Electron Tomography as a Tool to Study SARS-CoV-2 Morphology
Abstract
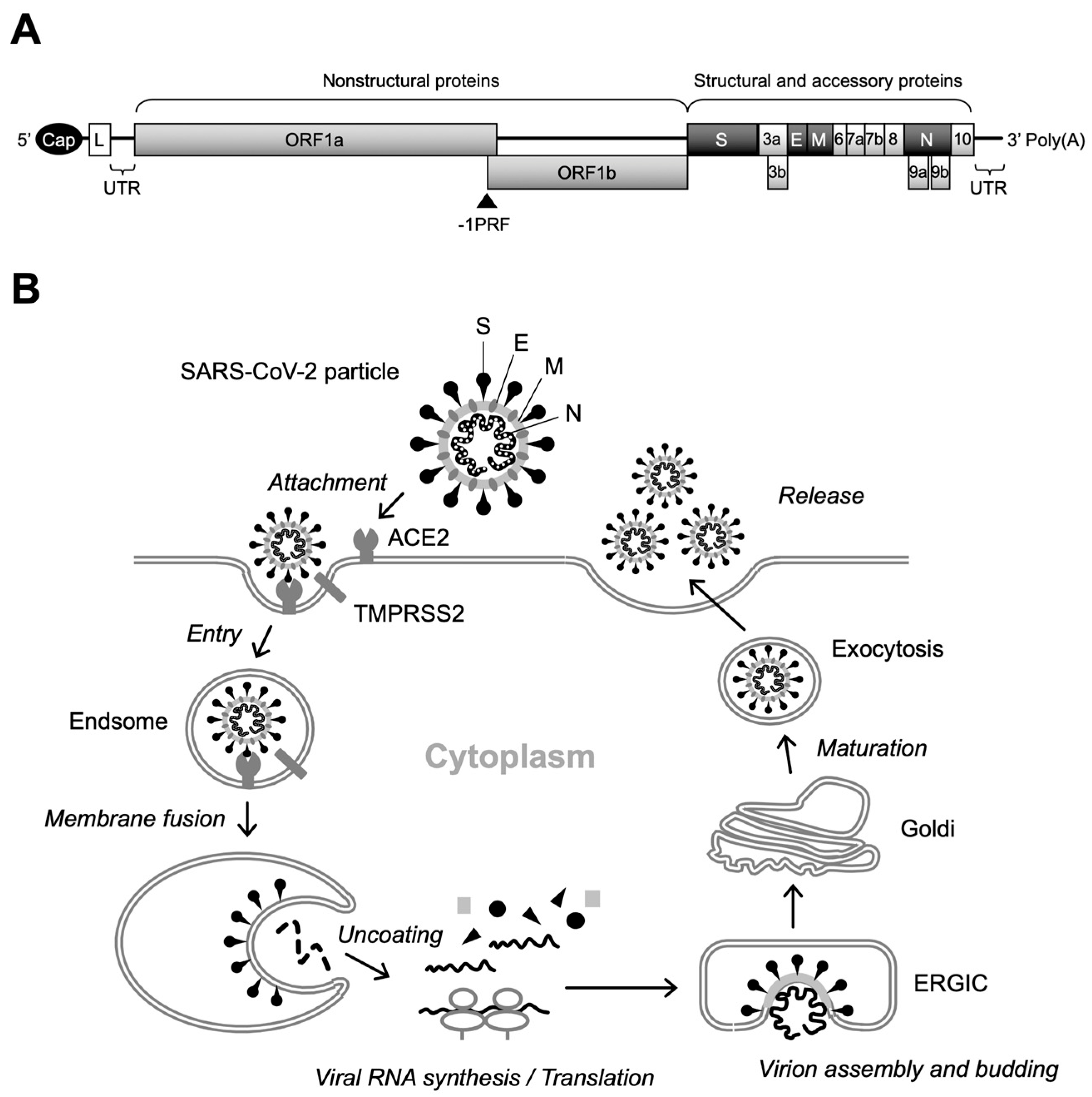
1. Introduction
2. Current Applications of TEM and ET in Viral Analysis
3. New Insights into SARS-Cov-2 Particle Formation Obtained by TEM and ET
4. EM as a Tool to Study the Morphological Integrity of Genetically Engineered Viruses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colantuoni, A.; Martini, R.; Caprari, P.; Ballestri, M.; Capecchi, P.L.; Gnasso, A.; Presti, R.L.; Marcoccia, A.; Rossi, M.; Caimi, G. COVID-19 Sepsis and Microcirculation Dysfunction. Front. Physiol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 August 2024).
