Supraspinatus Muscle Regeneration Following Rotator Cuff Tear: A Study of the Biomarkers Pax7, MyoD, and Myogenin
Abstract
1. Introduction
2. Results
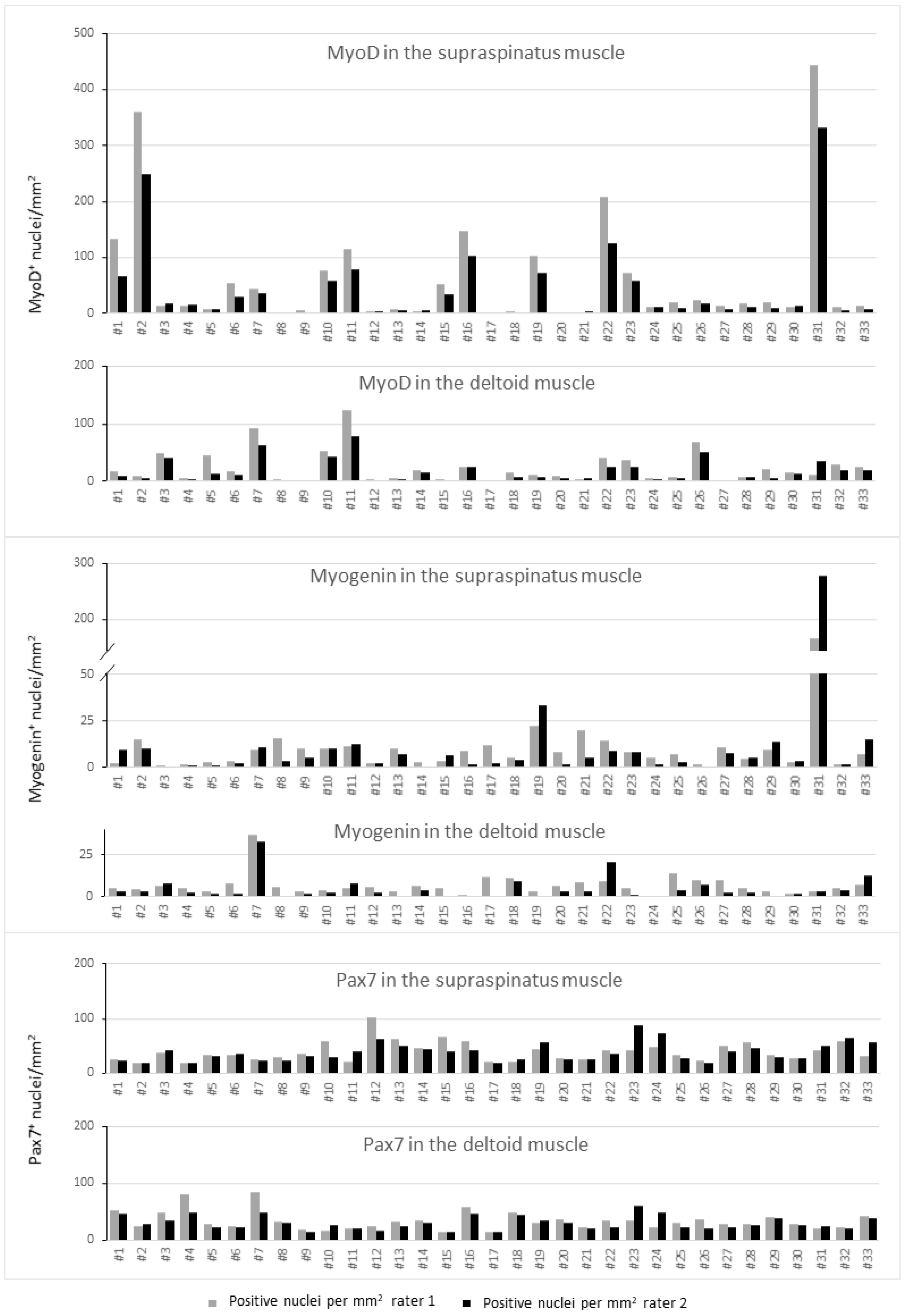
2.1. Inter-Rater r-Values
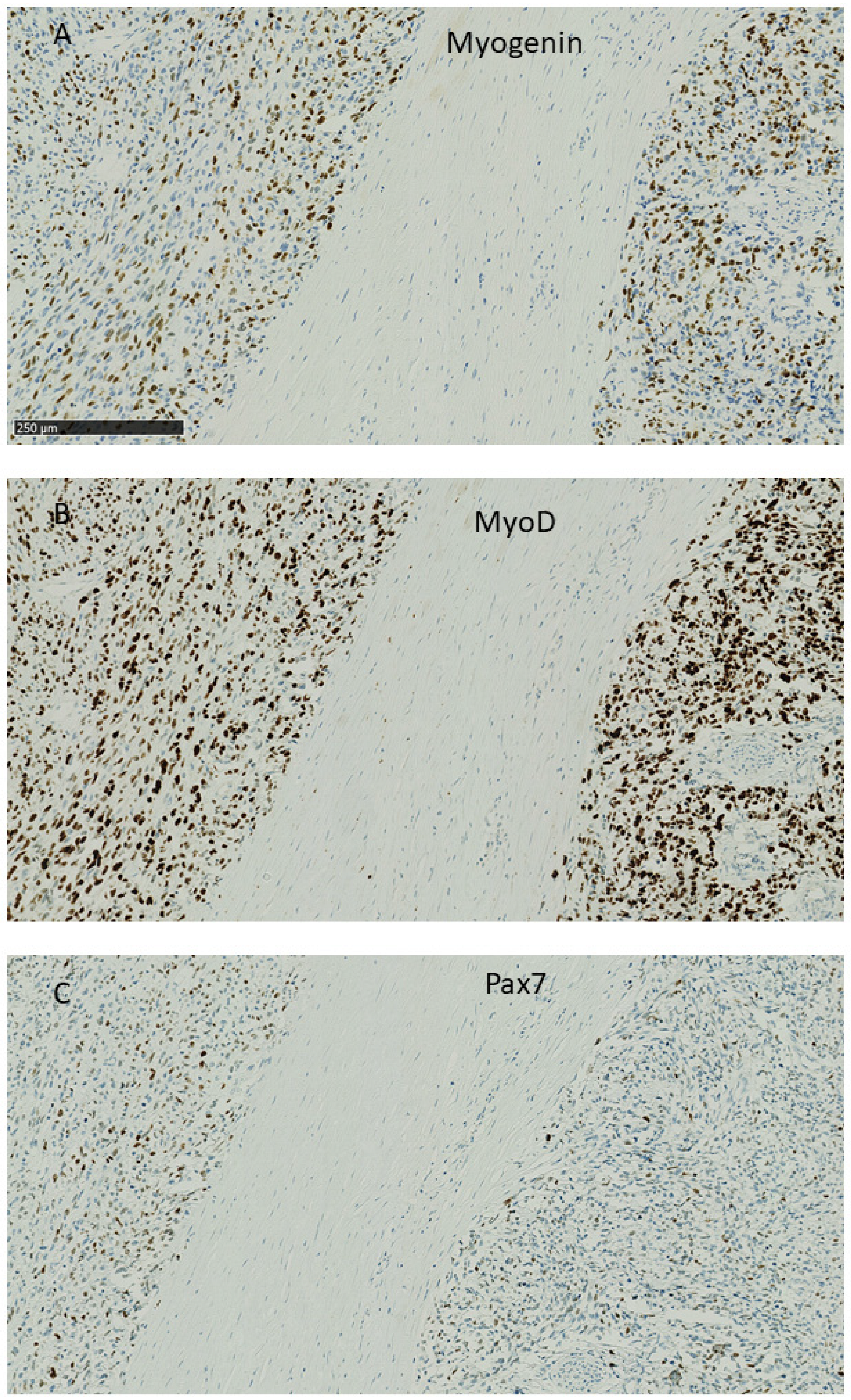
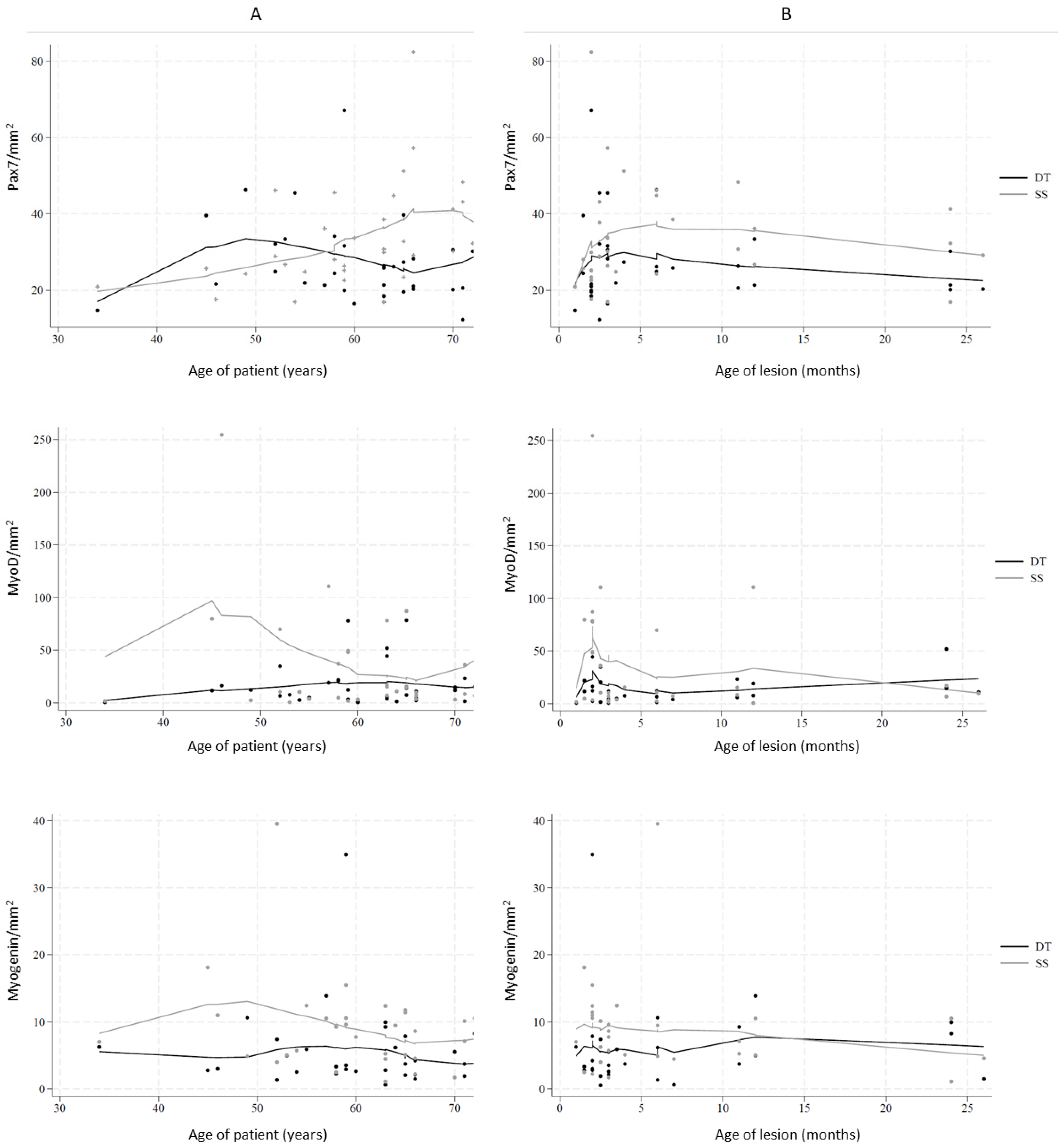
2.2. Myogenic Capacity and Activity
2.3. Density of Myogenic Factors
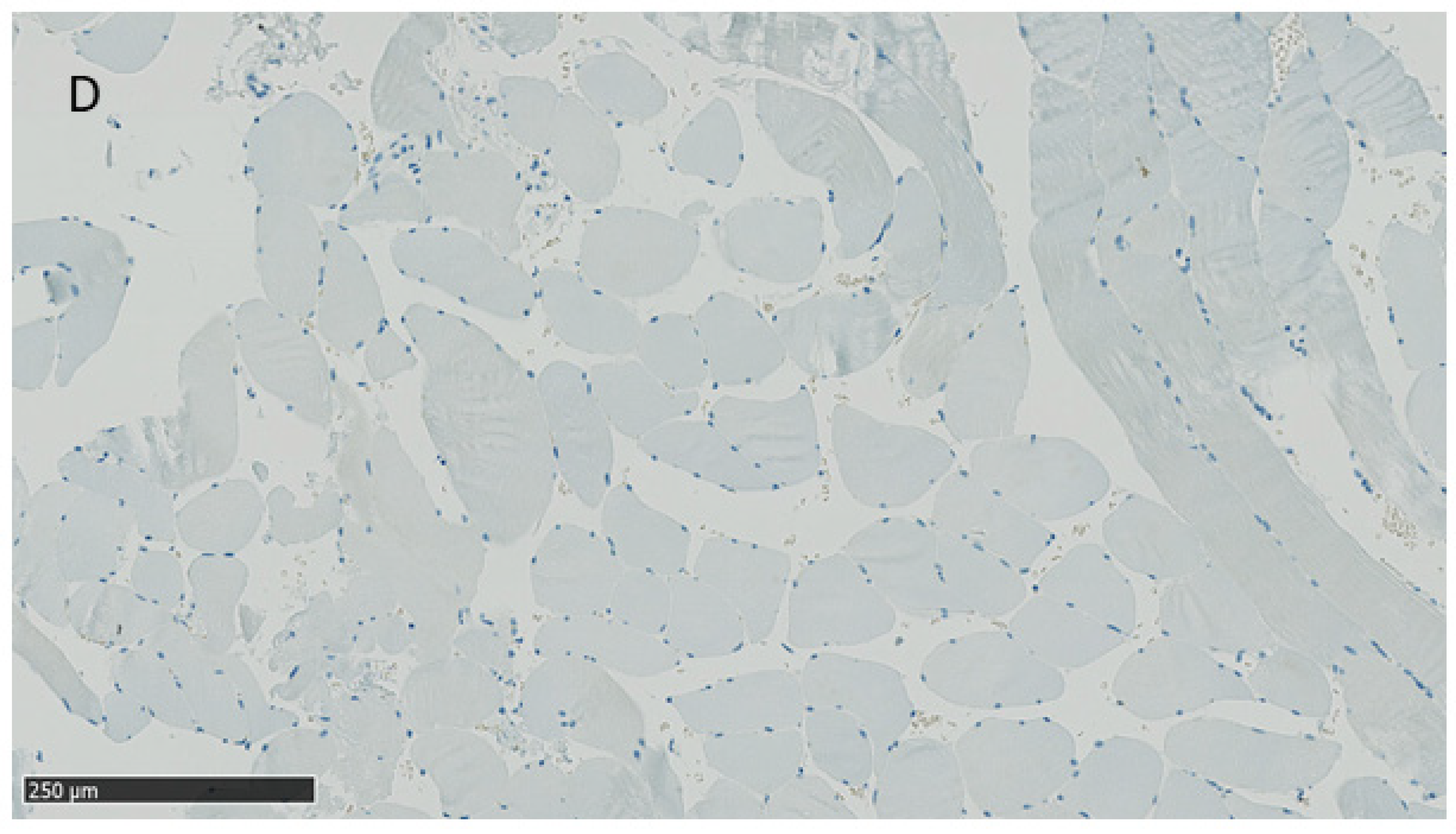
2.4. Presence of Internal Nuclei
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
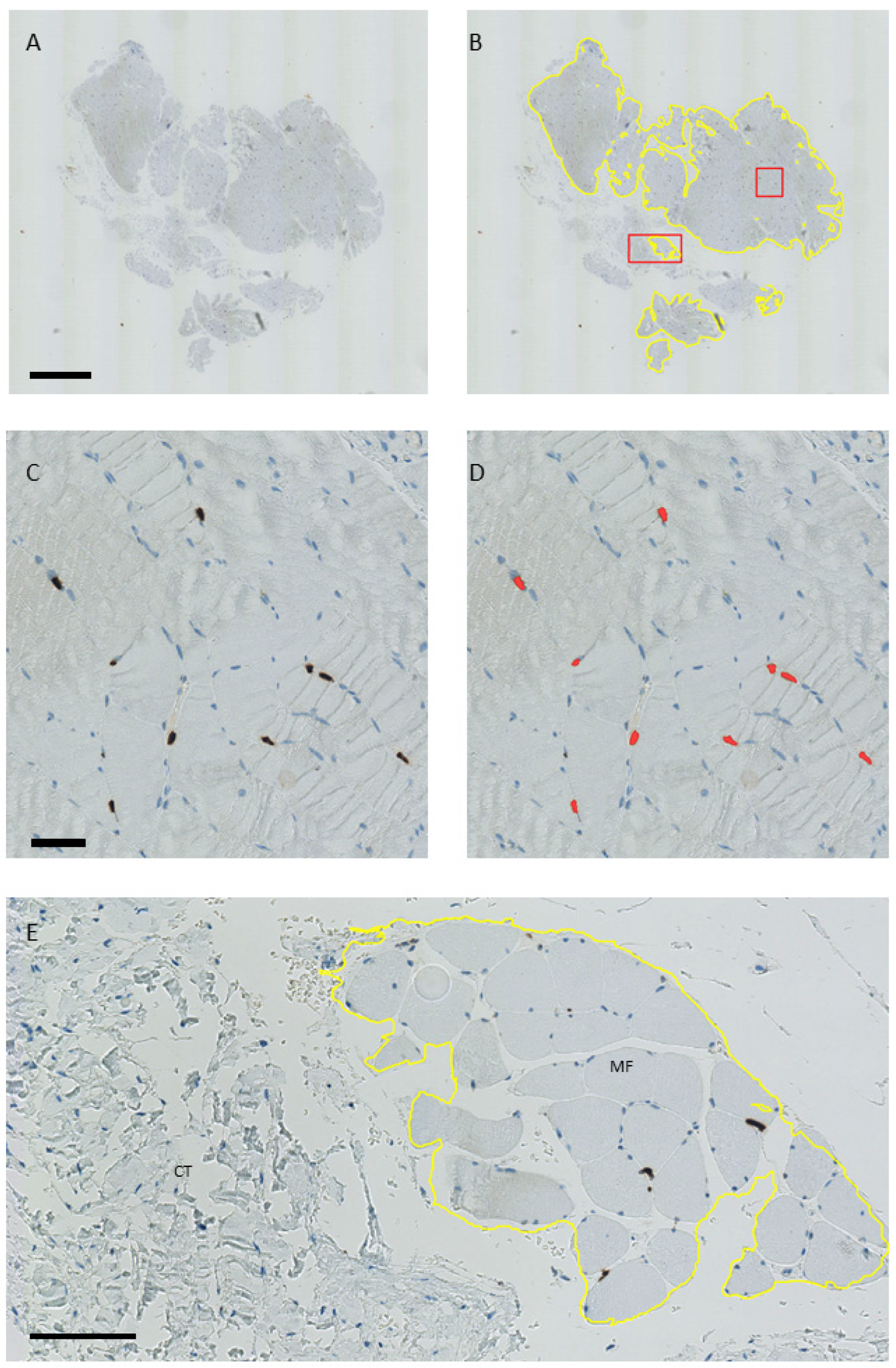
4.2. Histological Analyses
4.3. Estimation of the Number of Pax7, MyoD, and Myogenin-Positive Nuclei per Area
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Age of Lesion up to Three Months | Age of Lesion over Three Months | ||||
|---|---|---|---|---|---|
| Age of Patient | Age of Lesion | Internal Nuclei | Age of Patient | Age of Lesion | Internal Nuclei |
| (years) | (months) | (years) | (months) | ||
| 45 | 2 | 34 | 4 | yes | |
| 46 | 2 | 49 | 6 | yes | |
| 52 | 3 | yes | 52 | 6 | yes |
| 54 | 3 | yes | 53 | 12 | yes |
| 58 | 2 | yes | 55 | 3.5 | |
| 59 | 2 | 57 | 12 | yes | |
| 59 | 2 | yes | 58 | 72 | yes |
| 59 | 3 | 63 | 7 | ||
| 60 | 3 | 63 | 11 | ||
| 63 | 2 | 63 | 24 | ||
| 65 | 2 | 64 | 6 | yes | |
| 66 | 2 | 65 | 4 | yes | |
| 66 | 3 | 65 | 36 | yes | |
| 70 | 3 | 66 | 26 | yes | |
| 71 | 3 | yes | 70 | 24 | yes |
| 73 | 3 | yes | 71 | 11 | yes |
| 72 | 24 | ||||
Appendix B
References
