An Anthocyanin-Based Visual Reporter System for Genetic Transformation and Genome Editing in Cassava
Abstract
1. Introduction
2. Results
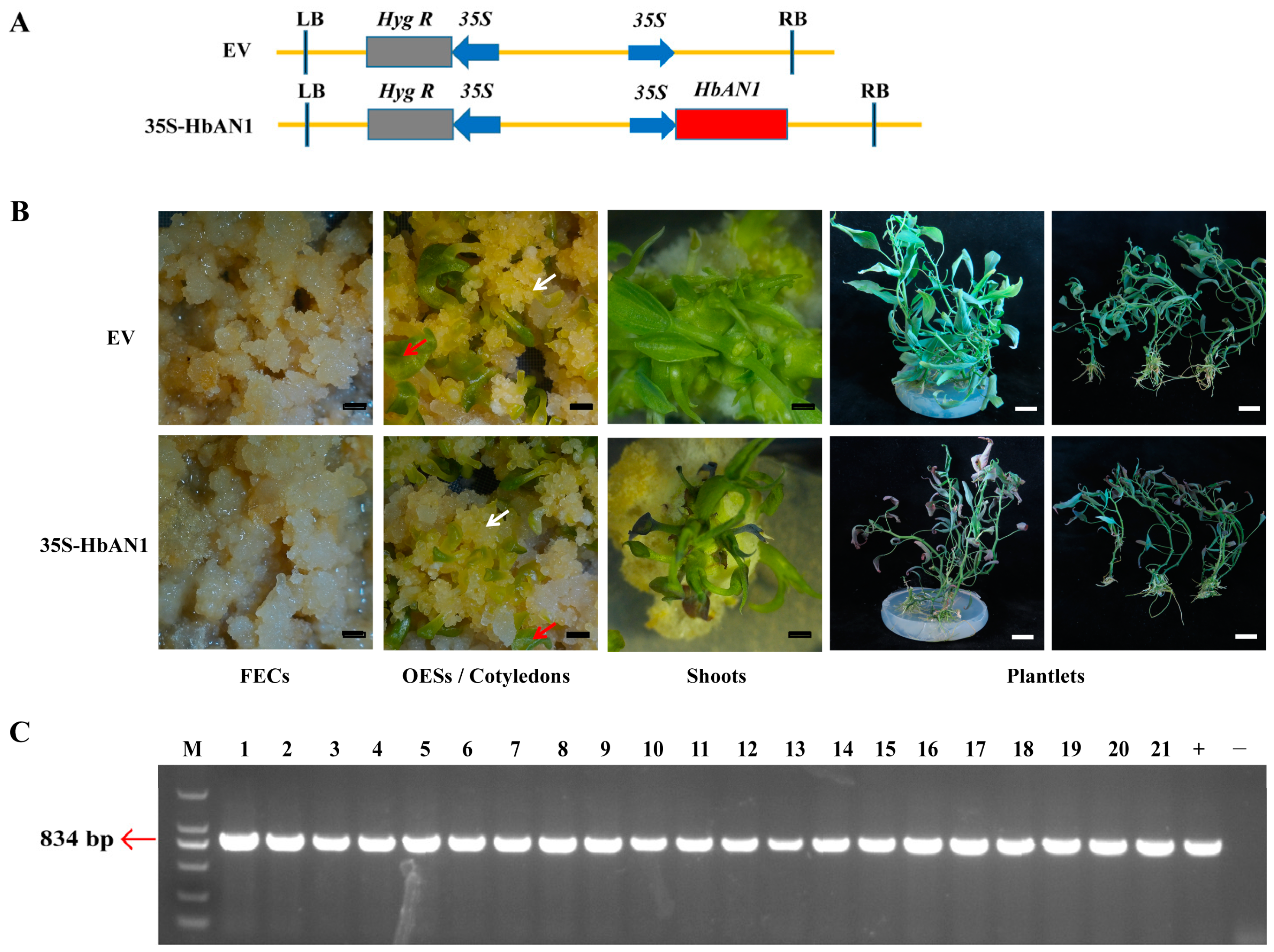
2.1. Acquisition of HbAN1-Overexpressing Transgenic Cassava
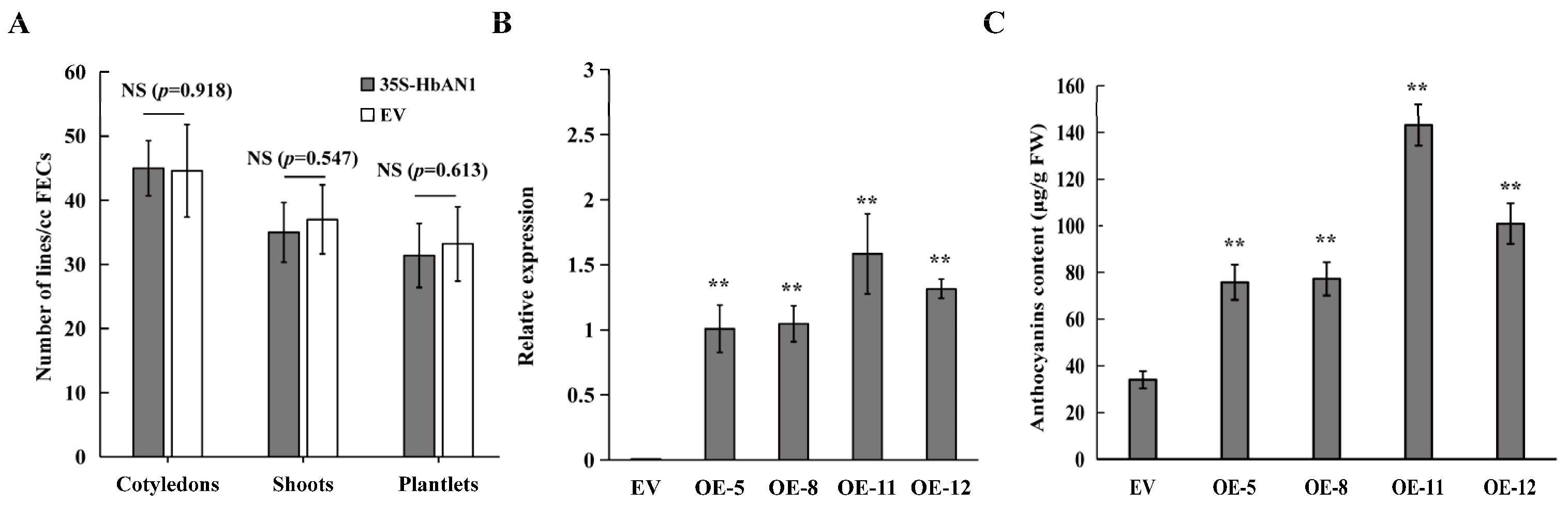
2.2. Evaluation of HbAN1-Overexpressing Genetic Transformation
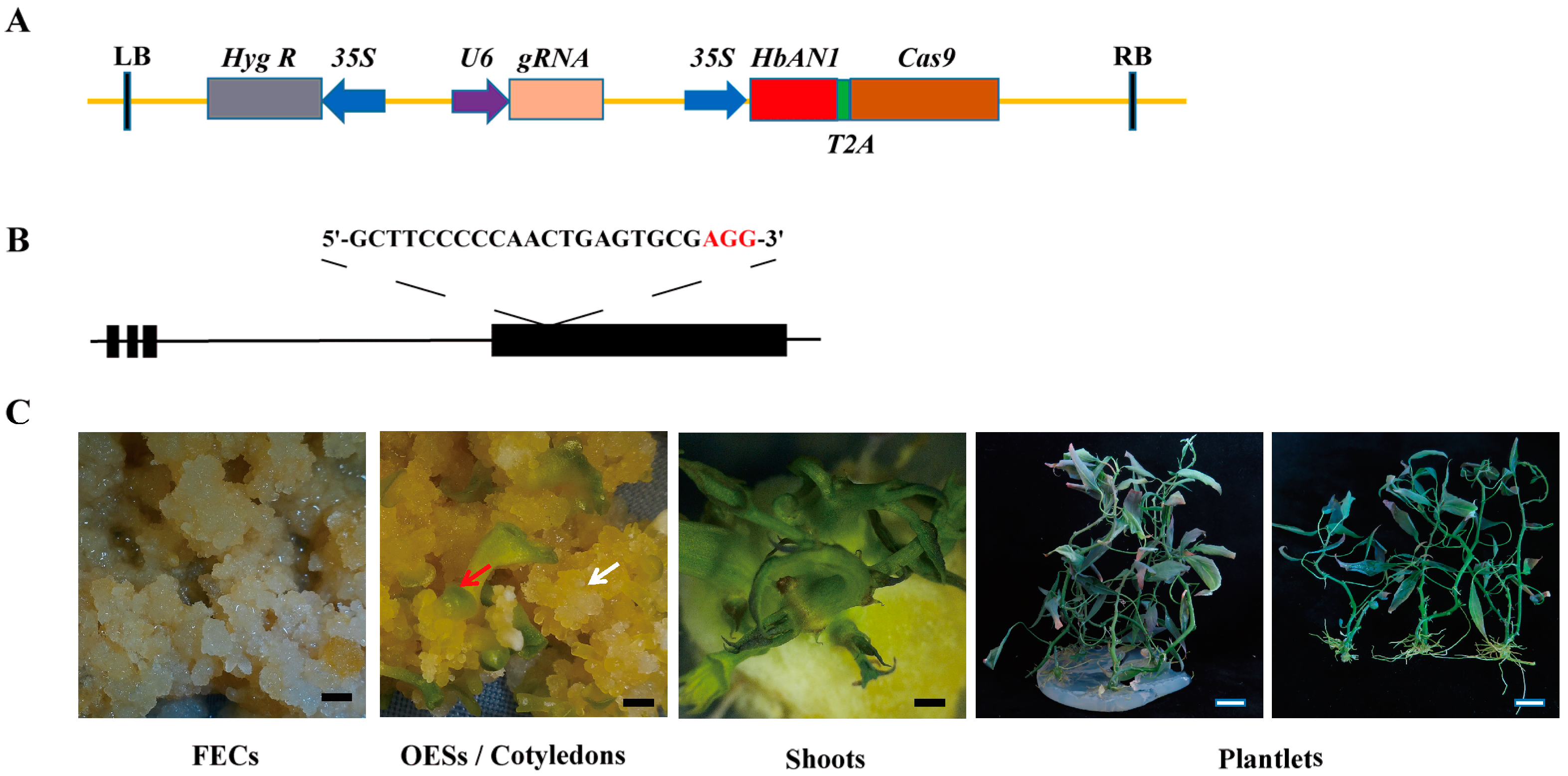
2.3. Construction of AR-CRISPR/Cas9-gRNA Editing Vector and Genetic Transformation
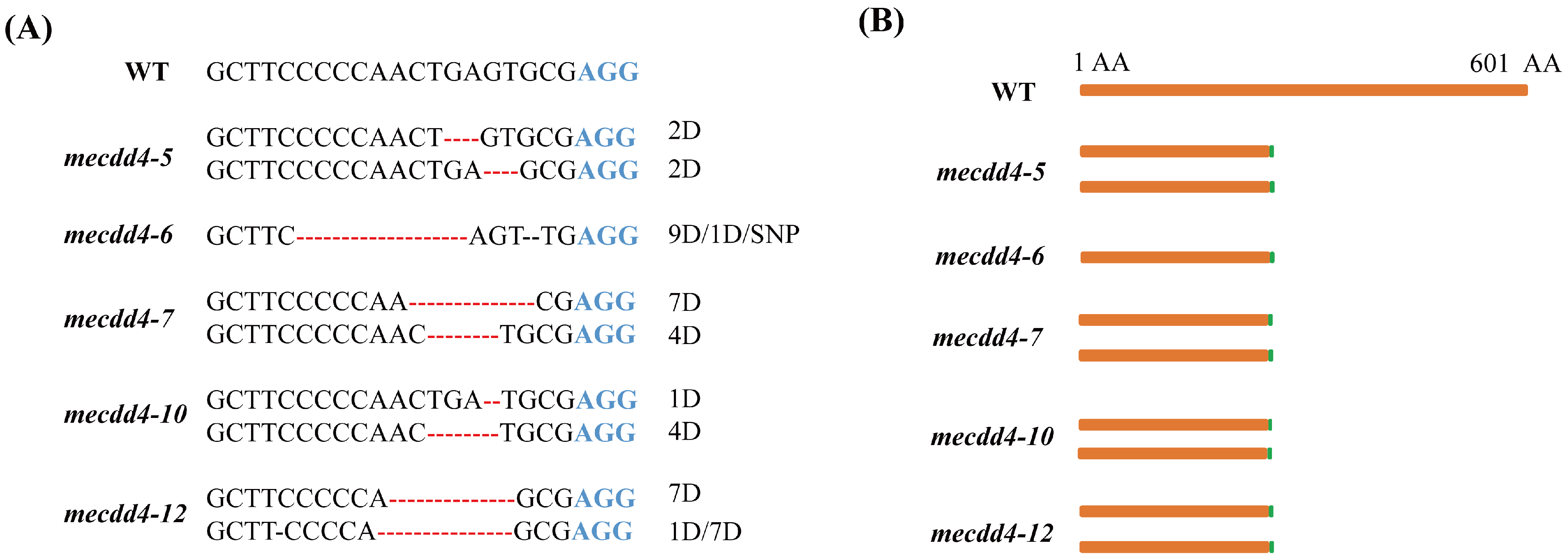
2.4. Analysis of the Editing Effects of the MeCDD4 Gene
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Construction of HbAN1 Expressing and AR-CRISPR/Cas9-MeCDD4-gRNA Editing Vectors
4.3. Cassava Genetic Transformation
4.4. Extraction and Measurement of Anthocyanins
4.5. Statistics of Cotyledons, Bud Regenerations and Seedlings of the Regenerated Plants
4.6. Hi-TOM Sequencing
4.7. qRT-PCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferraro, V.; Piccirillo, C.; Tomlins, K.; Pintado, M.E. Cassava (Manihot esculenta Crantz) and yam (Dioscorea spp.) crops and their derived foodstuffs: Safety, security and nutritional value. Crit. Rev. Food Sci. Nutr. 2015, 56, 2714–2727. [Google Scholar] [CrossRef] [PubMed]
- Enesi, R.O.; Hauser, S.; Pypers, P.; Kreye, C.; Tariku, M.; Six, J. Understanding changes in cassava root dry matter yield by different planting dates, crop ages at harvest, fertilizer application and varieties. Eur. J. Agron. 2022, 133, 126448. [Google Scholar] [CrossRef]
- Zanini, A.A.; Cuellar, W.J.; Celli, M.G.; Luque, A.V.; Medina, R.D.; Conci, V.C.; Feo, L.d.V.D. Distinct strains of the re-emergent Cassava common mosaic virus (genus: Potexvirus) infecting cassava in argentina. Plant Pathol. 2018, 67, 1814–1820. [Google Scholar] [CrossRef]
