Therapeutic Contribution of Tau-Binding Thiazoloflavonoid Hybrid Derivatives Against Glioblastoma Using Pharmacological Approach in 3D Spheroids
Abstract
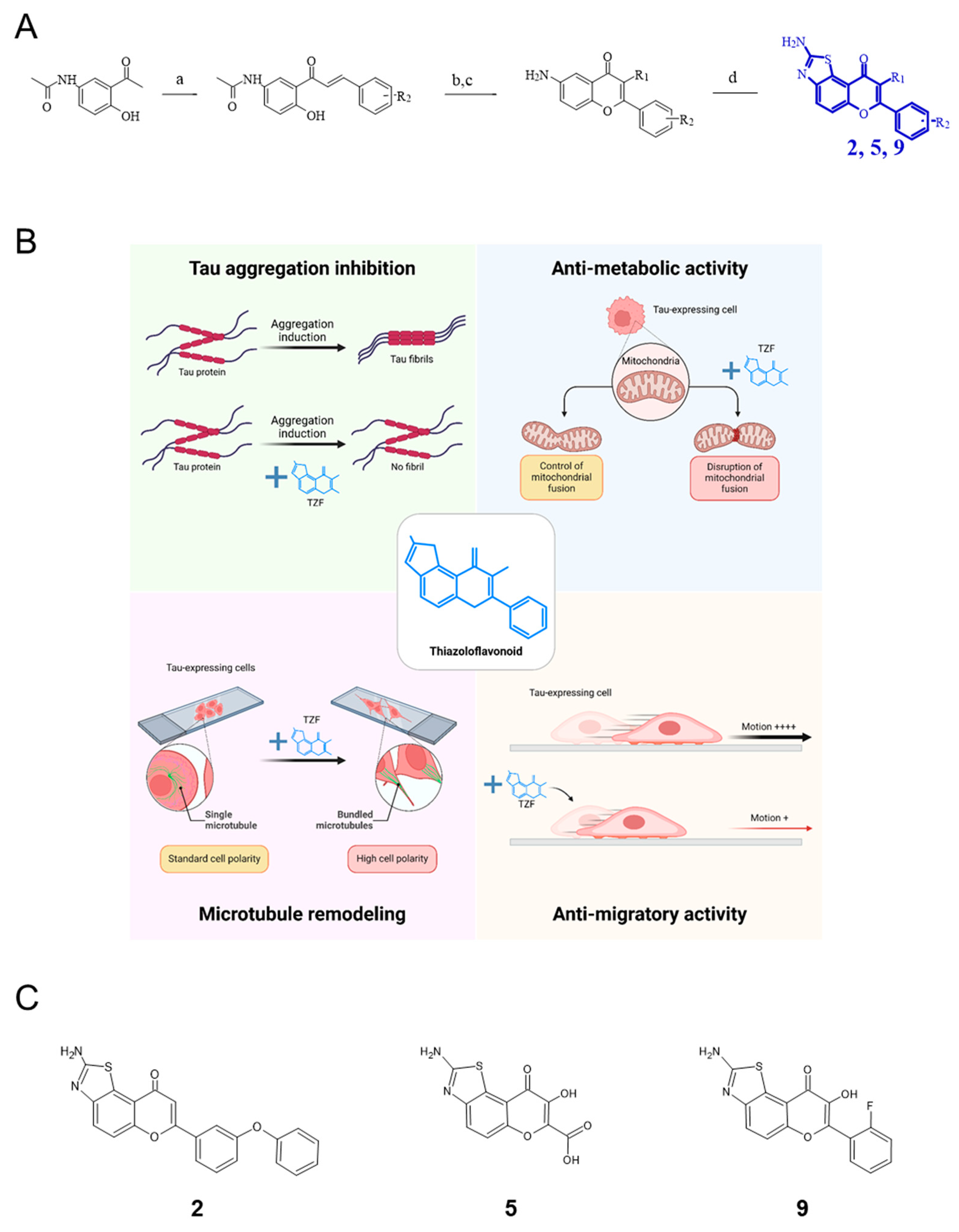
1. Introduction
2. Results
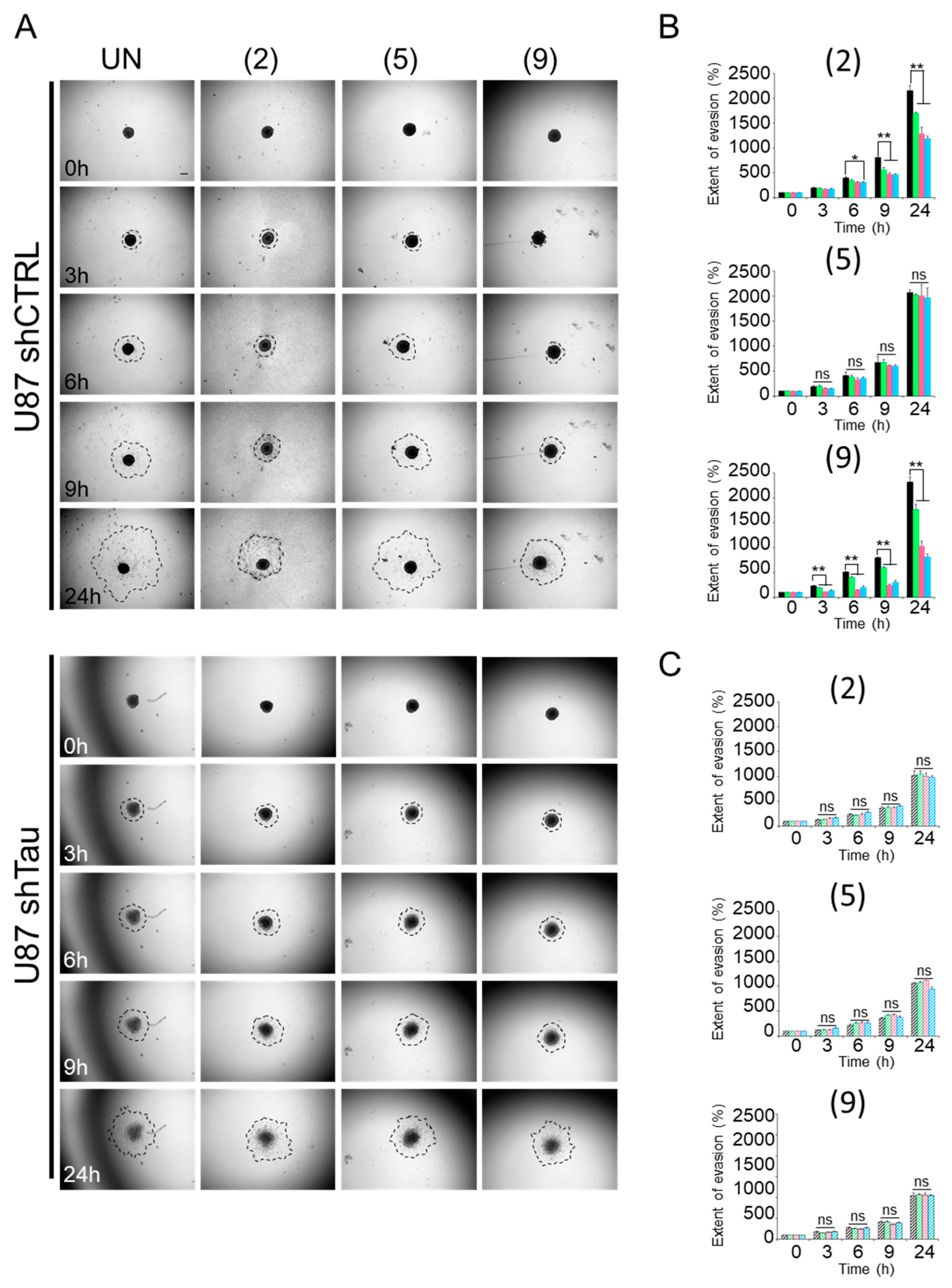
2.1. Compounds 2 and 9 Hinder Evasion of Tau-Expressing Cells from MCSs
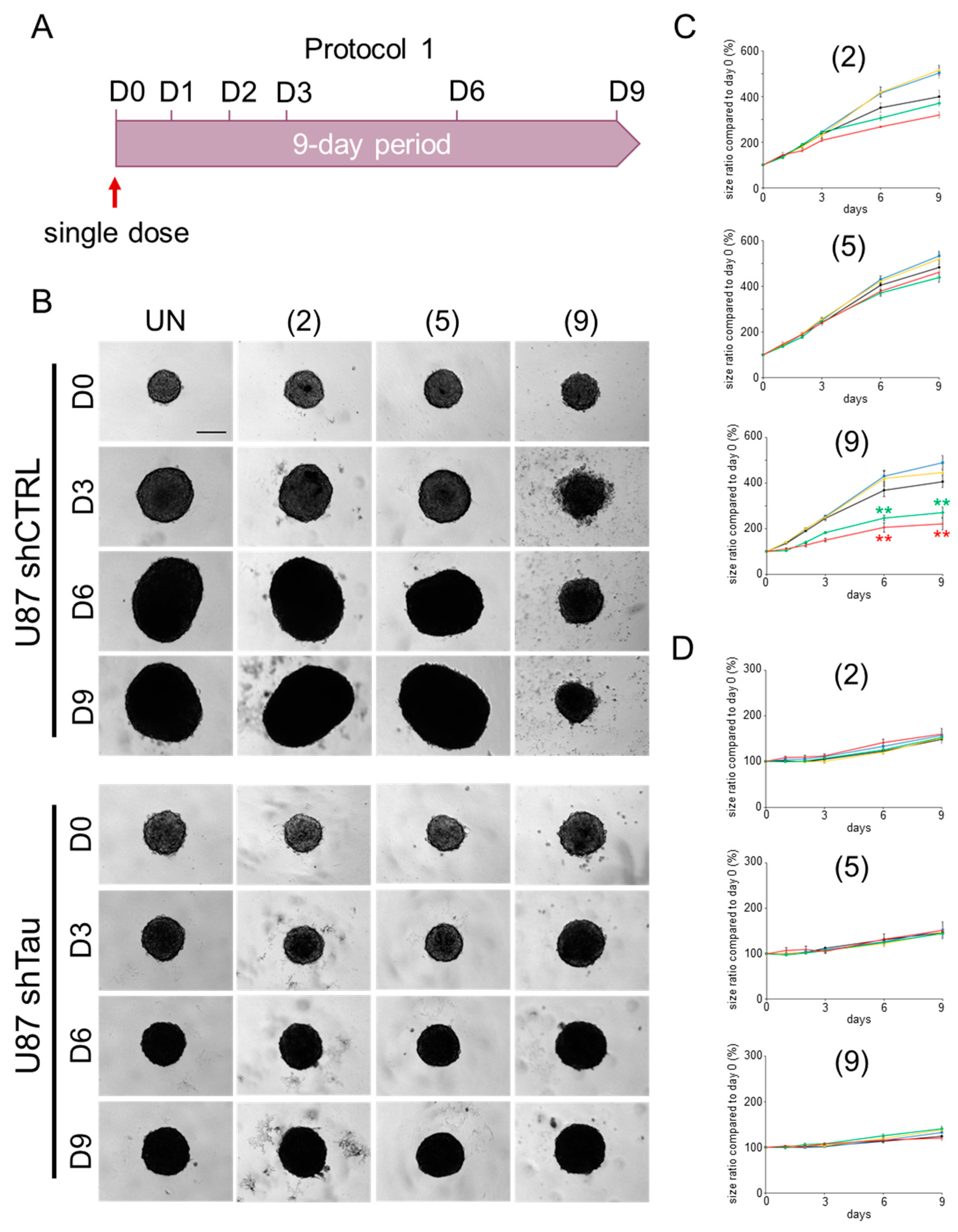
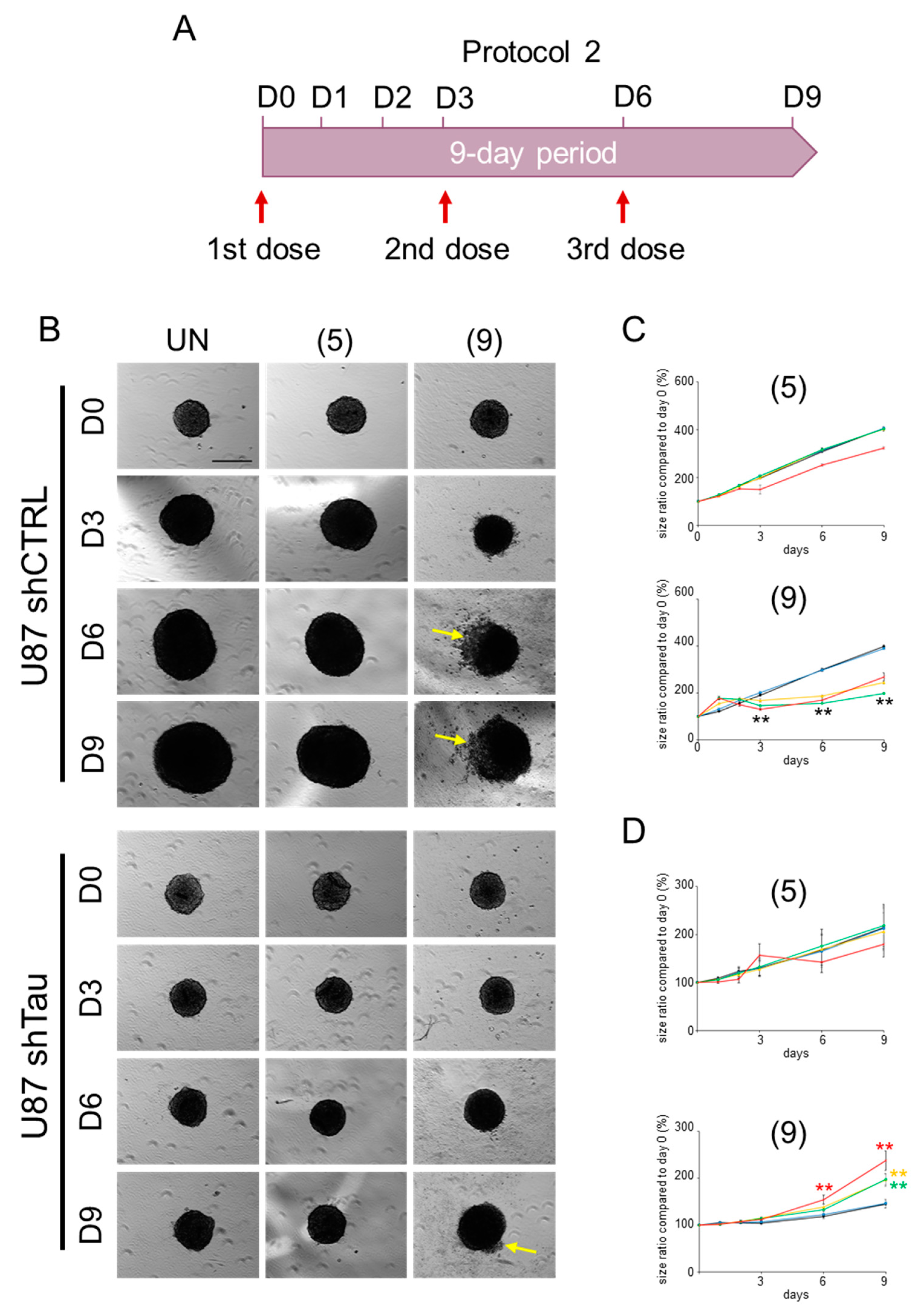
2.2. Compound 9 Affects the Growth of MCSs
2.2.1. Protocol 1: Impact of a Single-Compound Treatment on MCS Growth
2.2.2. Protocol 2: Impact of a Repeated Compound Treatment on MCS Growth
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Biology
4.2.1. Cell Culture
4.2.2. Multi-Cellular Spheroid (MCS) Cell Evasion Assay
4.2.3. MCS Growth Assay
4.2.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Witman, G.B.; Cleveland, D.W.; Weingarten, M.D.; Kirschner, M.W. Tubulin Requires Tau for Growth onto Microtubule Initiating Sites. Proc. Natl. Acad. Sci. USA 1976, 73, 4070–4074. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R. Expression of Separate Isoforms of Human Tau Protein: Correlation with the Tau Pattern in Brain and Effects on Tubulin Polymerization. EMBO J. 1990, 9, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; García-García, E.; Avila, J. Microtubule Depolymerization and Tau Phosphorylation. J. Alzheimers Dis. 2013, 37, 507–513. [Google Scholar] [CrossRef]
- Hochgräfe, K.; Sydow, A.; Mandelkow, E. Regulatable Transgenic Mouse Models of ALzheimer Disease: Onset, Reversibility and Spreading of T Au Pathology. FEBS J. 2013, 280, 4371–4381. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A. GSK-3 Is Essential in the Pathogenesis of Alzheimer’s Disease. J. Alzheimers Dis. 2006, 9, 309–317. [Google Scholar] [CrossRef]