- WHO Director-General’s Opening Remarks at the Media Briefing—5 May 2023. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing---5-may-2023 (accessed on 28 October 2024).
- Li, X.; Mi, Z.; Liu, Z.; Rong, P. SARS-CoV-2: Pathogenesis, Therapeutics, Variants, and Vaccines. Front. Microbiol. 2024, 15, 1334152. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Hardenbrook, N.J.; Zhang, P. A Structural View of the SARS-CoV-2 Virus and Its Assembly. Curr. Opin. Virol. 2022, 52, 123–134. [Google Scholar] [CrossRef]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef] [PubMed]
- Riegger, R.J.; Caliskan, N. Thinking Outside the Frame: Impacting Genomes Capacity by Programmed Ribosomal Frameshifting. Front. Mol. Biosci. 2022, 9, 842261. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-NCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, Y.; Sanyal, S. Membrane Heist: Coronavirus Host Membrane Remodeling during Replication. Biochimie 2020, 179, 229–236. [Google Scholar] [CrossRef]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 Structure and Replication Characterized by In Situ Cryo-Electron Tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef]
- Wolff, G.; Limpens, R.W.A.L.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; Jong, A.W.M.d.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A Molecular Pore Spans the Double Membrane of the Coronavirus Replication Organelle. Science 2020, 369, 1395–1398. [Google Scholar] [CrossRef]
- Cortese, M.; Lee, J.-Y.; Cerikan, B.; Neufeldt, C.J.; Oorschot, V.M.J.; Köhrer, S.; Hennies, J.; Schieber, N.L.; Ronchi, P.; Mizzon, G.; et al. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe. 2020, 28, 853–866.e5. [Google Scholar] [CrossRef]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535.e14. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.-F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Martines, R.B.; Ritter, J.M.; Matkovic, E.; Gary, J.; Bollweg, B.C.; Bullock, H.; Goldsmith, C.S.; Silva-Flannery, L.; Seixas, J.N.; Reagan-Steiner, S.; et al. Early Release–Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States–Volume 26, Number 9—September 2020–Emerging Infectious Diseases Journal–CDC. Emerg. Infect. Dis. 2020, 26, 2005–2015. [Google Scholar] [CrossRef]
- Dittmayer, C.; Meinhardt, J.; Radbruch, H.; Radke, J.; Heppner, B.I.; Heppner, F.L.; Stenzel, W.; Holland, G.; Laue, M. Why Misinterpretation of Electron Micrographs in SARS-CoV-2-Infected Tissue Goes Viral. Lancet 2020, 396, e64–e65. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Frank, J. “Just in Time”: The Role of Cryo-Electron Microscopy in Combating Recent Pandemics. Biochemistry 2021, 60, 3449–3451. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e13. [Google Scholar] [CrossRef]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and Distributions of SARS-CoV-2 Spike Proteins on Intact Virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Turoňová, B.; Sikora, M.; Schürmann, C.; Hagen, W.J.H.; Welsch, S.; Blanc, F.E.C.; Von Bülow, S.; Gecht, M.; Bagola, K.; Hörner, C.; et al. In Situ Structural Analysis of SARS-CoV-2 Spike Reveals Flexibility Mediated by Three Hinges. Science 2020, 370, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.; Howe, A.; Gilchrist, J.B.; Sheng, Y.; Sun, D.; Knight, M.L.; Zanetti-Domingues, L.C.; Bateman, B.; Krebs, A.-S.; Chen, L.; et al. Correlative Multi-Scale Cryo-Imaging Unveils SARS-CoV-2 Assembly and Egress. Nat. Commun. 2021, 12, 4629. [Google Scholar] [CrossRef]
- Wu, H.; Fujioka, Y.; Sakaguchi, S.; Suzuki, Y.; Nakano, T. Three-Dimensional Reconstruction by Electron Tomography for the Application to Ultrastructural Analysis of SARS-CoV-2 Particles. Med. Mol. Morphol. 2021, 55, 1–8. [Google Scholar] [CrossRef]
- Wu, H.; Fujioka, Y.; Sakaguchi, S.; Suzuki, Y.; Nakano, T. Morphological Analysis for Two Types of Viral Particles in Vacuoles of SARS-CoV-2-Infected Cells. Méd. Mol. Morphol. 2024, 57, 124–135. [Google Scholar] [CrossRef]
- Otegui, M.S.; Pennington, J.G. Electron Tomography in Plant Cell Biology. Microscopy 2019, 68, 69–79. [Google Scholar] [CrossRef]
- Saibil, H.R. Cryo-EM in Molecular and Cellular Biology. Mol. Cell 2022, 82, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, M.V. Transmission electron microscopy: Chemical fixation, freezing methods, and inmunolocalization. In The Maize Handbook; Springer: New York, NY, USA, 1994; pp. 118–134. [Google Scholar]
- Carter, M.; Essner, R.; Goldstein, N.; Iyer, M. Microscopy. In Guide to Research Techniques in Neuroscience, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 115–143. [Google Scholar]
- Cheng, Y. Single-Particle Cryo-EM at Crystallographic Resolution. Cell 2015, 161, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Taha, B.A.; Mashhadany, Y.A.; Al-Jumaily, A.H.J.; Zan, M.S.D.B.; Arsad, N. SARS-CoV-2 Morphometry Analysis and Prediction of Real Virus Levels Based on Full Recurrent Neural Network Using TEM Images. Viruses 2022, 14, 2386. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, M.; Baraldi, C.; Sopetto, G.B.; Finozzi, D.; Gentile, C.; Gentile, M.D.; Marconi, R.; Paladino, D.; Raoss, A.; Riedmiller, I.; et al. SARS-CoV-2 and the Host Cell: A Tale of Interactions. Front. Virol. 2022, 1, 815388. [Google Scholar] [CrossRef]
- Pinto, A.L.; Rai, R.K.; Brown, J.C.; Griffin, P.; Edgar, J.R.; Shah, A.; Singanayagam, A.; Hogg, C.; Barclay, W.S.; Futter, C.E.; et al. Ultrastructural Insight into SARS-CoV-2 Entry and Budding in Human Airway Epithelium. Nat. Commun. 2022, 13, 1609. [Google Scholar] [CrossRef]
- Knoops, K.; Kikkert, M.; Worm, S.H.E.V.D.; Zevenhoven-Dobbe, J.C.; Van Der Meer, Y.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. SARS-Coronavirus Replication Is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. PLoS Biol. 2008, 6, e226. [Google Scholar] [CrossRef]
