Vitamin K2 Protects Against SARS-CoV-2 Envelope Protein-Induced Cytotoxicity in Chronic Myeloid Leukemia Cells and Enhances Imatinib Activity
Abstract
1. Introduction
2. Results
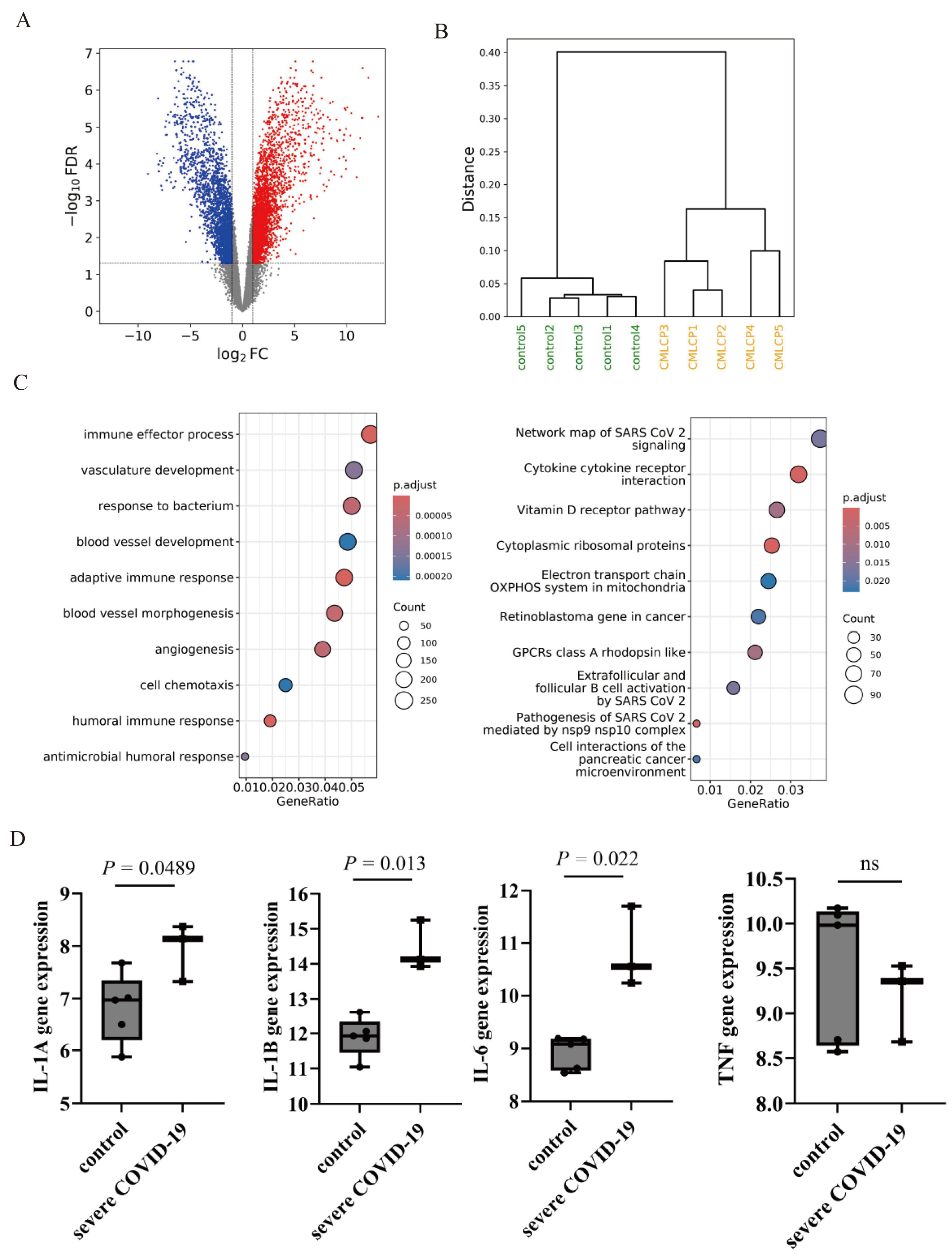
2.1. Gene Expression Analysis of COVID-19-Related Genes
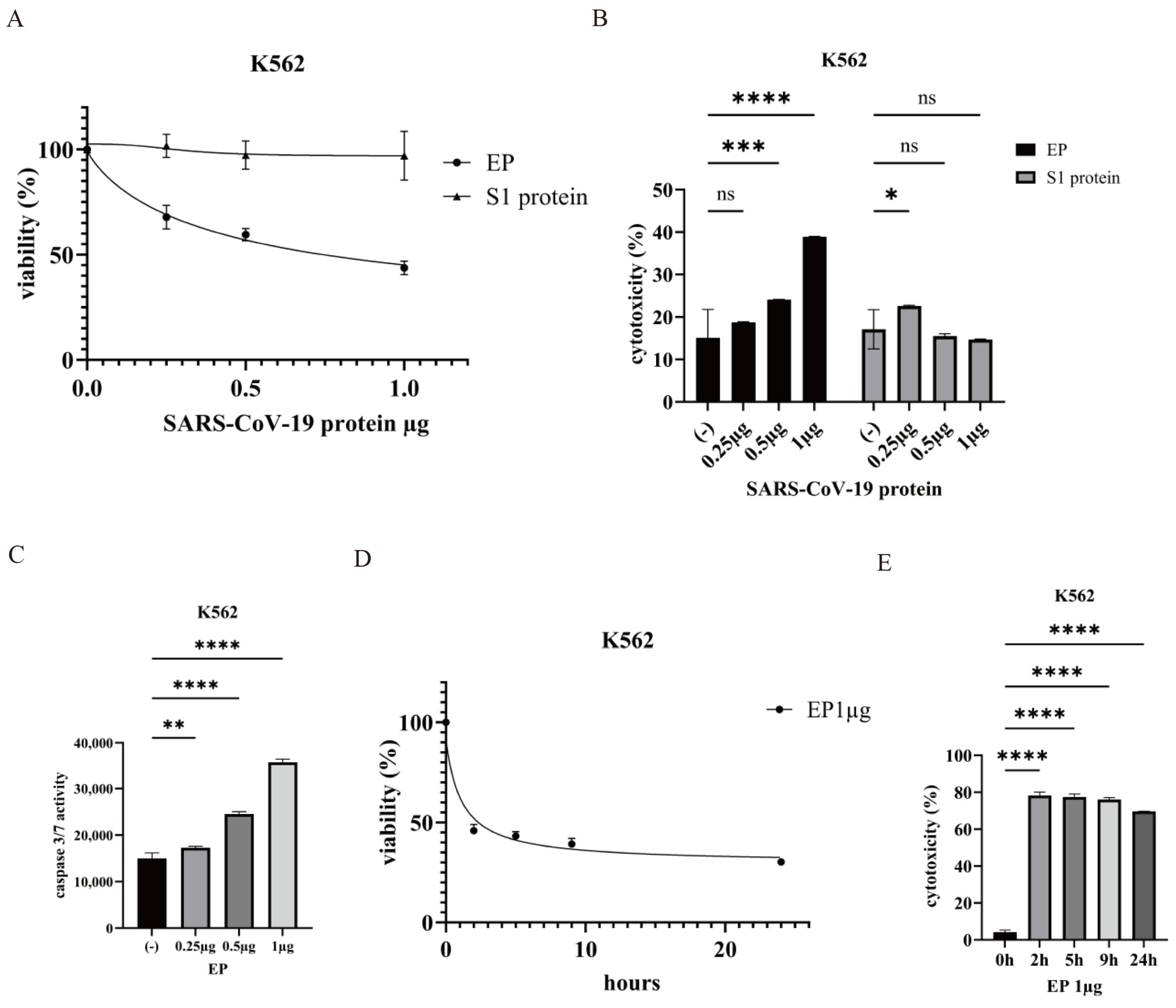
2.2. Effect of the SARS-CoV-2 Envelope Protein on CML Cells
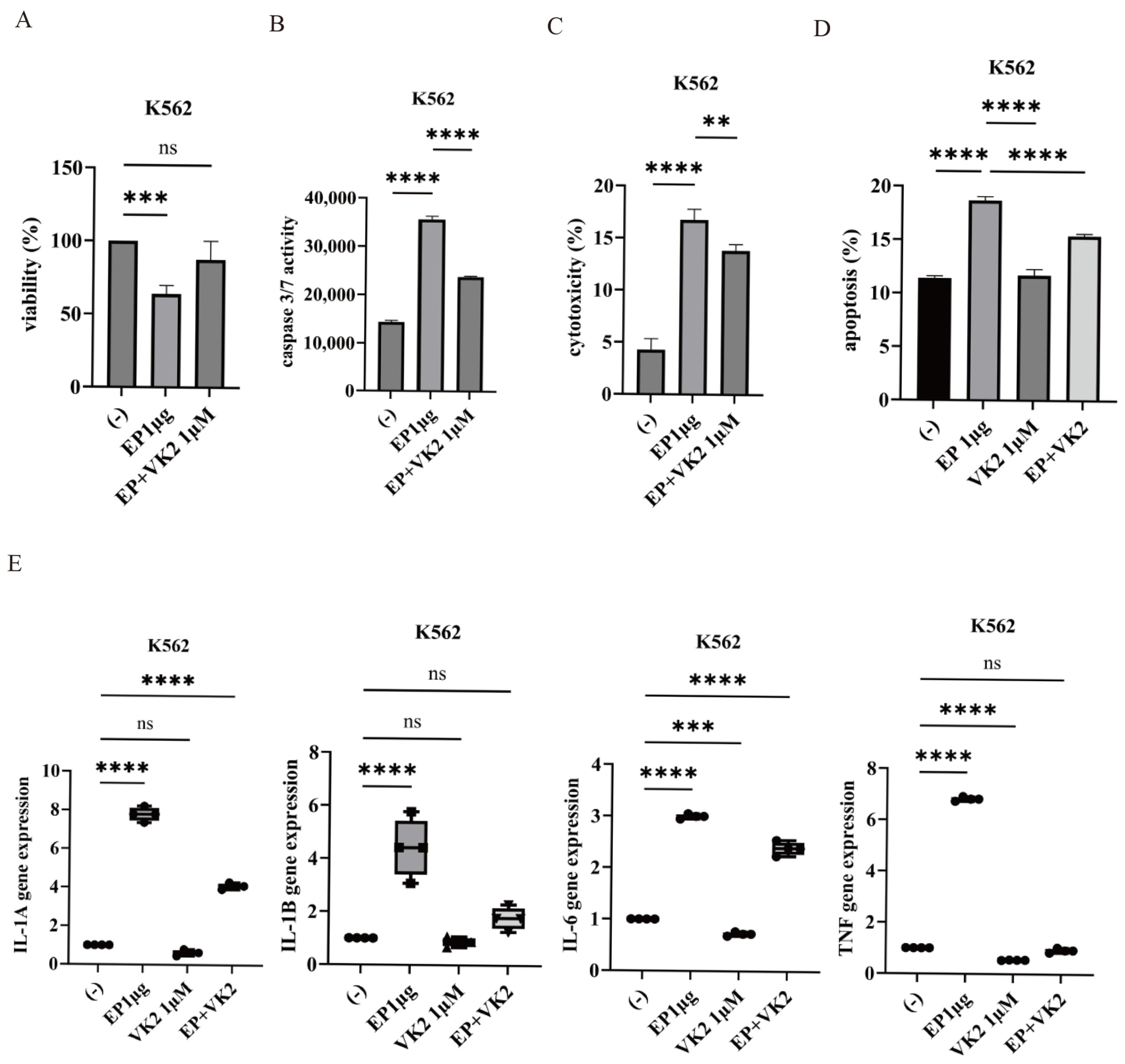
2.3. Effect of VK2 on SARS-CoV-2 Ep-Induced Cytotoxicity
2.4. Anti-Leukemic Effect of VK2 on CML Cells
2.5. Synergistic Effects of Imatinib and VK2 in CML Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture
4.3. Data Collection and Processing
4.4. Cell Proliferation Assay
4.5. Caspase 3/7 Activity
4.6. Cytotoxicity Assay
4.7. Colon Formation Assay
4.8. Apoptosis Assay
4.9. MMP
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jahirul Islam, M.; Nawal Islam, N.; Siddik Alom, M.; Kabir, M.; Halim, M.A. A review on structural, non-structural, and accessory proteins of SARS-CoV-2: Highlighting drug target sites. Immunobiology 2023, 228, 152302. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef]
- Denaro, M.; Ferro, E.; Barrano, G.; Meli, S.; Busacca, M.; Corallo, D.; Capici, A.; Zisa, A.; Cucuzza, L.; Gradante, S.; et al. Monitoring of SARS-CoV-2 Infection in Ragusa Area: Next Generation Sequencing and Serological Analysis. Int. J. Mol. Sci. 2023, 24, 4742. [Google Scholar] [CrossRef]