- Wagner, P.; Wang, B.; Clark, E.; Lee, H.; Rouzier, R.; Pusztai, L. Microtubule Associated Protein (MAP)-Tau: A Novel Mediator of Paclitaxel Sensitivity In Vitro and In Vivo. Cell Cycle 2005, 4, 1149–1152. [Google Scholar] [CrossRef]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-Associated Protein Tau: A Marker of Paclitaxel Sensitivity in Breast Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, N.; Shao, G.; Qian, J.; Shen, D.; Fei, Y.; Mao, W.; Wu, D. Relationship Between Gastric Cancer Tau Protein Expression and Paclitaxel Sensitivity. Pathol. Oncol. Res. 2013, 19, 429–435. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Denoulet, P.; Millot, G.; Tatoud, R.; Podgorniak, M.-P.; Tew, K.D.; Calvo, F.; Fellous, A. Estramustine Resistance Correlates with Tau Over-Expression in Human Prostatic Carcinoma Cells. Int. J. Cancer 1998, 77, 626–631. [Google Scholar] [CrossRef]
- Souter, S.; Lee, G. Microtubule-associated Protein Tau in Human Prostate Cancer Cells: Isoforms, Phosphorylation, and Interactions. J. Cell. Biochem. 2009, 108, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Ye, J.C.; Hsieh, C.H.; Chen, L.M.; Lin, T.Y.; Hung, Y.C.; Chang, W.C. PTEN, Tau-AP-3, Thymidylate Synthase Immunohistochemistry Scoring Expression in Patients with Uterine Leiomyomas, Uterine Smooth Muscle Tumors of Uncertain Malignancy Potential and Uterine Leiomyosarcomas. Eur. J. Gynaecol. Oncol. 2011, 32, 496–499. [Google Scholar] [PubMed]