- Wang, W.; Hostettler, C.E.; Damberger, F.F.; Kossmann, J.; Lloyd, J.R.; Zeeman, S.C. Modification of cassava root starch phosphorylation enhances starch functional properties. Front. Plant Sci. 2018, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Schöpke, N.T.C.; Carcamo, R.; de Schöpke, A.E.G.; Konan, N.; Marmey, P. Stable transformation of cassava (Manihot esculenta Crantz) by particle bombardment and by Agrobacterium. Afr. J. Root Tuber Crops 1997, 2, 187–193. [Google Scholar]
- Schöpke, N.T.C.; Cárcamo, R.; Konan, D.K.; Marmey, P.; Henshaw, G.G.; Beachy, R.N. Regeneration of transgenic cassava plants (Manihot esculenta Crantz) from microbombarded embryogenic suspension cultures. Nat. Biotechnol. 1996, 14, 731–735. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Beyene, G.; Kalyaeva, M.; Fauquet, C.M.; Taylor, N. Improvements in agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz) for large-scale production of transgenic plants. Plant Cell Tissue Organ Cult. PCTOC 2015, 121, 591–603. [Google Scholar] [CrossRef]
- Chetty, C.; Rossin, C.; Gruissem, W.; Vanderschuren, H.; Rey, M. Empowering biotechnology in southern africa: Establishment of a robust transformation platform for the production of transgenic industry-preferred cassava. New Biotechnol. 2013, 30, 136–143. [Google Scholar] [CrossRef]
- Nyaboga, E.N.; Njiru, J.M.; Tripathi, L. Factors influencing somatic embryogenesis, regeneration, and agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz) cultivar TME14. Front. Plant Sci. 2015, 6, 411. [Google Scholar] [CrossRef]
