Abstract
Leptin plays an indispensable role in energy homeostasis, and its involvement in metabolic activities has been extensively explored in fish. We generated mutant lines of leptina (−5 bp) and leptinb (+8 bp) in zebrafish using CRISPR/Cas9 technology to explore the metabolic characteristics of lepa and lepb mutant zebrafish in response to high glucose nutritional stress induced by high levels of carbohydrates. The results were as follows: the body weight and food intake of adult zebrafish of the two mutant species were increased; the visceral fat accumulation, whole-body crude lipid, and crude protein contents of lepb−/− were increased; and the visceral fat accumulation and crude lipid in lepa−/− zebrafish were decreased. The blood glucose levels of the two mutant zebrafish were increased, the mRNA expression levels of glycolytic genes pk and gck were decreased in the two mutant zebrafish, and there were differences between lepa−/− and lepb−/− zebrafish. The expressions of glycogen synthesis and decomposition genes were inhibited and promoted, respectively. The expression of adipose synthesis genes in the liver and muscle was stimulated in lepb−/− zebrafish but suppressed in lepa−/− zebrafish. Lipolysis and oxidation genes were also stimulated in lepa−/− zebrafish livers, while the livers of lepb−/− zebrafish were stimulated but muscle was inhibited. In conclusion, the results indicate that lepa plays a major role in glucose metabolism, which is conducive to promoting glucose utilization and lipogenesis, while lepb mainly promotes lipolysis and oxidation, regulates protein generation, and plays a minor role in glucose metabolism.
1. Introduction
As an important source of protein, the demand for fish is increasing. In order to increase fish production, improvement in feed utilization has become critical. Therefore, adjusting the composition of nutrients in feed is the key to improving feed utilization. However, fish feed with carbohydrate and fat levels at or above the upper limit usually trigger diseases such as diabetes or obesity, seriously affecting the utilization of feed by fish and, thus, yields. Obesity and type 2 diabetes mellitus (T2DM) are also among the most commonly encountered and difficult-to-cure health problems in humans today, and the World Health Organization (WHO) has declared both diseases to be global epidemics [1]. A key feature of these conditions is disrupted leptin signaling. Leptin is a hormone that is formed as a result of spontaneous mutations in the autosomal obesity gene (Ob) and plays a key role in energy homeostasis, as described by Zhang et al. (1994) [2].
Leptin consists of 167 amino acids and has a protein size of 16 kDa [3]. The sequence similarity of leptin between bony fish and mammals ranges from approximately 13% to 25% [4,5,6,7]. There is only one isoform of leptin and its receptor in mammals, but at least two paralogous homologs of leptin and its receptor have been found in fish [8]. Fish-specific whole-genome duplication (WGD) events lead to the multicopy generation of scleractinian genes [9]. In mammals, adipose tissue and the brain are generally recognized as major sites of leptin synthesis and receptor expression [10,11,12]. In bony fish, leptin is usually expressed in the liver, and the levels of leptin expression in adipose tissue differ depending on the fish species [13,14,15,16,17,18,19]. The expression patterns of the two isoforms of leptin also differ, with leptina (lepa) being predominantly expressed in the liver and leptinb (lepb) expression levels higher in the ovaries [4,15]. The specificity of tissue expression of leptin indicates that its biological function may also be specific.
In mammals, changes in leptin levels mainly affect food intake and energy expenditure [20]. Food intake levels are associated with changes in hypothalamic neuropeptide expression. In mammals, leptin plays an anorexic role by inhibiting the expression of neuropeptide Y (NPY) and agouti-related protein (AgRP) and stimulating the expression of pro-opiomelanocortin (POMC) and cocaine and amphetamine-regulated transcription (CART) [21,22,23]. Leptin also plays a role in feeding by acting on receptors synthesized in the hypothalamus of fish, influencing appetite factors [24].
