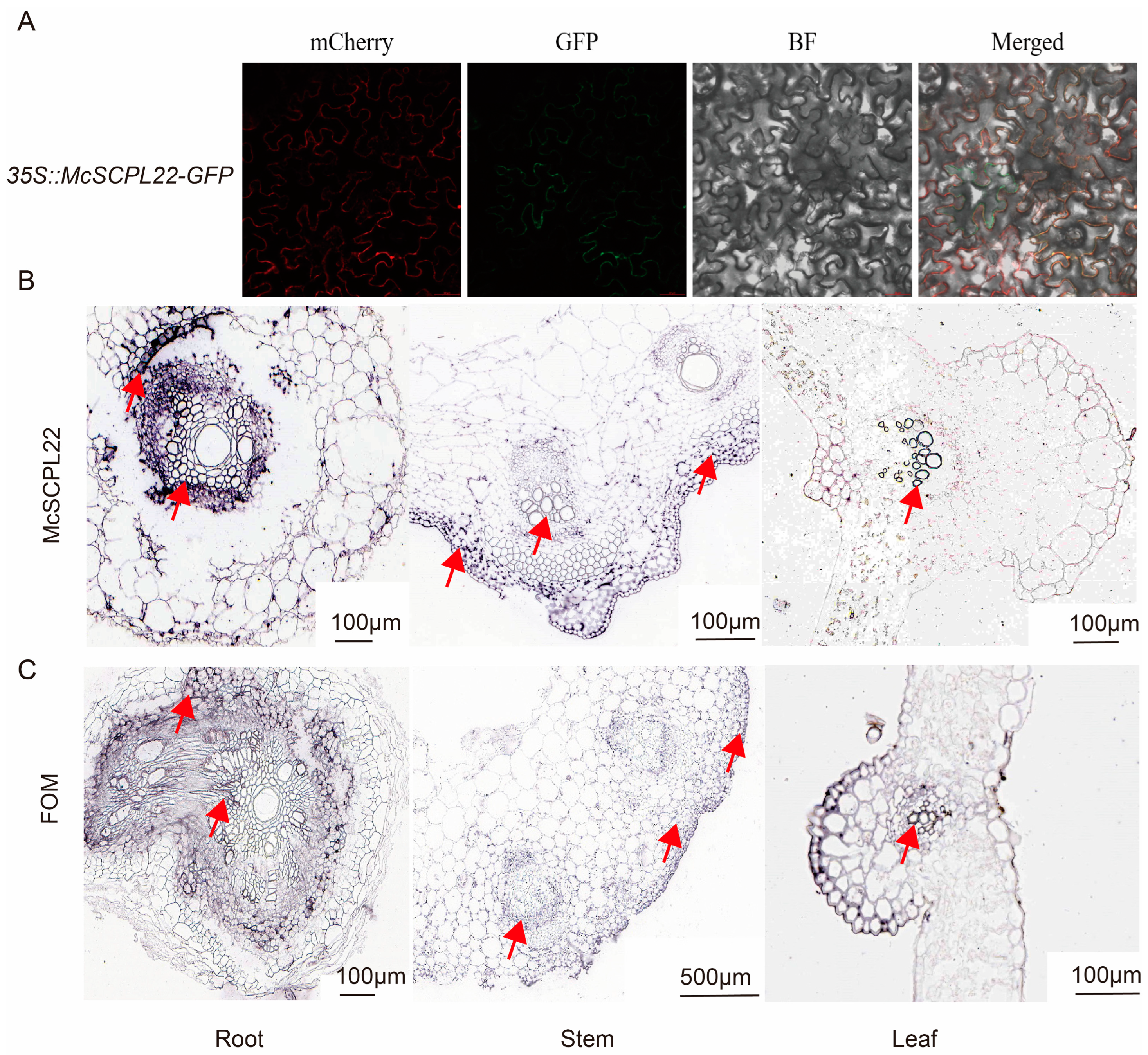
2.8. McSCPL22 May Respond to FOM Infection
3. Discussion
4. Methods
4.1. Plant Material
4.2. Identification of SCPL Genes
4.3. Phylogenetic Analysis of the SCPL Gene Family
4.4. Structure Analysis of the SCPL Gene Family
4.5. Expression Pattern of SCPL Genes Based on RNA Sequencing Data
4.6. Fungal Infection Assay and Treatment with SA and JA
4.7. Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Subcellular Localization of the McSCPL22 Protein
4.9. Analysis of Hydrogen Peroxide Accumulation
4.10. Toluidine Blue Staining
4.11. In Situ Hybridization Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Behera, T.K.; Behera, S.; Bharathi, L.K.; John, K.J.; Staub, J.E. Bitter Gourd: Botany, Horticulture, Breeding; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2015, 32, 181–202. [Google Scholar] [CrossRef]
- Dhillon, M.K.; Singh, R.; Naresh, J.S.; Sharma, H.C. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. J. Insect Sci. (Online) 2005, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Hou, Y.Y.; Peng, C.Y.; Wang, Y.Y.; He, B.L.; Gao, K.X. Genetic diversity and phylogenetic analysis of Fusarium oxysporium strains isolated from the Cucurbitaceae hosts revealed by SRAPs. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2017, 28, 947–956. [Google Scholar]
- Li, X.F.; Tian, Y.H.; Peng, H.Y.; He, B.L.; Gao, K.X. Isolation, screening and identification of anantagonistic actinomycetes to control Fusarium wilt of Momordica charantia. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2020, 31, 3869–3879. [Google Scholar]
- Guan, F.; Shi, B.; Zhang, J.; Wan, X. Transcriptome analysis provides insights into lignin synthesis and MAPK signaling pathway that strengthen the resistance of bitter gourd (Momordica charantia) to Fusarium wilt. Genomics 2023, 115, 110538. [Google Scholar] [CrossRef]
- Guan, F.; Shi, B.; Zhang, J.; Wan, X. Metabolome Revealed the Potential Mechanism of Fusarium Wilt Resistance in Bitter Gourd (Momordica charantia) Based on Liquid Chromatography with Mass Spectrometry. Plant Dis. 2024, 108, 920–929. [Google Scholar] [CrossRef]
- Milkowski, C.; Strack, D. Serine carboxypeptidase-like acyltransferases. Phytochemistry 2004, 65, 517–524. [Google Scholar] [CrossRef]
- Mugford, S.T.; Milkowski, C. Serine carboxypeptidase-like acyltransferases from plants. Nat. Prod. Biosynth. Microorg. Plants Pt B 2012, 516, 279–297. [Google Scholar]
- Agarwal, V.; Tikhonov, A.; Metlitskaya, A.; Severinov, K.; Nair, S.K. Structure and function of a serine carboxypeptidase adapted for degradation of the protein synthesis antibiotic microcin C7. Proc. Natl. Acad. Sci. USA 2012, 109, 4425–4430. [Google Scholar] [CrossRef]