- Barreto-Vieira, D.F.; da Silva, M.A.N.; de Almeida, A.L.T.; Rasinhas, A.d.C.; Monteiro, M.E.; Miranda, M.D.; Motta, F.C.; Siqueira, M.M.; Girard-Dias, W.; Archanjo, B.S.; et al. SARS-CoV-2: Ultrastructural Characterization of Morphogenesis in an In Vitro System. Viruses 2022, 14, 201. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, H.; Huang, W.; Zhou, J.; Qiu, M.; Deng, Z.; Chen, L.; Weng, Y.; Cai, L.; Gu, Y.; et al. Cell Morphological Analysis of SARS-CoV-2 Infection by Transmission Electron Microscopy. J. Thorac. Dis. 2020, 12, 4368–4373. [Google Scholar] [CrossRef]
- Calder, L.J.; Calcraft, T.; Hussain, S.; Harvey, R.; Rosenthal, P.B. Electron Cryotomography of SARS-CoV-2 Virions Reveals Cylinder-Shaped Particles with a Double Layer RNP Assembly. Commun. Biol. 2022, 5, 1210. [Google Scholar] [CrossRef]
- Vogt, V.M. Retroviral Virions and Genomes. In Retroviruses; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011. [Google Scholar]
- Takasaki, T.; Kurane, I.; Aihara, H.; Ohkawa, N.; Yamaguchi, J. Electron Microscopic Study of Human Immunodeficiency Virus Type 1 (HIV-1) Core Structure: Two RNA Strands in the Core of Mature and Budding Particles. Arch. Virol. 1997, 142, 375–382. [Google Scholar] [CrossRef]
- Saha, I.; Saffarian, S. Dynamics of the HIV Gag Lattice Detected by Localization Correlation Analysis and Time-Lapse IPALM. Biophys. J. 2020, 119, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Márquez, C.L.; Parker, M.W.; Böcking, A.T. Negative Staining Transmission Electron Microscopy of HIV Viral Particles Permeabilized with PFO and Capsid Stabilized with IP6. Bio Protoc. 2022, 12, e4536. [Google Scholar] [CrossRef] [PubMed]
- Márquez, C.L.; Lau, D.; Walsh, J.; Shah, V.; McGuinness, C.; Wong, A.; Aggarwal, A.; Parker, M.W.; Jacques, D.A.; Turville, S.; et al. Kinetics of HIV-1 Capsid Uncoating Revealed by Single-Molecule Analysis. eLife 2018, 7, e34772. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.; Ganser-Pornillos, B.K.; Tivol, W.F.; Sundquist, W.I.; Jensen, G.J. Three-Dimensional Structure of HIV-1 Virus-like Particles by Electron Cryotomography. J. Mol. Biol. 2005, 346, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.G.; Wilk, T.; Welker, R.; Kräusslich, H.; Fuller, S.D. Structural Organization of Authentic, Mature HIV-1 Virions and Cores. EMBO J. 2003, 22, 1707–1715. [Google Scholar] [CrossRef]
- Prasad, V.M.; Leaman, D.P.; Lovendahl, K.N.; Croft, J.T.; Benhaim, M.A.; Hodge, E.A.; Zwick, M.B.; Lee, K.K. Cryo-ET of Env on Intact HIV Virions Reveals Structural Variation and Positioning on the Gag Lattice. Cell 2022, 185, 641–653.e17. [Google Scholar] [CrossRef]
- Ward, A.E.; Kiessling, V.; Pornillos, O.; White, J.M.; Ganser-Pornillos, B.K.; Tamm, L.K. HIV-Cell Membrane Fusion Intermediates Are Restricted by Serincs as Revealed by Cryo-Electron and TIRF Microscopy. J. Biol. Chem. 2020, 295, 15183–15195. [Google Scholar] [CrossRef]