- Klimek, L.; Agache, I.; Cooke, E.; Jutel, M.; Akdis, C.A.; O’Hehir, R. COVID-19 vaccines-The way forward. Allergy 2022, 77, 15–16. [Google Scholar] [CrossRef]
- Sharafi, F.; Jafarzadeh Esfehani, R.; Moodi Ghalibaf, A.; Jarahi, L.; Shamshirian, A.; Mozdourian, M. Leukopenia and leukocytosis as strong predictors of COVID-19 severity: A cross-sectional study of the hematologic abnormalities and COVID-19 severity in hospitalized patients. Health Sci. Rep. 2023, 6, e1574. [Google Scholar] [CrossRef]
- Ahmadi, E.; Bagherpour, Z.; Zarei, E.; Omidkhoda, A. Pathological effects of SARS-CoV-2 on hematological and immunological cells: Alterations in count, morphology, and function. Pathol. Res. Pract. 2022, 231, 153782. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020, 95, 691–709. [Google Scholar] [CrossRef]
- Pane, F.; Intrieri, M.; Quintarelli, C.; Izzo, B.; Muccioli, G.C.; Salvatore, F. BCR/ABL genes and leukemic phenotype: From molecular mechanisms to clinical correlations. Oncogene 2002, 21, 8652–8667. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.P.; Ernst, T.; Branford, S.; Cayuela, J.M.; Deininger, M.; Fabarius, A.; Kim, D.D.H.; Machova Polakova, K.; Radich, J.P.; Hehlmann, R.; et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia 2023, 37, 2150–2167. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Hochhaus, A.; Branford, S.; Müller, M.C.; Kaeda, J.S.; Foroni, L.; Druker, B.J.; Guilhot, F.; Larson, R.A.; O’Brien, S.G.; et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the International Randomized Study of interferon and STI571 (IRIS). Blood 2010, 116, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Poudel, G.; Tolland, M.G.; Hughes, T.P.; Pagani, I.S. Mechanisms of resistance and implications for treatment strategies in chronic myeloid leukaemia. Cancers 2022, 14, 3300. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- Bus, K.; Szterk, A. Relationship between structure and biological activity of various vitamin K forms. Foods 2021, 10, 3136. [Google Scholar] [CrossRef]
- Welsh, J.; Bak, M.J.; Narvaez, C.J. New insights into vitamin K biology with relevance to cancer. Trends Mol. Med. 2022, 28, 864–881. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Wu, D.; Wang, Y.; Chen, J.; Duan, L.; Li, S.; Li, Y. Vitamin K: Infection, inflammation, and auto-immunity. J. Inflamm. Res. 2024, 17, 1147–1160. [Google Scholar]
- Thomas, P.D. The gene ontology and the meaning of biological function. Methods Mol. Biol. 2017, 1446, 15–24. [Google Scholar]
- Rovini, A.; Heslop, K.; Hunt, E.G.; Morris, M.E.; Fang, D.; Gooz, M.; Gerencser, A.A.; Maldonado, E.N. Quantitative analysis of mitochondrial membrane potential heterogeneity in unsynchronized and synchronized cancer cells. FASEB J. 2021, 35, e21148. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, P.; Li, M.; Chen, Z.; Xin, H.; Reilly, S.; Zhang, X. SARS-CoV-2 E protein: Pathogenesis and potential therapeutic development. Biomed. Pharmacother. 