- Yamaguchi, K.; Ditsios, K.; Middleton, W.D.; Hildebolt, C.F.; Galatz, L.M.; Teefey, S.A. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J. Bone Jt. Surg. Am. Vol. 2006, 88, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Freygant, M.; Dziurzyńska-Białek, E.; Guz, W.; Samojedny, A.; Gołofit, A.; Kostkiewicz, A.; Terpin, K. Magnetic resonance imaging of rotator cuff tears in shoulder impingement syndrome. Pol. J. Radiol. 2014, 79, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.J.; Lansdown, D.A.; Cheung, S.; Feeley, B.T.; Ma, C.B. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J. Shoulder Elb. Surg. 2013, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.T.; McDonnell, S.M.; Knowles, H.J.; Rees, J.L.; Carr, A.J.; Hulley, P.A. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J. Bone Jt. Surg. Br. Vol. 2010, 92, 448–453. [Google Scholar] [CrossRef]
- Gladstone, J.N.; Bishop, J.Y.; Lo, I.K.; Flatow, E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am. J. Sports Med. 2007, 35, 719–728. [Google Scholar] [CrossRef]
- Matthews, T.J.; Hand, G.C.; Rees, J.L.; Athanasou, N.A.; Carr, A.J. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J. Bone Jt. Surg. Br. Vol. 2006, 88, 489–495. [Google Scholar] [CrossRef]
- Godenèche, A.; Elia, F.; Kempf, J.F.; Nich, C.; Berhouet, J.; Saffarini, M.; Collin, P. Fatty infiltration of stage 1 or higher significantly compromises long-term healing of supraspinatus repairs. J. Shoulder Elb. Surg. 2017, 26, 1818–1825. [Google Scholar] [CrossRef]