- Ciarkowska, A.; Ostrowski, M.; Starzynska, E.; Jakubowska, A. Plant SCPL acyltransferases: Multiplicity of enzymes with various functions in secondary metabolism. Phytochem. Rev. 2019, 18, 303–316. [Google Scholar] [CrossRef]
- Remington, S.J. Serine carboxypeptidases: A new and versatile family of enzymes. Curr. Opin. Biotechnol. 1993, 4, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Zhang, H.; Yang, Y.; Ge, X.; Song, F. A rice serine carboxypeptidase-like gene OsBISCPL1 is involved in regulation of defense responses against biotic and oxidative stress. Gene 2008, 420, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mugford, S.T.; Qi, X.; Bakht, S.; Hill, L.; Wegel, E.; Hughes, R.K.; Papadopoulou, K.; Melton, R.; Philo, M.; Sainsbury, F.; et al. A serine carboxypeptidase-like acyltransferase is required for synthesis of antimicrobial compounds and disease resistance in oats. Plant Cell 2009, 21, 2473–2484. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.N.; Han, S.J. Genome-wide analysis of serine carboxypeptidase-like genes in soybean and their roles in stress resistance. Int. J. Mol. Sci. 2024, 25, 6712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Deng, X.; Wang, P.; Geng, S.; Gao, W.; Guo, P.; Chen, Q.; Li, C.; Qu, Y. Genome-wide analysis of serine carboxypeptidase-like protein (SCPL) family and functional validation of Gh_SCPL42 unchromosome conferring cotton Verticillium der Verticillium wilt stress in Gossypium hirsutum. BMC Plant Biol. 2022, 22, 421. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Q.P.; Jia, J.; Xue, Q.Z. Genomic analysis of serine carboxypeptidase-like protein family of Arabidopsis thaliana. Yi Chuan Xue Bao Acta Genet. Sin. 2005, 32, 864–873. [Google Scholar]
- Fraser, C.M.; Rider, L.W.; Chapple, C. An expression and bioinformatics analysis of the Arabidopsis serine carboxypeptidase-like gene family. Plant Physiol. 2005, 138, 1136–1148. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, Q. The serine carboxypeptidase like gene family of rice (Oryza sativa L. ssp. japonica). Funct. Integr. Genom. 2006, 6, 14–24. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Chu, W.Y.; Wang, Y.J.; Yan, H.W.; Chen, Z.; Xiang, Y. Genome-wide identification, classification and expression analysis of the serine carboxypeptidase-like protein family in poplar. Physiol. Plant. 2018, 162, 333–352. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Li, P.; She, G.; Xia, E.; Benedito, V.A.; Wan, X.C.; Zhao, J. Genome-wide analysis of serine carboxypeptidase-like acyltransferase gene family for evolution and characterization of enzymes involved in the biosynthesis of galloylated catechins in the tea plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Zhang, L.L.; Zhao, W.; Fu, L.; Han, Y.X.; Wang, K.K.; Yan, L.Y.; Li, Y.; Zhang, X.-H.; Min, D.-H. Genome-wide analysis of the serine carboxypeptidase-like protein family in Triticum aestivum reveals TaSCPL184-6D is involved in abiotic stress response. BMC Genom. 2021, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ce, F.; Tang, H.; Tian, G.F.; Yang, L.; Qian, W.; Dong, H.L. Genome-wide analysis of the serine carboxypeptidase-like (SCPL) proteins in Brassica napus L. Plant Physiol. Biochem. 2022, 186, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.Y.; Chen, B.J.; Pei, X.X.; Wang, X.Y.; Wang, X.; Nazir, M.F.; Wang, J.J.; Zhang, X.; Xing, A.; Pan, Z.; et al. Genome-wide analysis of the serine carboxypeptidase-like protein family reveals Ga09G1039 is involved in fiber elongation in cotton. Plant Physiol. Biochem. 2023, 201, 107759. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.S.; Bergey, D.R.; Ryan, C.A. Characterization and localization of a wound-inducible type I serine-carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill.). Planta 2001, 212, 222–230. [Google Scholar] [CrossRef]
- Sheahan, J.J. Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 1996, 83, 679–686. [Google Scholar] [CrossRef]
- Shirley, A.M.; McMichael, C.M.; Chapple, C. The sng2 mutant of Arabidopsis is defective in the gene encoding the serine carboxypeptidase-like protein sinapoylglucose:choline sinapoyltransferase. Plant J. 2001, 28, 83–94. [Google Scholar] [CrossRef]
- Lehfeldt, C.; Shirley, A.M.; Meyer, K.; Ruegger, M.O.; Cusumano, J.C.; Viitanen, P.V.; Strack, D.; Chapple, C. Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 2000, 12, 1295–1306. [Google Scholar] [CrossRef]
- Fraser, C.M.; Thompson, M.G.; Shirley, A.M.; Ralph, J.; Schoenherr, J.A.; Sinlapadech, T.; Hall, M.C.; Chapple, C. Related Arabidopsis serine carboxypeptidase-like sinapoylglucose acyltransferases display distinct but overlapping substrate specificities. Plant Physiol. 2007, 144, 1986–1999. [Google Scholar] [CrossRef]
- Urasaki, N.; Takagi, H.; Natsume, S.; Uemura, A.; Taniai, N.; Miyagi, N.; Fukushima, M.; Suzuki, S.; Tarora, K.; Tamaki, M.; et al. Draft genome sequence of bitter gourd (Momordica charantia), a vegetable and medicinal plant in tropical and subtropical regions. DNA Res. 2017, 24, 51–58. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res. 2010, 38, D211–D222. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Guan, F.; Zhang, J.Y.; Shi, B.; Wan, X.J.; Huang, C.L. Identification and fungicides screening of bitter gourd fusarium wilt pathogen. Acta Agric. Jiangxi 2021, 33, 68–72. [Google Scholar]