2023, 159, 114242. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Prueller, F.; Dawczynski, C.; Curcic, P.; Sloup, Z.; Holter, M.; Herrmann, M.; Meinitzer, A. Dramatic decrease of vitamin K2 subtype Menaquinone-7 in COVID-19 Patients. Antioxidants 2022, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and COVID-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy 2020, 18, 23. [Google Scholar] [CrossRef]
- Stępień, A.; Koziarska-Rościszewska, M.; Rysz, J.; Stępień, M. Biological role of vitamin K-with particular emphasis on cardiovascular and renal aspects. Nutrients 2022, 14, 262. [Google Scholar] [CrossRef]
- Okabe, S.; Gotoh, A. Effect of asciminib and vitamin K2 on Abelson tyrosine-kinase-inhibitor-resistant chronic myelogenous leukemia cells. BMC Cancer 2023, 23, 827. [Google Scholar] [CrossRef]
- Okabe, S.; Tanaka, Y.; Moriyama, M.; Gotoh, A. Efficacy of dasatinib against ponatinib-resistant chronic myeloid leukemia cells. Leuk. Lymphoma 2020, 61, 237–239. [Google Scholar] [CrossRef]
- Li, S.Q.; Liu, J.; Zhang, J.; Wang, X.L.; Chen, D.; Wang, Y.; Xu, Y.M.; Huang, B.; Lin, J.; Li, J.; et al. Transcriptome profiling reveals the high incidence of hnRNPA1 exon 8 inclusion in chronic myeloid leukemia. J. Adv. Res. 2020, 24, 301–310. [Google Scholar] [CrossRef]
- Chimote, A.A.; Alshwimi, A.O.; Chirra, M.; Gawali, V.S.; Powers-Fletcher, M.V.; Hudock, K.M.; Conforti, L. Immune and ionic mechanisms mediating the effect of dexamethasone in severe COVID-19. Front. Immunol. 2023, 14, 1143350. [Google Scholar] [CrossRef]