The homeostasis of energy, i.e., the metabolism maintained by animals, is the basis of normal life activities, and the imbalance of homeostasis can lead to obesity or diabetes [25]. Feeding and energy metabolism in mammals can be regulated by neurons with glucose-sensing properties, which are distributed in the hypothalamus [26,27]. Leptin promotes energy homeostasis by regulating lipid and carbohydrate metabolism in peripheral tissues [28]. Leptin also mobilizes lipids for energy use by promoting lipolysis, inhibiting lipogenesis, and up-regulating fatty acid oxidation in the liver, muscle, and adipose tissue [29,30,31,32]. Dysfunctional metabolism; decreased body temperature and oxygen consumption; accumulation of lipids in tissues; and the development of insulin resistance, leading to elevated plasma glucose levels, have been reported in leptin-deficient obese mice [33].
Although leptin is well known in mammals for its adipose-inhibitory effects, it also regulates glucose homeostasis—an effect that is independent of its influence on adiposity. To date, the mechanisms by which leptin exerts its glucoregulatory effects remain largely unknown, especially in fish, and the effect on fat is also ambiguous. There are two distinct isoforms of leptin in bony fish, and we hypothesize that some of the functions of the two isoforms may overlap with those of mammals but that a particular isoform may play its own unique role. In order to study the function of the leptin gene, the CRISPR/Cas9 system was utilized to mutate the leptin gene in zebrafish. Zebrafish with a loss of function of the leptin gene were used as experimental subjects to investigate the differences in feeding, energy metabolism, and other aspects related to the leptin gene in zebrafish and to determine the role of leptin in their feeding and metabolism. This research also aimed to analyze the functional differentiation of the lepa and lepb genes in zebrafish and to provide effective evidence for the study of the different physiological roles exerted by leptin in fish. It further aims to improve the understanding of the role of leptin in non-mammals and is expected to provide theoretical references for solving the obesity and diabetes problems currently faced by human beings.
2. Results
2.1. Construction of Leptin Mutant in Zebrafish
In this study, mutant lepa−/− and lepb−/− zebrafish strains were obtained. The lepa−/− mutant lacks five bases, while the lepb−/− mutant adds eight bases (Figure 1). By comparing the gene sequences and predicted protein structures with wild-type samples, it was found that the lepa−/− (−5 bp) mutant had a translation termination at 67 amino acids, while the lepb−/− (+8 bp) mutant had a translation termination at 154 amino acids. All of these mutants have deletions in their protein domains.
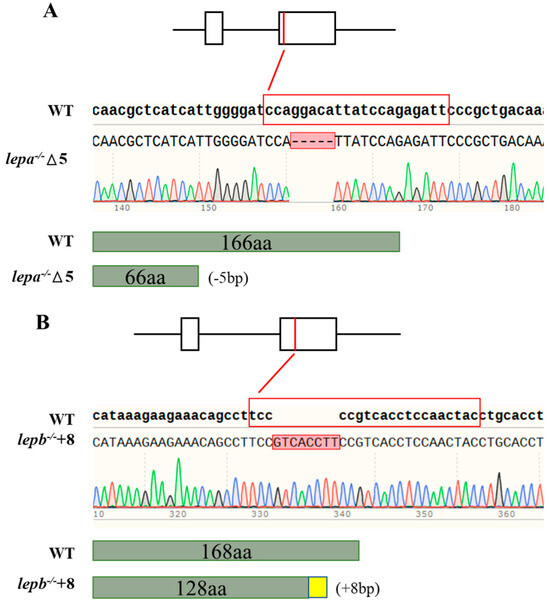
Figure 1.
(A,B) The sequence result of mutant genes. Dotted lines represent missing bases, and red squares represent inserted bases. The sgRNA sequences are highlighted in red, and the −5 bp and +8 bp deletions are indicated by sequencing validation.
2.2. Analysis of Growth Performance of Lepa−/− and Lepb−/− Mutants Under High-Glucose Diet Induction
After 60 days of high-glucose diet induction, it was found that compared with wild-type zebrafish (Table 1), the body weight of lepa−/− and lepb−/− zebrafish increased by 137.18% (p < 0.05) and 135.51% (p < 0.05), respectively (Table 1). In terms of the entero–lipid ratio, that of the lepa−/− zebrafish ratio was significantly decreased (p < 0.05), while that of the lepb−/− zebrafish was distinctly increased (p < 0.05).

Table 1.
Growth performance and whole-body proximate composition of three genotypes of zebrafish fed a high-glucose diet for 60 days.