- Ye, J.; Zhang, Z.; Sun, L.; Fang, Y.; Xu, X.; Zhou, G. miR-186 Regulates Chemo-Sensitivity to Paclitaxel via Targeting MAPT in Non-Small Cell Lung Cancer (NSCLC). Mol. Biosyst. 2016, 12, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Gargini, R.; Segura-Collar, B.; Sánchez-Gómez, P. Novel Functions of the Neurodegenerative-Related Gene Tau in Cancer. Front. Aging Neurosci. 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Gargini, R.; Segura-Collar, B.; Herránz, B.; García-Escudero, V.; Romero-Bravo, A.; Núñez, F.J.; García-Pérez, D.; Gutiérrez-Guamán, J.; Ayuso-Sacido, A.; Seoane, J.; et al. The IDH-TAU-EGFR Triad Defines the Neovascular Landscape of Diffuse Gliomas. Sci. Transl. Med. 2020, 12, eaax1501. [Google Scholar] [CrossRef]
- Breuzard, G.; Pagano, A.; Bastonero, S.; Malesinski, S.; Parat, F.; Barbier, P.; Peyrot, V.; Kovacic, H. Tau Regulates the Microtubule-Dependent Migration of Glioblastoma Cells via the Rho-ROCK Signaling Pathway. J. Cell Sci. 2019, 132, jcs222851. [Google Scholar] [CrossRef]
- Pagano, A.; Breuzard, G.; Parat, F.; Tchoghandjian, A.; Figarella-Branger, D.; De Bessa, T.C.; Garrouste, F.; Douence, A.; Barbier, P.; Kovacic, H. Tau Regulates Glioblastoma Progression, 3D Cell Organization, Growth and Migration via the PI3K-AKT Axis. Cancers 2021, 13, 5818. [Google Scholar] [CrossRef]
- Patil, V.; Pal, J.; Somasundaram, K. Elucidating the Cancer-Specific Genetic Alteration Spectrum of Glioblastoma Derived Cell Lines from Whole Exome and RNA Sequencing. Oncotarget 2015, 6, 43452–43471. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Hedna, R.; Di Maio, A.; Robin, M.; Allegro, D.; Tatoni, M.; Peyrot, V.; Barbier, P.; Kovacic, H.; Breuzard, G. 2-Aminothiazole-Flavonoid Hybrid Derivatives Binding to Tau Protein and Responsible for Antitumor Activity in Glioblastoma. Int. J. Mol. Sci. 2023, 24, 15050. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the Formation, Use and Understanding of Multi-Cellular Spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Achilli, T.-M.; McCalla, S.; Tripathi, A.; Morgan, J.R. Quantification of the Kinetics and Extent of Self-Sorting in Three Dimensional Spheroids. Tissue Eng. Part C Methods 2012, 18, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Hwo, S.-Y.; Kirschner, M.W. Physical and Chemical Properties of Purified Tau Factor and the Role of Tau in Microtubule Assembly. J. Mol. Biol. 1977, 116, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Hwo, S.-Y.; Kirschner, M.W. Purification of Tau, a Microtubule-Associated Protein That Induces Assembly of Microtubules from Purified Tubulin. J. Mol. Biol. 1977, 116, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Hedna, R.; Kovacic, H.; Pagano, A.; Peyrot, V.; Robin, M.; Devred, F.; Breuzard, G. Tau Protein as Therapeutic Target for Cancer? Focus on Glioblastoma. Cancers 2022, 14, 5386. [Google Scholar] [CrossRef]
- Couchie, D.; Fages, C.; Bridoux, A.M.; Rolland, B.; Tardy, M.; Nunez, J. Microtubule-Associated Proteins and in Vitro Astrocyte Differentiation. J. Cell Biol. 1985, 101, 2095–2103. [Google Scholar] [CrossRef]
- Couchie, D.; Nunez, J. Immunological Characterization of Microtubule-associated Proteins Specific for the Immature Brain. FEBS Lett. 1985, 188, 331–335. [Google Scholar] [CrossRef]
- Miyazono, M.; Iwaki, T.; Kitamoto, T.; Shin, R.-W.; Fukui, M.; Tateishi, J. Widespread Distribution of Tau in the Astrocytic Elements of Glial Tumors. Acta Neuropathol. 1993, 86, 236–241. [Google Scholar] [CrossRef]