- Passos, D.O.; Li, M.; Yang, R.; Rebensburg, S.V.; Ghirlando, R.; Jeon, Y.; Shkriabai, N.; Kvaratskhelia, M.; Craigie, R.; Lyumkis, D. Cryo-EM Structures and Atomic Model of the HIV-1 Strand Transfer Complex Intasome. Science 2017, 355, 89–92. [Google Scholar] [CrossRef]
- Philippe, N.; Legendre, M.; Doutre, G.; Couté, Y.; Poirot, O.; Lescot, M.; Arslan, D.; Seltzer, V.; Bertaux, L.; Bruley, C.; et al. Pandoraviruses: Amoeba Viruses with Genomes Up to 2.5 Mb Reaching That of Parasitic Eukaryotes. Science 2013, 341, 281–286. [Google Scholar] [CrossRef]
- Burton-Smith, R.N.; Murata, K. Cryo-Electron Microscopy of the Giant Viruses. Microscopy 2021, 70, 477–486. [Google Scholar] [CrossRef]
- Xiao, C.; Fischer, M.G.; Bolotaulo, D.M.; Ulloa-Rondeau, N.; Avila, G.A.; Suttle, C.A. Cryo-EM Reconstruction of the Cafeteria Roenbergensis Virus Capsid Suggests Novel Assembly Pathway for Giant Viruses. Sci. Rep. 2017, 7, 5484. [Google Scholar] [CrossRef] [PubMed]
- Chihara, A.; Burton-Smith, R.N.; Kajimura, N.; Mitsuoka, K.; Okamoto, K.; Song, C.; Murata, K. A Novel Capsid Protein Network Allows the Characteristic Internal Membrane Structure of Marseilleviridae Giant Viruses. Sci. Rep. 2022, 12, 21428. [Google Scholar] [CrossRef]
- Downing, K.H.; Glaeser, R.M. Estimating the Effect of Finite Depth of Field in Single-Particle Cryo-EM. Ultramicroscopy 2018, 184, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced Isolation of SARS-CoV-2 by TMPRSS2-Expressing Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Mastronarde, D.N. Automated Electron Microscope Tomography Using Robust Prediction of Specimen Movements. J. Struct. Biol. 2005, 152, 36–51. [Google Scholar] [CrossRef]
- Gan, L.; Jensen, G.J. Electron Tomography of Cells. Q. Rev. Biophys. 2012, 45, 27–56. [Google Scholar] [CrossRef]
- McEwen, B.F.; Marko, M. The Emergence of Electron Tomography as an Important Tool for Investigating Cellular Ultrastructure. J. Histochem. Cytochem. 2000, 49, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Low, J.G.H.; Lee, L.S.; Ooi, E.E.; Ethirajulu, K.; Yeo, P.; Matter, A.; Connolly, J.E.; Skibinski, D.A.G.; Saudan, P.; Bachmann, M.; et al. Safety and Immunogenicity of a Virus-like Particle Pandemic Influenza A (H1N1) 2009 Vaccine: Results from a Double-Blinded, Randomized Phase I Clinical Trial in Healthy Asian Volunteers. Vaccine 2014, 32, 5041–5048. [Google Scholar] [CrossRef][Green Version]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major Findings and Recent Advances in Virus–like Particle (VLP)-Based Vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Chen, G.L.; Coates, E.E.; Plummer, S.H.; Carter, C.A.; Berkowitz, N.; Conan-Cibotti, M.; Cox, J.H.; Beck, A.; O’Callahan, M.; Andrews, C.; et al. Effect of a Chikungunya Virus–Like Particle Vaccine on Safety and Tolerability Outcomes. JAMA 2020, 323, 1369–1377. [Google Scholar] [CrossRef]