- Greenspoon, J.A.; Petri, M.; Warth, R.J.; Millett, P.J. Massive rotator cuff tears: Pathomechanics, current treatment options, and clinical outcomes. J. Shoulder Elb. Surg. 2015, 24, 1493–1505. [Google Scholar] [CrossRef]
- Frich, L.H.; Fernandes, L.R.; Schrøder, H.D.; Hejbøl, E.K.; Nielsen, P.V.; Jørgensen, P.H.; Stensballe, A.; Lambertsen, K.L. The inflammatory response of the supraspinatus muscle in rotator cuff tear conditions. J. Shoulder Elb. Surg. 2021, 30, e261–e275. [Google Scholar] [CrossRef]
- Gibbons, M.C.; Singh, A.; Anakwenze, O.; Cheng, T.; Pomerantz, M.; Schenk, S.; Engler, A.J.; Ward, S.R. Histological Evidence of Muscle Degeneration in Advanced Human Rotator Cuff Disease. J. Bone Jt. Surg. Am. Vol 2017, 99, 190–199. [Google Scholar] [CrossRef]
- Gerber, C.; Schneeberger, A.G.; Hoppeler, H.; Meyer, D.C. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: A study in thirteen patients. J. Shoulder Elb. Surg. 2007, 16, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Hill, R.J.; Krasney, P.A.; O’Conner, B.; Peirce, N.; Greenhaff, P.L. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004, 18, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.O.; Pavlath, G.K. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1753–C1762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, G.Y.; Kwon, D.R.; Lee, S.C. Regeneration of Full-Thickness Rotator Cuff Tendon Tear After Ultrasound-Guided Injection with Umbilical Cord Blood-Derived Mesenchymal Stem Cells in a Rabbit Model. Stem Cells Transl. Med. 2015, 4, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Flood, M.D.; Roche, S.M.; Sugg, K.B.; Momoh, A.O.; Kosnik, P.E.; Bedi, A.; Mendias, C.L. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int. Orthop. 2016, 40, 759–764. [Google Scholar] [CrossRef]
- Davies, M.R.; Garcia, S.; Tamaki, S.; Liu, X.; Lee, S.; Jose, A.; Pomerantz, J.H.; Feeley, B.T. Muscle stem cell activation in a mouse model of rotator cuff injury. J. Orthop. Res. 2018, 36, 1370–1376. [Google Scholar] [CrossRef]
- Güleçyüz, M.F.; Macha, K.; Pietschmann, M.F.; Ficklscherer, A.; Sievers, B.; Roßbach, B.P.; Jansson, V.; Müller, P.E. Allogenic Myocytes and Mesenchymal Stem Cells Partially Improve Fatty Rotator Cuff Degeneration in a Rat Model. Stem Cell Rev. Rep. 2018, 14, 847–859. [Google Scholar] [CrossRef]
- Koide, M.; Hagiwara, Y.; Tsuchiya, M.; Kanzaki, M.; Hatakeyama, H.; Tanaka, Y.; Minowa, T.; Takemura, T.; Ando, A.; Sekiguchi, T.; et al. Retained Myogenic Potency of Human Satellite Cells from Torn Rotator Cuff Muscles Despite Fatty Infiltration. Tohoku J. Exp. Med. 2018, 244, 15–24. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Dumont, N.A.; Rudnicki, M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013, 14, 1062–1072. [Google Scholar] [CrossRef]