- Jin, F.; Jin-Lee, H.J.; Johnson, A.J. Mouse Models of Experimental Glioblastoma. In Gliomas; Debinski, W., Ed.; Brain Tumor Center of Excellence, Wake Forest Baptist Medical Center Comprehensive Cancer Center: Winston Salem, NC, USA; Exon Publications: Brisbane, Australia, 2021; pp. 15–46. ISBN 978-0-645-00174-7. [Google Scholar]
- Korur, S.; Huber, R.M.; Sivasankaran, B.; Petrich, M.; Morin, P.; Hemmings, B.A.; Merlo, A.; Lino, M.M. GSK3β Regulates Differentiation and Growth Arrest in Glioblastoma. PLoS ONE 2009, 4, e7443. [Google Scholar] [CrossRef]
- Kotliarova, S.; Pastorino, S.; Kovell, L.C.; Kotliarov, Y.; Song, H.; Zhang, W.; Bailey, R.; Maric, D.; Zenklusen, J.C.; Lee, J.; et al. Glycogen Synthase Kinase-3 Inhibition Induces Glioma Cell Death through c-MYC, Nuclear Factor-κB, and Glucose Regulation. Cancer Res. 2008, 68, 6643–6651. [Google Scholar] [CrossRef]
- Yushan, R.; Wenjie, C.; Suning, H.; Yiwu, D.; Tengfei, Z.; Madushi, W.M.; Feifei, L.; Changwen, Z.; Xin, W.; Roodrajeetsing, G.; et al. Insights into the Clinical Value of Cyclin-Dependent Kinase 5 in Glioma: A Retrospective Study. World, J. Surg. Oncol. 2015, 13, 223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Do, P.A.; Lee, C.H. The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers 2020, 13, 101. [Google Scholar] [CrossRef]
- Danielson, P.B. The Cytochrome P450 Superfamily: Biochemistry, Evolution and Drug Metabolism in Humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef]
- Dubois, C.; Dufour, R.; Daumar, P.; Aubel, C.; Szczepaniak, C.; Blavignac, C.; Mounetou, E.; Penault-Llorca, F.; Bamdad, M. Development and Cytotoxic Response of Two Proliferative MDA-MB-231 and Non-Proliferative SUM1315 Three-Dimensional Cell Culture Models of Triple-Negative Basal-like Breast Cancer Cell Lines. Oncotarget 2017, 8, 95316–95331. [Google