Further observation of the changes in body components of lepa−/− and lepb−/− zebrafish showed that there was a tendency for an elevated crude protein content in lepa−/− zebrafish with no statistically significant differences and a significant elevation in lepb−/− zebrafish (p < 0.05) compared to the control group. The data indicated that the crude fat content of lepa−/− zebrafish was prominently reduced (p < 0.05), while the visceral fat deposition of lepb−/− zebrafish was elevated (p < 0.05).
2.3. Gene Expression Related to Appetite and Food Intake
After high-glucose induction, the weight-gain rate of the mutants was significantly higher than that of the wild type, which may be due to abnormal leptin levels in the mutants, blocked appetite-inhibiting signals, or stimulated appetite-promoting signals. Therefore, we tested the feeding rates of the three genotypes of zebrafish, and the results indicated that the feeding rates of lepa−/− and lepb−/− zebrafish were significantly higher than those of wild-type zebrafish (p < 0.05) (Figure 2A). The mRNA expression levels of agrp and npy in lepa−/− and lepb−/− zebrafish were significantly elevated compared to those of wild-type zebrafish (p < 0.05) (Figure 2B), while the mRNA expression levels of appetite-suppressing genes pomc and cart in lepa−/− and lepb−/− zebrafish were not significantly different from those in wild-type zebrafish (p > 0.05).
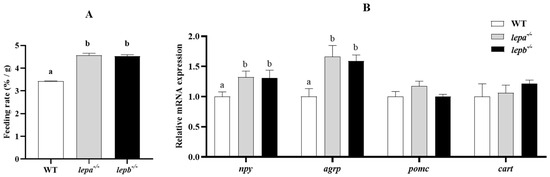
Figure 2.
Genee expression related to appetite and food intake of lepa−/−, lepb−/−, and wild-type zebrafish. (A) Food intake of lepa−/−, lepb−/−, and wild-type zebrafish. (B) Expression levels of appetite-related genes in the brain of the three genotypes of zebrafish. Different letters indicate significant differences (p < 0.05).
2.4. Changes of Blood Physiological and Biochemical Related Indicators in Lepa−/− and Lepb−/− Zebrafish
We further explored the impact of leptin on blood glucose and lipid levels, as shown in Figure 3. The blood glucose levels of the mutant zebrafish were obviously higher than those of the wild-type zebrafish (p < 0.05), and the blood glucose levels of lepa−/− zebrafish were higher than those of lepb−/− zebrafish (p < 0.05) (Figure 3A). Compared with wild-type zebrafish, the levels of triglycerides (TG) and cholesterol in the blood of lepa−/− zebrafish were significantly reduced (p < 0.05), while those in lepb−/− zebrafish were significantly increased (p < 0.05) (Figure 3B,C).
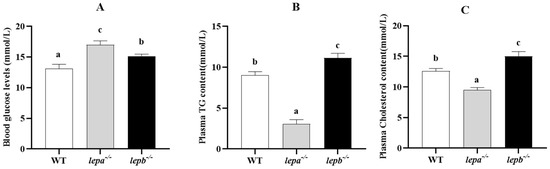
Figure 3.
The effect of leptin on blood glucose and lipid levels. (A) Blood glucose levels, (B) plasma TG levels, and (C) plasma cholesterol levels in the three genotypes of zebrafish. Different letters indicate significant differences (p < 0.05), with n = 6 for each genotype.
2.5. Lepa−/− and Lepb−/− Zebrafish Liver and Muscle Glycogen and Fat Contents
The glycogen and fat contents in the liver and muscle tissues of lepa−/−, lepb−/−, and wild-type zebrafish were detected by staining with Oil Red O and AB-PAS. The results of oil red O staining showed that the fat content of lepa−/− zebrafish was lower, while that of lepb−/− zebrafish was higher in muscle and liver compared to the wild-type fish (Figure 4A,B). AB-PAS staining revealed no significant differences in glycogen content among the three genotypes in liver and muscle tissues (Figure 4C,D).
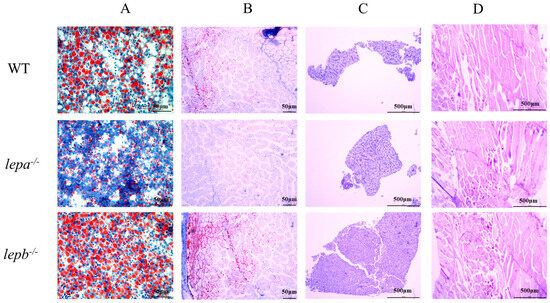
Figure 4.