- Yan, Z.; Choi, S.; Liu, X.; Zhang, M.; Schageman, J.J.; Lee, S.Y.; Hart, R.; Lin, L.; Thurmond, F.A.; Williams, R.S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 2003, 278, 8826–8836. [Google Scholar] [CrossRef] [PubMed]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. In Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Cao, Y.; Kumar, R.M.; Penn, B.H.; Berkes, C.A.; Kooperberg, C.; Boyer, L.A.; Young, R.A.; Tapscott, S.J. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 2006, 25, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Meyer, G.A.; Farris, A.L.; Sato, E.; Gibbons, M.; Lane, J.G.; Ward, S.R.; Engler, A.J. Muscle progenitor cell regenerative capacity in the torn rotator cuff. J. Orthop. Res. 2015, 33, 421–429. [Google Scholar] [CrossRef]
- Fuchs, B.; Weishaupt, D.; Zanetti, M.; Hodler, J.; Gerber, C. Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J. Shoulder Elb. Surg. 1999, 8, 599–605. [Google Scholar] [CrossRef]
- Sciorati, C.; Clementi, E.; Manfredi, A.A.; Rovere-Querini, P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: Cellular and molecular players. Cell. Mol. Life Sci. 2015, 72, 2135–2156. [Google Scholar] [CrossRef]
- Renault, V.; Thornell, L.E.; Eriksson, P.O.; Butler-Browne, G.; Mouly, V. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002, 1, 132–139. [Google Scholar] [CrossRef]
- Gibson, M.C.; Schultz, E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 1983, 6, 574–580. [Google Scholar] [CrossRef]