- Lentz, E.M.; Eisner, S.; McCallum, E.J.; Schlegel, K.; Campos, F.d.A.d.P.; Gruissem, W.; Vanderschuren, H. Genetic transformation of recalcitrant cassava by embryo selection and increased hormone levels. Methods Protoc. 2018, 1, 42. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Lu, X.-H.; Zhen, X.-H.; Yang, H.; Che, Y.-N.; Hou, J.-Y.; Geng, M.-T.; Liu, J.; Hu, X.-W.; Li, R.-M.; et al. A transformation and genome editing system for cassava cultivar SC8. Genes 2022, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Odipio, J.; Alicai, T.; Ingelbrecht, I.; Nusinow, D.A.; Bart, R.; Taylor, N.J. Efficient crispr/cas9 genome editing of phytoene desaturase in cassava. Front. Plant Sci. 2017, 8, 1780. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous crispr/cas9-mediated editing of cassava eif4e isoforms ncbp-1 and ncbp-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2018, 17, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ma, Q.; Zhong, Y.; Jing, J.; Wei, Z.; Zhou, W.; Lu, X.; Tian, Y.; Zhang, P. Editing of the starch branching enzyme gene sbe2 generates high-amylose storage roots in cassava. Plant Mol. Biol. 2021, 108, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.-E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS- and PTST1- edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef]
- Schreuder, M.; Raemakers, C.; Jacobsen, E.; Visser, R. Efficient production of transgenic plants by agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz). Euphytica 2001, 120, 35–42. [Google Scholar] [CrossRef]
- Putten, H.J.J.K.-V.; Wolters, A.-M.A.; Pereira-Bertram, I.M.; Berg, H.H.J.v.D.; van der Krol, A.R.; Visser, R.G.F. Cloning and characterization of a tuberous root-specific promoter from cassava (Manihot esculenta Crantz). Planta 2012, 236, 1955–1965. [Google Scholar] [CrossRef]
- Okwuonu, I.C.; Achi, O.K.; Egesi, C.N.; Taylor, N.J. Evaluation of red fluorescent protein (dsred) as alternative visual marker of genetic transformation in cassava (Manihot esculenta Crantz). In Vitr. Cell. Dev. Biol.—Plant 2015, 51, 571–579. [Google Scholar] [CrossRef]