- Li, H.; Wang, X.M.; Chen, L.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Considine, M.J.; Yu, J.Q.; Zhou, Y.H. Growth temperature-induced changes in biomass accumulation, photosynthesis and glutathione redox homeostasis as influenced by hydrogen peroxide in cucumber. Plant Physiol. Biochem. PPB 2013, 71, 1–10. [Google Scholar] [CrossRef]
- Meenakshi, G.; Solomon, J.J.; Sulochana, C.B. Toluidine blue metachromasia; a means for farly detection of plant virus infection. Stain. Technol. 2009, 47, 267–268. [Google Scholar] [CrossRef]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain technology 2009, 48, 247–249. [Google Scholar] [CrossRef]
- Trinh, L.A.; McCutchen, M.D.; Bonner-Fraser, M.; Fraser, S.E.; Bumm, L.A.; McCauley, D.W. Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. BioTechniques 2018, 42, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Hanna, W.M.; Kwok, K. Chromogenic hybridization: A viable alternative to fluorescence hybridization in the HER2 testing algorithm. Mod. Pathol. 2006, 19, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N. Detection of c-myc amplification in formalin-fixed paraffin-embedded tumor tissue by chromogenic in situ hybridization (CISH). Methods Mol. Biol. 2013, 1012, 249–254. [Google Scholar] [PubMed]

| NCBI_ID | Renamed | CDD | SMART | Length | MW (Da) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| LOC111013428 | McSCPL1 | + | + | 569 | 63,021.2 | 4.87 | Vac |
| LOC111007166 | McSCPL2 | + | + | 433 | 48,972.9 | 8.8 | Vac |
| LOC111010259 | McSCPL3 | + | + | 456 | 51,938.3 | 7.22 | Vac |
| LOC111010625 | McSCPL4 | + | + | 482 | 54,264.1 | 5.53 | Vac |
| LOC111014585 | McSCPL5 | + | + | 480 | 54,343.4 | 8.31 | Vac |
| LOC111020681 | McSCPL6 | + | + | 472 | 52,963.4 | 7.09 | Vac |
| LOC111022214 | McSCPL7 | + | + | 470 | 52,931.4 | 8.41 | Vac |
| LOC111014800 | McSCPL8 | + | + | 512 | 57,242.1 | 7.25 | Vac |
| LOC111018253 | McSCPL9 | + | + | 505 | 56,220.9 | 6.32 | Pero |
| LOC111011999 | McSCPL10 | + | + | 480 | 53,752.8 | 6.24 | Vac |
| LOC111006031 | McSCPL11 | + | + | 509 | 57,031.1 | 5.2 | Pero, Vac |
| LOC111020491 | McSCPL12 | + | + | 506 | 56,443.7 | 5.44 | Pero, Vac |
| LOC111021720 | McSCPL13 | + | + | 477 | 53,267.2 | 6.94 | Vac |
| LOC111020263 | McSCPL14 | + | + | 479 | 54,015.8 | 6.79 | Vac |
| LOC111024700 | McSCPL15 | + | + | 471 | 53,692.9 | 5.09 | Vac |