- Swann, H.; Sharma, A.; Preece, B.; Peterson, A.; Eldredge, C.; Belnap, D.M.; Vershinin, M.; Saffarian, S. Minimal System for Assembly of SARS-CoV-2 Virus like Particles. Sci. Rep. 2020, 10, 21877. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.M.; Taha, T.Y.; Tabata, T.; Chen, I.P.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; Chen, P.-Y.; Hayashi, J.M.; Soczek, K.M.; et al. Rapid Assessment of SARS-CoV-2 Evolved Variants Using Virus-like Particles. Science 2021, 374, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.M.; Ciling, A.; Taha, T.Y.; Chen, I.P.; Khalid, M.M.; Sreekumar, B.; Chen, P.-Y.; Kumar, G.R.; Suryawanshi, R.; Silva, I.; et al. Omicron Mutations Enhance Infectivity and Reduce Antibody Neutralization of SARS-CoV-2 Virus-like Particles. Proc. Natl. Acad. Sci. USA 2022, 119, e2200592119. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System. Front. Bioeng. Biotechnol. 2020, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Suzuki, Y.; Ishida, K.; Arakawa, M.; Wu, H.; Fujioka, Y.; Emi, A.; Maeda, K.; Hamajima, R.; Nakano, T.; et al. Distinct Motifs in the E Protein Are Required for SARS-CoV-2 Virus Particle Formation and Lysosomal Deacidification in Host Cells. J. Virol. 2023, 97, e00426-23. [Google Scholar] [CrossRef]
- Racaniello, V.R.; Baltimore, D. Cloned Poliovirus Complementary DNA Is Infectious in Mammalian Cells. Science 1981, 214, 916–919. [Google Scholar] [CrossRef]
- Ávila-Pérez, G.; Nogales, A.; Martín, V.; Almazán, F.; Martínez-Sobrido, L. Reverse Genetic Approaches for the Generation of Recombinant Zika Virus. Viruses 2018, 10, 597. [Google Scholar] [CrossRef]
- Aubry, F.; Nougairède, A.; Gould, E.A.; Lamballerie, X. de Flavivirus Reverse Genetic Systems, Construction Techniques and Applications: A Historical Perspective. Antivir. Res. 2015, 114, 67–85. [Google Scholar] [CrossRef]
- Kurhade, C.; Xie, X.; Shi, P.-Y. Reverse Genetic Systems of SARS-CoV-2 for Antiviral Research. Antivir. Res. 2023, 210, 105486. [Google Scholar] [CrossRef]
- Kril, V.; Aïqui-Reboul-Paviet, O.; Briant, L.; Amara, A. New Insights into Chikungunya Virus Infection and Pathogenesis. Annu. Rev. Virol. 2021, 8, 327–347. [Google Scholar] [CrossRef]
- Halstead, S.B. Reappearance of Chikungunya, Formerly Called Dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tanaka, A.; Maeda, Y.; Emi, A.; Fujioka, Y.; Sakaguchi, S.; Vasudevan, S.G.; Kobayashi, T.; Lim, C.-K.; Takasaki, T.; et al. Construction and Characterization of an Infectious Clone Generated from Chikungunya Virus SL11131 Strain. Virology 2021, 552, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Nakano, T.; Sakuragi, S.; Shioda, T.; Sano, K.; Sakuragi, J. The Relationship between HIV-1 Genome RNA Dimerization, Virion Maturation and Infectivity. Nucleic Acids Res. 2011, 39, 3404–3417. [Google Scholar] [CrossRef]