- Rosellini, D. Selectable markers and reporter genes: A well furnished toolbox for plant science and genetic engineering. Crit. Rev. Plant Sci. 2012, 31, 401–453. [Google Scholar] [CrossRef]
- Ye, S.; Cai, C.; Ren, H.; Wang, W.; Xiang, M.; Tang, X.; Zhu, C.; Yin, T.; Zhang, L.; Zhu, Q. An efficient plant regeneration and transformation system of ma bamboo (Dendrocalamus latiflorus munro) started from young shoot as explant. Front. Plant Sci. 2017, 8, 1298. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez De Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2016, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Kortstee, A.J.; Khan, S.A.; Helderman, C.; Trindade, L.M.; Wu, Y.; Visser, R.G.F.; Brendolise, C.; Allan, A.; Schouten, H.J.; Jacobsen, E. Anthocyanin production as a potential visual selection marker during plant transformation. Transgenic Res. 2011, 20, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-S.; Nguyen, V.P.; Jeon, H.-W.; Kim, M.-H.; Eom, S.H.; Lim, Y.J.; Kim, W.-C.; Park, E.-J.; Choi, Y.-I.; Ko, J.-H. Overexpression ofptrmyb119, a r2r3-myb transcription factor frompopulus trichocarpa, promotes anthocyanin production in hybrid poplar. Tree Physiol. 2016, 36, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xi, X.; Zong, Y.; Li, S.; Li, Y.; Cao, D.; Liu, B. Overexpression of thmyc4e enhances anthocyanin biosynthesis in common wheat. Int. J. Mol. Sci. 2019, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Jiang, H.; Mao, Z.; Wang, N.; Jiang, S.; Xu, H.; Yang, G.; Zhang, Z.; Chen, X. The r2r3-myb transcription factor mdmyb24-like is involved in methyl jasmonate-induced anthocyanin biosynthesis in apple. Plant Physiol. Biochem. 2019, 139, 273–282. [Google Scholar] [CrossRef]