Oil red O and AB-PAS staining of liver and muscle tissue of three genotypes zebrafish. (A) Oil red O staining of the liver. (B) Oil red O staining of muscle. (C) AB-PAS staining of the liver. (D) AB-PAS staining of muscle.
The contents of glycogen and TG in the muscle and liver of zebrafish were detected by a liver/muscle glycogen assay kit and TG assay kit. The glycogen contents of lepa−/− and lepb−/− zebrafish in the liver were significantly lower than those of wild-type zebrafish (p < 0.05) (Figure 5A). In muscle tissue, there was no significant difference in glycogen content among lepa−/−, lepb−/−, and wild-type zebrafish (p > 0.05) (Figure 5B). Compared to the TG contents of wild-type zebrafish, the TG contents of lepa−/− zebrafish in both muscle and the liver were significantly decreased (p < 0.05), while the TG contents of lepb−/− zebrafish were significantly increased (p < 0.05) (Figure 5C,D).
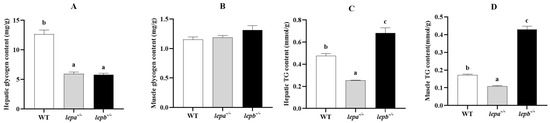
Figure 5.
The levels of glycogen and triglyceride (TG) in the liver and muscle of three genotypes of zebrafish. (A) Glycogen levels in the liver. (B) Glycogen levels in muscle. (C) TG levels in the liver. (D) TG levels in muscle. The letters a, b, and c in the bar chart represent significant differences for each index among lepa−/−, lepb−/−, and wild-type zebrafish (p < 0.05), with n = 6 for each genotype.
2.6. Expression Analysis of Genes Related to Glucose and Lipid Metabolism
We further examined genetic changes related to lipid metabolism, glucose metabolism, and protein synthesis. Compared with the wild-type zebrafish, the mRNA expression levels of glycolysis genes glucokinase (gck) and pyruvate kinase (pk) in the mutants were significantly decreased (p < 0.05) (Figure 6A). The expressions of gluconeogenesis genes phosphoenolpyruvate carboxykinase 1 (pck1) and glucose-6-phosphatase a catalytic subunit tandem duplicate 1 (g6pca.1) were significantly increased (p < 0.05) (Figure 6A). The expression of glycogen phosphorylase gene glycogen phosphorylase (gp) was significantly higher (p < 0.05), and that of glycogen synthase 2 (gys2) was significantly lower (p < 0.05) (Figure 6B). These findings are consistent with the results of glycogen content assays conducted in liver tissue.
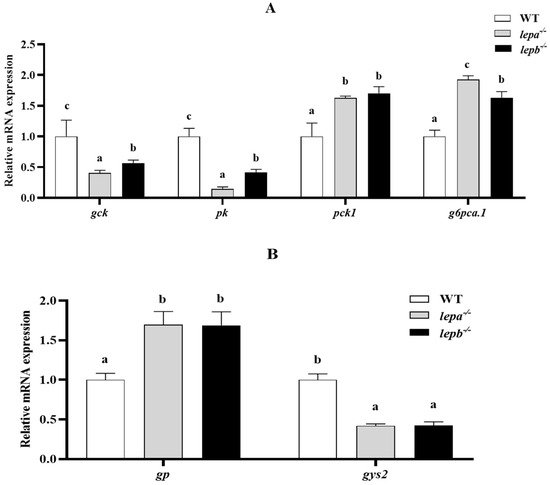
Figure 6.
Expression levels of genes related to glucose metabolism in the liver. (A) Glycolysis and gluconeogenesis genes. (B) Glycogen metabolism gene. The letters a, b, and c in the bar chart represent significant differences foreach index among lepa−/−, lepb−/−, and wild-type zebrafish (p < 0.05), with n = 6 for each genotype.