- Monath, T.P.; Barrett, A.D.T. Pathogenesis and Pathophysiology of Yellow Fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [CrossRef]
- Monath, T.P. Treatment of Yellow Fever. Antivir. Res. 2008, 78, 116–124. [Google Scholar] [CrossRef]
- Bodakuntla, S.; Kuhn, C.C.; Biertümpfel, C.; Mizuno, N. Cryo-Electron Microscopy in the Fight against COVID-19—Mechanism of Virus Entry. Front. Mol. Biosci. 2023, 10, 1252529. [Google Scholar] [CrossRef]
- Saville, J.W.; Berezuk, A.M.; Srivastava, S.S.; Subramaniam, S. Three-Dimensional Visualization of Viral Structure, Entry, and Replication Underlying the Spread of SARS-CoV-2. Chem. Rev. 2022, 122, 14066–14084. [Google Scholar] [CrossRef] [PubMed]
- Leigh, K.E.; Modis, Y. Imaging and Visualizing SARS-CoV-2 in a New Era for Structural Biology. Interface Focus 2021, 11, 20210019. [Google Scholar] [CrossRef]
- Liu, C.; Mendonça, L.; Yang, Y.; Gao, Y.; Shen, C.; Liu, J.; Ni, T.; Ju, B.; Liu, C.; Tang, X.; et al. The Architecture of Inactivated SARS-CoV-2 with Postfusion Spikes Revealed by Cryo-EM and Cryo-ET. Structure 2020, 28, 1218–1224.e4. [Google Scholar] [CrossRef]
- Calder, L.J.; Wasilewski, S.; Berriman, J.A.; Rosenthal, P.B. Structural Organization of a Filamentous Influenza A Virus. Proc. Natl. Acad. Sci. USA 2010, 107, 10685–10690. [Google Scholar] [CrossRef]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor Binding and Priming of the Spike Protein of SARS-CoV-2 for Membrane Fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, Y.; Kume, N.P.; Sakata, K.; Sato, S.B. Fine Structure of Influenza A Virus Observed by Electron Cryo-microscopy. Embo J. 1994, 13, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Song, L.; Dunker, A.K.; Foster, J.A.; Uversky, V.N.; Goh, G.K.-M. A Comparative Experimental and Computational Study on the Nature of the Pangolin-CoV and COVID-19 Omicron. Int. J. Mol. Sci. 2024, 25, 7537. [Google Scholar] [CrossRef]
- Johnson, B.A.; Zhou, Y.; Lokugamage, K.G.; Vu, M.N.; Bopp, N.; Crocquet-Valdes, P.A.; Kalveram, B.; Schindewolf, C.; Liu, Y.; Scharton, D.; et al. Nucleocapsid Mutations in SARS-CoV-2 Augment Replication and Pathogenesis. PLoS Pathog. 2022, 18, e1010627. [Google Scholar] [CrossRef]


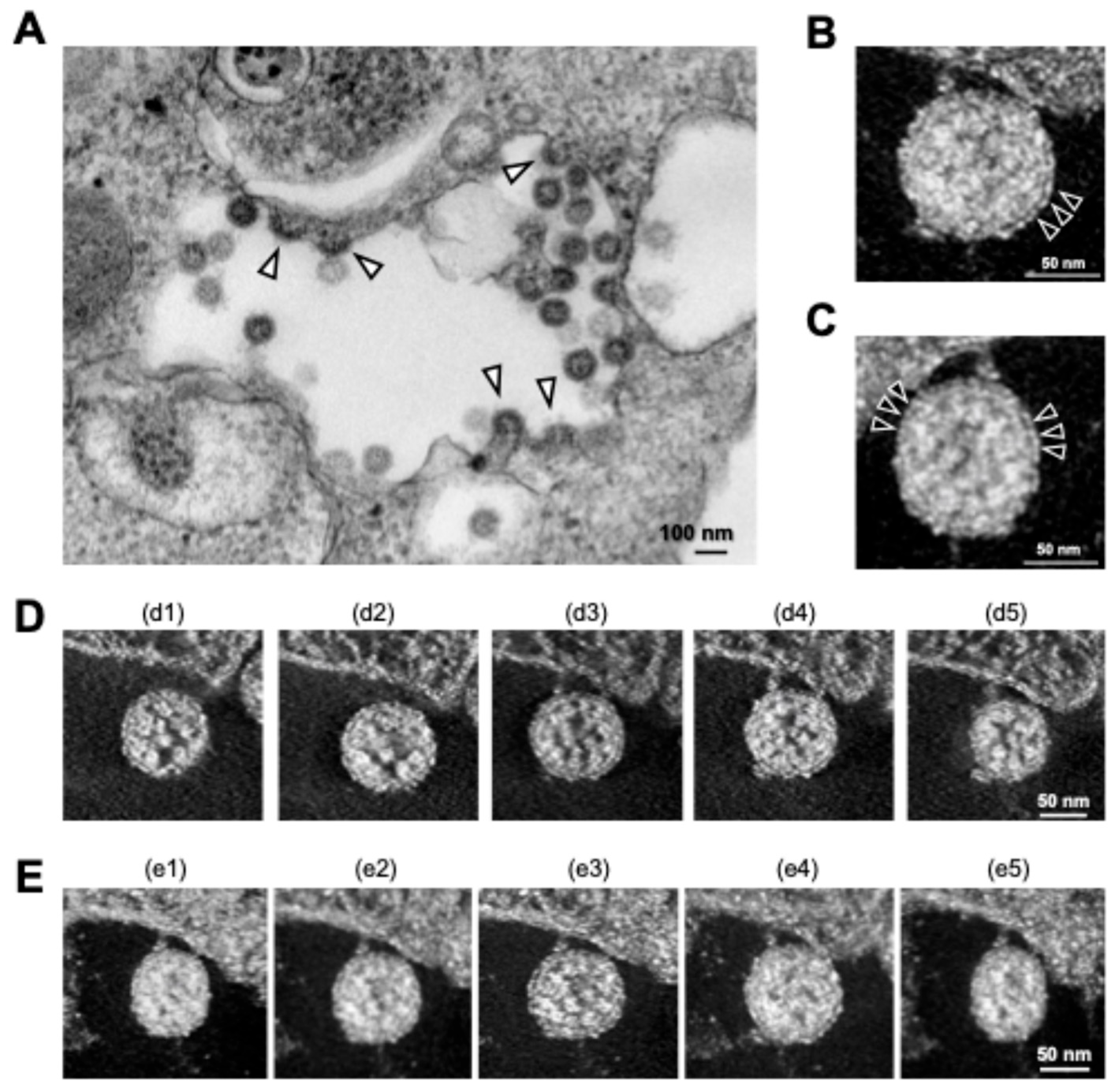
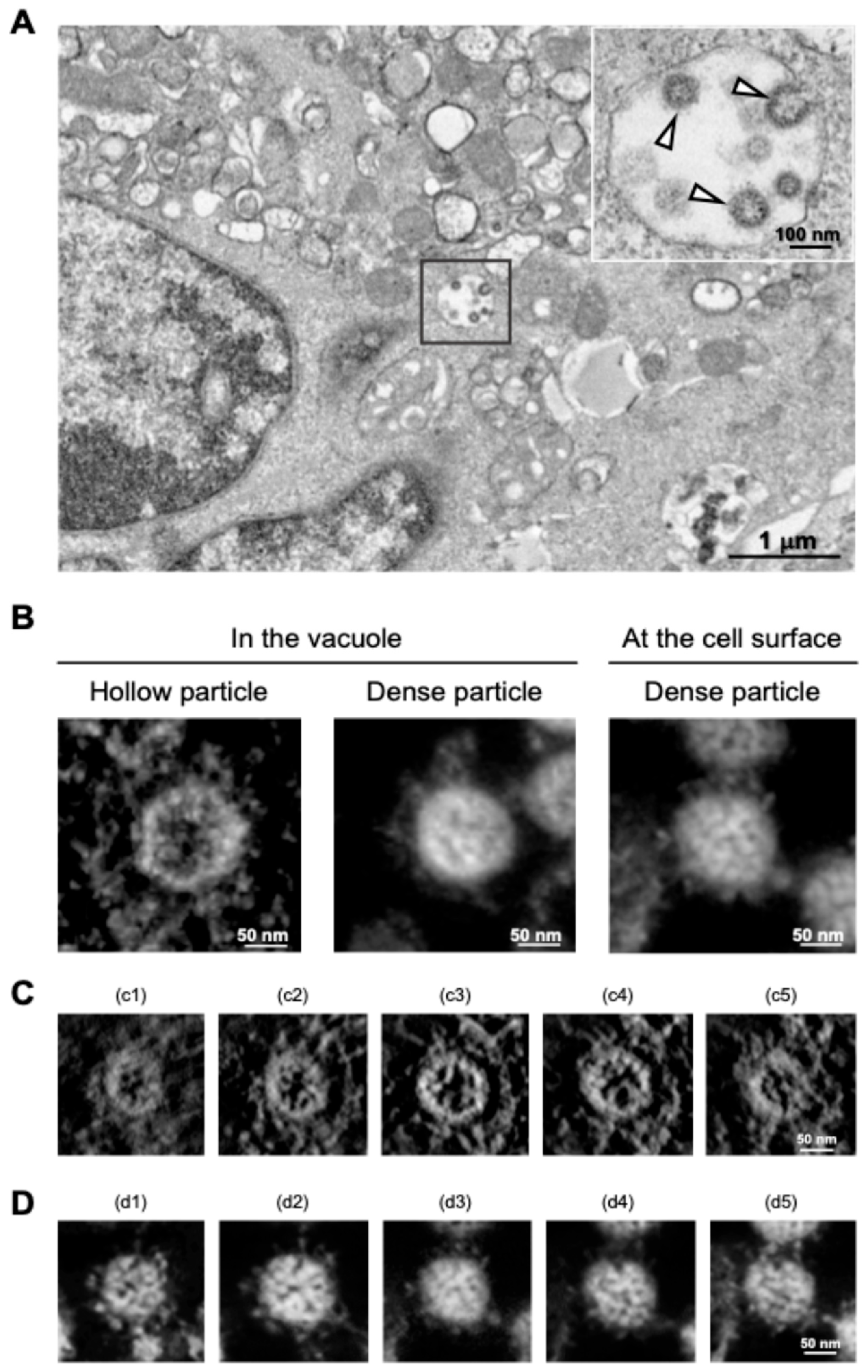
| Virus | EM Methods | Findings | References |
|---|---|---|---|
| SARS-CoV-2 | TEM | SARS-CoV-2 virions are spherical or pleomorphic, with diameters ranging from 80 to 120 nm. | [37] |
| TEM | The internal structure of SARS-CoV-2, including the helical nucleocapsid and the replication of the virus within the cytoplasm. | [18,39] | |
| TEM | The exocytosis of virus particles and ultrastructural changes in infected cells. | [41] | |
| Cryo-ET | The roughly spherical shape of the virus, with a diameter of approximately 89.8 nm, and the conformational states of the spike proteins. | [16] | |
| Cryo-ET | How the virus packs its 30 kb-long RNA genome. | [26,43] | |