- Xie, Y.-G.; Ma, Y.-Y.; Bi, P.-P.; Wei, W.; Liu, J.; Hu, Y.; Gou, Y.-J.; Zhu, D.; Wen, Y.-Q.; Feng, J.-Y. Transcription factor fvtcp9 promotes strawberry fruit ripening by regulating the biosynthesis of abscisic acid and anthocyanins. Plant Physiol. Biochem. 2020, 146, 374–383. [Google Scholar] [CrossRef]
- Huang, T.; Fang, Y.; Chang, J.; Chen, T.; Xin, S.; Ko, N.C.K.; Huang, H.; Hua, Y. Cloning and Function Analysis of R2R3-MYB Regulatory Factor for Anthocyanin Biosynthesis in Hevea brasiliensis. Chin. J. Trop. Crops 2017, 38, 2285–2293. [Google Scholar] [CrossRef]
- Huang, T.; Xin, S.; Fang, Y.; Chen, T.; Chang, J.; Ko, N.C.K.; Huang, H.; Hua, Y. Use of a novel r2r3-myb transcriptional activator of anthocyanin biosynthesis as visual selection marker for rubber tree (Hevea brasiliensis) transformation. Ind. Crops Prod. 2021, 174, 114225. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, H.; Xu, N.; Zhang, B.; Gou, F.; Zhu, J.-K. Application of the crispr–cas system for efficient genome engineering in plants. Mol. Plant 2013, 6, 2008–2011. [Google Scholar] [CrossRef]
- Sharma, P.; Yan, F.; Doronina, V.A.; Escuin-Ordinas, H.; Ryan, M.D.; Brown, J.D. 2a peptides provide distinct solutions to driving stop-carry on translational recoding. Nucleic Acids Res. 2012, 40, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel r2r3-myb transcription factor famyb5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, C.; Waurich, V.; Wagner, H.; Ramos, R.; Oiza, N.; Muñoz, P.; Triviño, J.C.; Caruana, J.; Liu, Z.; Cobo, N.; et al. Allelic variation of myb10 is the major force controlling natural variation in skin and flesh color in strawberry (Fragaria spp.) fruit. Plant Cell 2020, 32, 3723–3749. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Yu, X.; Yang, H.; Gao, Q.; Ji, H.; Wang, Y.; Yan, G.; Peng, Y.; Luo, H.; Liu, K.; et al. Development and validation of an effective crispr/cas9 vector for efficiently isolating positive transformants and transgene-free mutants in a wide range of plant species. Front. Plant Sci. 2018, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, J.; Yuan, C.; Guo, Y.; Yu, H.; Li, Y.; Huang, C. Cas9-pf, an early flowering and visual selection marker system, enhances the frequency of editing event occurrence and expedites the isolation of genome-edited and transgene-free plants. Plant Biotechnol. J. 2019, 17, 1191–1193. [Google Scholar] [CrossRef]
| Line Name | Mutation Sequence | Reads Count | Reads Ratio | Mutation Type | Mutant Base |
|---|---|---|---|---|---|
| WT | GCTTCCCCCAACTGAGTGCGAGG | 68,606 | 83.04% | WT | |
| meccd4-1 | GCTTCCCCCAA-----------TGCGAGG | 26,593 | 46.25% | 5D | CTGAG |
| GCTTCCCCCAACTG------GCGAGG | 24,928 | 43.36% | 3D | AGT | |
| meccd4-2 | GCTTCCCCCA--------TGCGAGG | 56,300 | 84.10% | 6D | ACTGAG |
| meccd4-4 | GCTT------------------------------------------ | 33,361 | 49.97% | 26D | CCCCCAACTGAGTGCGAGGTGATTGA |
| GCTTCC--------------------------CGAGG | 26,650 | 39.92% | 12D | CCCAACTGAGTG | |
| meccd4-5 | GCTTCCCCCAACT----GTGCGAGG | 42,805 | 51.02% | 2D | GA |
| GCTTCCCCCAACTGA----GCGAGG | 36,498 | 43.50% | 2D | GT | |
| meccd4-6 | GCTTC---------------------AGT-TGAGG | 23,776 | 90.84% | 9D, 1D, SNP | CCCCAACTG,G,C->T |
| meccd4-7 | GCTTCCCCCAA---------------CGAGG | 32,807 | 45.03% | 7D | CTGAGTG |
| GCTTCCCCCAAC--------TGCGAGG | 30,596 | 41.99% | 4D | TGAG | |
| meccd4-8 | GCTTCCCCCAACTGA----GCGAGG | 33,855 | 52.31% | 2D | GT |
| GCTTCCCCCAACTG------GCGAGG | 13,453 | 20.78% | 3D | AGT | |
| GCTTCCCCCAACT----GTGCGAGG | 3718 | 05.74% | 2D | GA | |
| GCTTCCCCCAACTGAGT----GAGG | 3604 | 05.57% | 2D | GC | |
| meccd4-9 | GCTTCCCCCA-------------TGCGAGG | 22,175 | 36.57% | 6D | ACTGAG |