- Prieto, C.; Barrios, D. RaNA-Seq: Interactive RNA-Seq analysis from FASTQ files to functional analysis. Bioinformatics 2019, 36, 1955–1956. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okabe, S.; Arai, Y.; Gotoh, A. Vitamin K2 Protects Against SARS-CoV-2 Envelope Protein-Induced Cytotoxicity in Chronic Myeloid Leukemia Cells and Enhances Imatinib Activity. Int. J. Mol. Sci. 2024, 25, 11800. https://doi.org/10.3390/ijms252111800
Okabe S, Arai Y, Gotoh A. Vitamin K2 Protects Against SARS-CoV-2 Envelope Protein-Induced Cytotoxicity in Chronic Myeloid Leukemia Cells and Enhances Imatinib Activity. International Journal of Molecular Sciences. 2024; 25(21):11800. https://doi.org/10.3390/ijms252111800
Chicago/Turabian StyleOkabe, Seiichi, Yuya Arai, and Akihiko Gotoh. 2024. "Vitamin K2 Protects Against SARS-CoV-2 Envelope Protein-Induced Cytotoxicity in Chronic Myeloid Leukemia Cells and Enhances Imatinib Activity" International Journal of Molecular Sciences 25, no. 21: 11800. https://doi.org/10.3390/ijms252111800
APA StyleOkabe, S., Arai, Y., & Gotoh, A. (2024). Vitamin K2 Protects Against SARS-CoV-2 Envelope Protein-Induced Cytotoxicity in Chronic Myeloid Leukemia Cells and Enhances Imatinib Activity. International Journal of Molecular Sciences, 25(21), 11800. https://doi.org/10.3390/ijms252111800