- Corbu, A.; Scaramozza, A.; Badiali-DeGiorgi, L.; Tarantino, L.; Papa, V.; Rinaldi, R.; D’Alessandro, R.; Zavatta, M.; Laus, M.; Lattanzi, G.; et al. Satellite cell characterization from aging human muscle. Neurol. Res. 2010, 32, 63–72. [Google Scholar] [CrossRef]
- Suetta, C.; Frandsen, U.; Jensen, L.; Jensen, M.M.; Jespersen, J.G.; Hvid, L.G.; Bayer, M.; Petersson, S.J.; Schrøder, H.D.; Andersen, J.L.; et al. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS ONE 2012, 7, e51238. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.Y.; Nielsen, J.L.; Schrøder, H.D.; Jacobsen, M.; Boyle, E.; Jørgensen, A.N.; Bech, R.D.; Frandsen, U.; Aagaard, P.; Diederichsen, L.P. Lack of muscle stem cell proliferation and myocellular hypertrophy in sIBM patients following blood-flow restricted resistance training. Neuromuscul. Disord. 2022, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.C.; Petersson, S.J.; Jørgensen, L.H.; Bollen, P.; Jensen, P.B.; Teisner, B.; Schroeder, H.D.; Jensen, C.H. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells 2009, 27, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.H.; Jensen, C.H.; Wewer, U.M.; Schrøder, H.D. Transgenic overexpression of ADAM12 suppresses muscle regeneration and aggravates dystrophy in aged mdx mice. Am. J. Pathol. 2007, 171, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.A.; Afshar, P.; Abrams, R.A.; Lieber, R.L. Skeletal muscle response to tenotomy. Muscle Nerve 2000, 23, 851–862. [Google Scholar] [CrossRef]