| GCTTCCCCCAACTGA----GCGAGG | 17,791 | 29.34% | 2D | GT | |
| GCTTCCCCCAACT-------------GAGG | 14,399 | 23.75% | 6D | GAGTGC | |
| meccd4-10 | GCTTCCCCCAACTGA--TGCGAGG | 25,004 | 45.79% | 1D | G |
| GCTTCCCCCAAC---------TGCGAGG | 23,782 | 43.55% | 4D | TGAG | |
| meccd4-12 | GCTTCCCCCA---------------GCGAGG | 32,546 | 44.66% | 7D | ACTGAGT |
| GCTT-CCCCA----------------GCGAGG | 32,364 | 44.41% | 1D,7D | C,ACTGAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, X.-H.; Pan, R.-R.; Lu, X.-H.; Ge, Y.-J.; Li, R.-M.; Liu, J.; Wang, Y.-J.; Yi, K.-X.; Li, C.-X.; Guo, J.-C.; et al. An Anthocyanin-Based Visual Reporter System for Genetic Transformation and Genome Editing in Cassava. Int. J. Mol. Sci. 2024, 25, 11808. https://doi.org/10.3390/ijms252111808
Zhen X-H, Pan R-R, Lu X-H, Ge Y-J, Li R-M, Liu J, Wang Y-J, Yi K-X, Li C-X, Guo J-C, et al. An Anthocyanin-Based Visual Reporter System for Genetic Transformation and Genome Editing in Cassava. International Journal of Molecular Sciences. 2024; 25(21):11808. https://doi.org/10.3390/ijms252111808
Chicago/Turabian StyleZhen, Xing-Hou, Ran-Ran Pan, Xiao-Hua Lu, Yu-Jian Ge, Rui-Mei Li, Jiao Liu, Ya-Jie Wang, Ke-Xian Yi, Chun-Xia Li, Jian-Chun Guo, and et al. 2024. "An Anthocyanin-Based Visual Reporter System for Genetic Transformation and Genome Editing in Cassava" International Journal of Molecular Sciences 25, no. 21: 11808. https://doi.org/10.3390/ijms252111808
APA StyleZhen, X.-H., Pan, R.-R., Lu, X.-H., Ge, Y.-J., Li, R.-M., Liu, J., Wang, Y.-J., Yi, K.-X., Li, C.-X., Guo, J.-C., Yao, Y., & Geng, M.-T. (2024). An Anthocyanin-Based Visual Reporter System for Genetic Transformation and Genome Editing in Cassava. International Journal of Molecular Sciences, 25(21), 11808. https://doi.org/10.3390/ijms252111808