| Cryo-ET | Cryo-ET showed the formation of DMVs within infected cells and the assembly and budding of virions at the ERGIC. | [29] | |
| HIV | TEM | HIV particles are spherical, possess an envelope derived from the host cell membrane, and contain a cone-shaped core encapsulating the viral RNA genome. | [44] |
| TEM | Mature HIV-1 particles contain two copies of RNA strands within the core, forming an interwound, coiling structure. | [45] | |
| TEM | The pleomorphic nature of HIV capsids. | [46] | |
| TEM | Inositol hexakisphosphate (IP6) stabilized the capsid structure, preventing premature disassembly during sample preparation. | [47,48] | |
| Cryo-ET | High-resolution 3D views of HIV, revealing the Env glycoproteins on the virion surface and a conical core encapsulating the viral genome. | [49,50] | |
| Cryo-ET | The structural heterogeneity of Env trimers, with a flexible stalk that allows for variable exposure of neutralizing epitopes, contributing to immune-evasion strategies. | [51] | |
| Cryo-ET | Fusion intermediates were visualized by using giant plasma membrane vesicles. Serinc3 and Serinc5 affect the progression of fusion at multiple steps. | [52] | |
| Cryo-ET | The structure of the HIV-1 intasome, providing high-resolution insights into how integrase self-associates to form a functional complex. | [53] | |
| Giant Viruses | Light microscopy and TEM | The morphology and lifecycle of Pandoraviruses within host cells, showing viral particles 1 µm in length and 0.5 µm in diameter. The viral particles are encased in a unique tegument-like envelope and their entry into host cells occurs through phagocytosis. | [54] |
| Cryo-EM | Novel capsid protein networks and scaffold proteins of the Tokyo virus, which are critical in capsid assembly and size regulation. | [57] | |
| Cryo-EM | The capsid structure of the Cafeteria roenbergensis virus and suggested novel assembly pathway for giant viruses. | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Fujioka, Y.; Sakaguchi, S.; Suzuki, Y.; Nakano, T. Electron Tomography as a Tool to Study SARS-CoV-2 Morphology. Int. J. Mol. Sci. 2024, 25, 11762. https://doi.org/10.3390/ijms252111762
Wu H, Fujioka Y, Sakaguchi S, Suzuki Y, Nakano T. Electron Tomography as a Tool to Study SARS-CoV-2 Morphology. International Journal of Molecular Sciences. 2024; 25(21):11762. https://doi.org/10.3390/ijms252111762
Chicago/Turabian StyleWu, Hong, Yoshihiko Fujioka, Shoichi Sakaguchi, Youichi Suzuki, and Takashi Nakano. 2024. "Electron Tomography as a Tool to Study SARS-CoV-2 Morphology" International Journal of Molecular Sciences 25, no. 21: 11762. https://doi.org/10.3390/ijms252111762
APA StyleWu, H., Fujioka, Y., Sakaguchi, S., Suzuki, Y., & Nakano, T. (2024). Electron Tomography as a Tool to Study SARS-CoV-2 Morphology. International Journal of Molecular Sciences, 25(21), 11762. https://doi.org/10.3390/ijms252111762