- Moosmayer, S.; Lund, G.; Seljom, U.S.; Haldorsen, B.; Svege, I.C.; Hennig, T.; Pripp, A.H.; Smith, H.J. At a 10-Year Follow-up, Tendon Repair Is Superior to Physiotherapy in the Treatment of Small and Medium-Sized Rotator Cuff Tears. J. Bone Jt. Surg. Am. Vol. 2019, 101, 1050–1060. [Google Scholar] [CrossRef]
- Ravn, M.K.; Ostergaard, T.I.; Schroeder, H.D.; Nyengaard, J.R.; Lambertsen, K.L.; Frich, L.H. Supraspinatus and deltoid muscle fiber composition in rotator cuff tear conditions. JSES Int. 2020, 4, 431–437. [Google Scholar] [CrossRef]
- Davidson, J.; Burkhart, S.S. The geometric classification of rotator cuff tears: A system linking tear pattern to treatment and prognosis. Arthroscopy 2010, 26, 417–424. [Google Scholar] [CrossRef]
- Gilbert, F.; Böhm, D.; Eden, L.; Schmalzl, J.; Meffert, R.H.; Köstler, H.; Weng, A.M.; Ziegler, D. Comparing the MRI-based Goutallier Classification to an experimental quantitative MR spectroscopic fat measurement of the supraspinatus muscle. BMC Musculoskelet. Disord. 2016, 17, 355. [Google Scholar] [CrossRef]
- Dubowitz, V.; Oldfors, A.; Sewry, C.A. Muscle Biopsy: A Practical Approach, 5th ed.; Elsevier: London, UK, 2021. [Google Scholar]
- The Danish Health and Medicines Authority. National Klinisk Retningslinje for Diagnostik og Behandling af Patienter med Udvalgte Skulderlidelser. Available online: https://www.ortopaedi.dk/fileadmin/DSSAK/Fagligt/Impingement_fag_visit_retn_8_dec_2011-UDGIVELSE.pdf (accessed on 20 October 2024).