Scholar] [CrossRef]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in Drug Sensitivity between Two-Dimensional and Three-Dimensional Culture Systems in Triple-Negative Breast Cancer Cell Lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Eder, T.; Weber, A.; Neuwirt, H.; Grünbacher, G.; Ploner, C.; Klocker, H.; Sampson, N.; Eder, I. Cancer-Associated Fibroblasts Modify the Response of Prostate Cancer Cells to Androgen and Anti-Androgens in Three-Dimensional Spheroid Culture. Int. J. Mol. Sci. 2016, 17, 1458. [Google Scholar] [CrossRef]
- De Simone, U.; Roccio, M.; Gribaldo, L.; Spinillo, A.; Caloni, F.; Coccini, T. Human 3D Cultures as Models for Evaluating Magnetic Nanoparticle CNS Cytotoxicity after Short- and Repeated Long-Term Exposure. Int. J. Mol. Sci. 2018, 19, 1993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, F.; Bulloj, A.; Zhang, Y.-W.; Tong, G.; Zhang, Z.; Liao, F.-F.; Xu, H.; Zhang, X.; Li, F.; et al. Tumor Suppressor PTEN Affects Tau Phosphorylation, Aggregation, and Binding to Microtubules. FASEB J. 2006, 20, 1272–1274. [Google Scholar] [CrossRef]
- Avila, J.; Hernández, F. GSK-3 Inhibitors for Alzheimer’s Disease. Expert Rev. Neurother. 2007, 7, 1527–1533. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The P53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Sola, M.; Magrin, C.; Pedrioli, G.; Pinton, S.; Salvadè, A.; Papin, S.; Paganetti, P. Tau Affects P53 Function and Cell Fate during the DNA Damage Response. Commun. Biol. 2020, 3, 245. [Google Scholar] [CrossRef] [PubMed]