| LOC111024586 | McSCPL16 | + | + | 498 | 55,155.1 | 6.67 | Vac |
| LOC111007100 | McSCPL17 | + | + | 467 | 52,517.2 | 7.05 | Vac |
| LOC111023593 | McSCPL18 | + | + | 465 | 51,808.3 | 6.7 | Vac |
| LOC111021347 | McSCPL19 | + | + | 480 | 53,215.9 | 6.79 | Vac |
| LOC111025862 | McSCPL20 | + | 470 | 53,137.9 | 6.1 | Vac | |
| LOC111025866 | McSCPL21 | + | 463 | 51,972.6 | 6.98 | Vac | |
| LOC111019830 | McSCPL22 | + | + | 498 | 55,001.1 | 8.58 | Vac |
| LOC111015180 | McSCPL23 | + | + | 395 | 44,625.3 | 6.85 | Vac |
| LOC111025759 | McSCPL24 | + | 498 | 56,128.5 | 6.97 | Vac | |
| LOC111018332 | McSCPL25 | + | 456 | 50,580.4 | 6.42 | Vac | |
| LOC111013427 | McSCPL26 | + | + | 366 | 40,877.8 | 4.85 | Pero |
| LOC111020504 | McSCPL27 | + | 467 | 52,041.8 | 6.79 | Vac | |
| LOC111011240 | McSCPL28 | + | 462 | 51,115.8 | 5.79 | Vac | |
| LOC111011335 | McSCPL29 | + | 433 | 48,114.7 | 6.27 | Vac | |
| LOC111011336 | McSCPL30 | + | 433 | 47,813.4 | 7.86 | Vac | |
| LOC111019252 | McSCPL31 | + | 1457 | 161,625.4 | 7.24 | Vac | |
| LOC111008438 | McSCPL32 | + | 268 | 31,091.9 | 8.34 | Vac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, F.; Yang, X.; Shi, B.; Wang, K.; Zhang, J.; Xie, Y.; Wan, X. Genome-Wide Analysis of the Serine Carboxypeptidase-like (SCPL) Protein Family of Bitter Gourd and Functional Validation of McSCPL22 in Fusarium oxysporum f. sp. Momordicae (FOM) Resistance. Int. J. Mol. Sci. 2024, 25, 11816. https://doi.org/10.3390/ijms252111816
Guan F, Yang X, Shi B, Wang K, Zhang J, Xie Y, Wan X. Genome-Wide Analysis of the Serine Carboxypeptidase-like (SCPL) Protein Family of Bitter Gourd and Functional Validation of McSCPL22 in Fusarium oxysporum f. sp. Momordicae (FOM) Resistance. International Journal of Molecular Sciences. 2024; 25(21):11816. https://doi.org/10.3390/ijms252111816
Chicago/Turabian StyleGuan, Feng, Xuetong Yang, Bo Shi, Kai Wang, Jingyun Zhang, Yuanyuan Xie, and Xinjian Wan. 2024. "Genome-Wide Analysis of the Serine Carboxypeptidase-like (SCPL) Protein Family of Bitter Gourd and Functional Validation of McSCPL22 in Fusarium oxysporum f. sp. Momordicae (FOM) Resistance" International Journal of Molecular Sciences 25, no. 21: 11816. https://doi.org/10.3390/ijms252111816
APA StyleGuan, F., Yang, X., Shi, B., Wang, K., Zhang, J., Xie, Y., & Wan, X. (2024). Genome-Wide Analysis of the Serine Carboxypeptidase-like (SCPL) Protein Family of Bitter Gourd and Functional Validation of McSCPL22 in Fusarium oxysporum f. sp. Momordicae (FOM) Resistance. International Journal of Molecular Sciences, 25(21), 11816. https://doi.org/10.3390/ijms252111816