| Adjusted Coefstr | Adjusted p Value | |
|---|---|---|
| Pax7 | 4.29 (−0.15; 8.72) | 0.058 |
| MyoD | 47.17(29.95; 64.40) | <0.001 |
| Myogenin | 17.80 (5.88; 29.73) | 0.003 |
| Muscle | Variable | Adjusted Coefstr | Adjusted p Value | |
|---|---|---|---|---|
| Pax7 | deltoid | Age of patient | −0.12 (−0.54; 0.31) | 0.585 |
| Pax7 | deltoid | Age of lesion > 3 months | 35.60 (10.13; 61.07) | 0.006 |
| Pax7 | supraspinatus | Age of patient | 0.60 (0.16; 1.04) | 0.007 |
| Pax7 | supraspinatus | Age of lesion > 3 months | −4.36 (−30.68; 21.96) | 0.746 |
| MyoD | deltoid | Age of patient | 0.31 (−0.45; 1.08) | 0.418 |
| MyoD | deltoid | Age of lesion > 3 months | 2.01 (−43.69; 47.71) | 0.931 |
| MyoD | supraspinatus | Age of patient | −1.04 (−3.04; 0.96) | 0.309 |
| MyoD | supraspinatus | Age of lesion > 3 months | 108.09 (−12.19; 228.37) | 0.078 |
| MyoG | deltoid | Age of patient | −0.08 (−0.37; 0.21) | 0.595 |
| MyoG | deltoid | Age of lesion > 3 months | 10.24 (−7.69; 28.17) | 0.263 |
| MyoG | supraspinatus | Age of patient | −0.32 (−0.65; 0.02) | 0.064 |
| MyoG | supraspinatus | Age of lesion > 3 months | 27.87 (7.54; 48.20) | 0.007 |
| Age of Lesion up to Three Months | Age of Lesion over Three Months | ||||||
|---|---|---|---|---|---|---|---|
| # | Age of Patient | Age of Lesion | Gender | # | Age of Patient | Age of Lesion | Gender |
| (years) | (months) | (years) | (months) | ||||
| 1 | 45 | 2 | f | 17 | 34 | 4 | m |
| 2 | 46 | 2 | m | 18 | 49 | 6 | m |
| 3 | 52 | 3 | f | 19 | 52 | 6 | m |
| 4 | 54 | 3 | m | 20 | 53 | 12 | m |
| 5 | 58 | 2 | m | 21 | 55 | 3.5 | m |
| 6 | 59 | 2 | f | 22 | 57 | 12 | m |
| 7 | 59 | 2 | m | 23 | 58 | 72 | m |
| 8 | 59 | 3 | f | 24 | 63 | 7 | f |
| 9 | 60 | 3 | m | 25 | 63 | 11 | m |
| 10 | 63 | 2 | m | 26 | 63 | 24 | m |
| 11 | 65 | 2 | m | 27 | 64 | 6 | f |
| 12 | 66 | 2 | m | 28 | 65 | 4 | m |
| 13 | 66 | 3 | m | 29 | 65 | 36 | f |
| 14 | 70 | 3 | m | 30 | 66 | 26 | m |
| 15 | 71 | 3 | m | 31 | 70 | 24 | f |
| 16 | 73 | 3 | m | 32 | 71 | 11 | m |
| 33 | 72 | 24 | f | ||||
| Mean | 60 | 2 | 60 | 17 | |||
| Std.dev. | 8.3 | 0.5 | 9.5 | 17.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejbøl, E.K.; Andkjær, S.W.; Dybdal, J.; Klindt, M.; Möller, S.; Lambertsen, K.L.; Schrøder, H.D.; Frich, L.H. Supraspinatus Muscle Regeneration Following Rotator Cuff Tear: A Study of the Biomarkers Pax7, MyoD, and Myogenin. Int. J. Mol. Sci. 2024, 25, 11742. https://doi.org/10.3390/ijms252111742
Hejbøl EK, Andkjær SW, Dybdal J, Klindt M, Möller S, Lambertsen KL, Schrøder HD, Frich LH. Supraspinatus Muscle Regeneration Following Rotator Cuff Tear: A Study of the Biomarkers Pax7, MyoD, and Myogenin. International Journal of Molecular Sciences. 2024; 25(21):11742. https://doi.org/10.3390/ijms252111742
Chicago/Turabian StyleHejbøl, Eva Kildall, Stephanie Wej Andkjær, Julie Dybdal, Marie Klindt, Sören Möller, Kate Lykke Lambertsen, Henrik Daa Schrøder, and Lars Henrik Frich. 2024. "Supraspinatus Muscle Regeneration Following Rotator Cuff Tear: A Study of the Biomarkers Pax7, MyoD, and Myogenin" International Journal of Molecular Sciences 25, no. 21: 11742. https://doi.org/10.3390/ijms252111742
APA StyleHejbøl, E. K., Andkjær, S. W., Dybdal, J., Klindt, M., Möller, S., Lambertsen, K. L., Schrøder, H. D., & Frich, L. H. (2024). Supraspinatus Muscle Regeneration Following Rotator Cuff Tear: A Study of the Biomarkers Pax7, MyoD, and Myogenin. International Journal of Molecular Sciences, 25(21), 11742. https://doi.org/10.3390/ijms252111742