- JazvinšćakJembrek, M.; Slade, N.; Hof, P.R.; Šimić, G. The Interactions of P53 with Tau and Aß as Potential Therapeutic Targets for Alzheimer’s Disease. Prog. Neurobiol. 2018, 168, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Li, H.-L.; Liu, R.; Zhang, Y.; Liao, K.; Wang, Q.; Wang, J.-Z.; Liu, S.-J. Tau Overexpression Inhibits Cell Apoptosis with the Mechanisms Involving Multiple Viability-Related Factors. J. Alzheimers Dis. 2010, 21, 167–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relave, E.T.; Hedna, R.; Di Maio, A.; Devred, F.; Kovacic, H.; Robin, M.; Breuzard, G. Therapeutic Contribution of Tau-Binding Thiazoloflavonoid Hybrid Derivatives Against Glioblastoma Using Pharmacological Approach in 3D Spheroids. Int. J. Mol. Sci. 2024, 25, 11785. https://doi.org/10.3390/ijms252111785
Relave ET, Hedna R, Di Maio A, Devred F, Kovacic H, Robin M, Breuzard G. Therapeutic Contribution of Tau-Binding Thiazoloflavonoid Hybrid Derivatives Against Glioblastoma Using Pharmacological Approach in 3D Spheroids. International Journal of Molecular Sciences. 2024; 25(21):11785. https://doi.org/10.3390/ijms252111785
Chicago/Turabian StyleRelave, Emmanuelle T., Rayane Hedna, Attilio Di Maio, François Devred, Hervé Kovacic, Maxime Robin, and Gilles Breuzard. 2024. "Therapeutic Contribution of Tau-Binding Thiazoloflavonoid Hybrid Derivatives Against Glioblastoma Using Pharmacological Approach in 3D Spheroids" International Journal of Molecular Sciences 25, no. 21: 11785. https://doi.org/10.3390/ijms252111785
APA StyleRelave, E. T., Hedna, R., Di Maio, A., Devred, F., Kovacic, H., Robin, M., & Breuzard, G. (2024). Therapeutic Contribution of Tau-Binding Thiazoloflavonoid Hybrid Derivatives Against Glioblastoma Using Pharmacological Approach in 3D Spheroids. International Journal of Molecular Sciences, 25(21), 11785. https://doi.org/10.3390/ijms252111785