In the assay of lipid metabolism-related genes, compared to wild-type zebrafish, acetyl-CoA carboxylase alpha (acc), fatty acid synthase (fas), and peroxisome proliferator-activated receptor gamma (pparγ) contents were significantly elevated in lepb−/− zebrafish liver and muscle tissues (p < 0.05), while they were significantly lower in lepa−/− zebrafish liver and muscle tissues (p < 0.05) (Figure 7). Carnitine palmitoyl transferase 1B (cpt1b) was significantly increased (p < 0.05) in both the liver and muscle of lepa−/− zebrafish, whereas the results for peroxisome proliferator-activated receptor alpha (pparα), a key transcription factor in fat oxidation, followed the same trend as cpt1b in lepa−/− zebrafish liver and muscle tissues. Interestingly, cpt1b was significantly increased (p < 0.05) in the liver of lepb−/− zebrafish but significantly decreased (p < 0.05) in muscle.
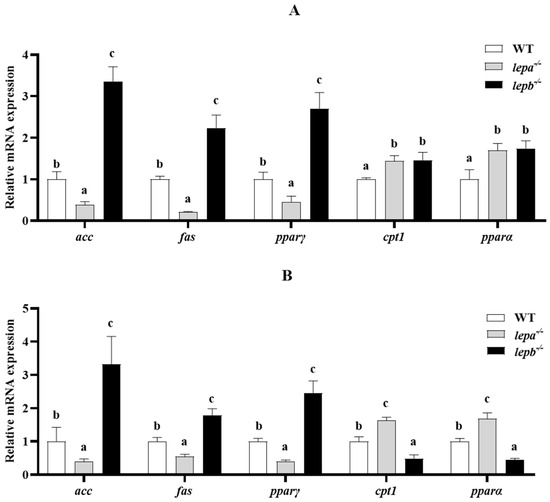
Figure 7.
Expression levels of lipid metabolism genes in the liver (A) and muscle (B) of three genotypes of zebrafish. The letters a, b, and c in the bar chart represent significant differences for each index among lepa−/−, lepb−/−, and wild-type zebrafish (p < 0.05), with n = 6 for each genotype.
3. Discussion
Leptin plays an important role in controlling food intake and growth energy metabolism in mammals, and little research has been performed on this function in fish or in non-mammals in general [34]. In this experiment, we explored the role of leptin in nutrient acquisition and energy allocation using pure lepa−/− and lepb−/− zebrafish mutants constructed by CRISPR/Cas9 gene editing.
Previous studies have shown that leptin in fish such as goldfish [35], rainbow trout [14,36], and Mandarin fish [37] is involved in the regulation of body feeding and body weight. Both NPY and AgRP are potent appetite stimulants. Their expression levels in the hypothalamus have been extensively demonstrated to be inhibited by leptin in mammals [34,38,39,40,41,42,43,44], and their appetite-stimulating effects have been studied in zebrafish [45,46,47]. Consistent with these observations, lepa−/− and lepb−/− zebrafish showed significantly higher rates of body weight gain and food intake than wild-type zebrafish following a high-glucose diet. Elevated feeding in lepa−/− and lepb−/− zebrafish and significantly higher levels of appetitive npy and agrp mRNA in lepa−/− and lepa−/− zebrafish than in wild-type zebrafish suggest that up-regulation of appetitive genes in the mutants may enhance their feeding.
Fewer studies have been conducted to explore whether lepa and lepb differ in glucose metabolism in fish. In the present study, lepa and lepb mutants were fed a high-glucose diet and found to have elevated blood glucose levels in both mutant fish, with lepa−/− zebrafish having higher blood glucose levels than lepb−/− zebrafish. We also observed changes in glycogen content by AB-PAS staining of zebrafish liver and muscle tissues; both mutant zebrafish had reduced liver glycogen contents and no changes in muscle tissue glycogen compared to the wild-type zebrafish, similar to the results obtained with leptin treatment in goldfish [35]. The expression of glycolysis-related genes pk and gck, as well as gluconeogenesis-related genes pck1 and g6pca, was reduced in the livers of both mutants compared to the wild-type zebrafish. One of the most important findings in our study was that lepa−/− zebrafish were more affected than lepb−/− zebrafish by the processes of glycolysis and gluconeogenesis. This also explains the higher blood glucose levels in both mutant zebrafish compared to the wild-type zebrafish, as well as the higher blood glucose levels in lepa−/− zebrafish compared to lepb−/− zebrafish—a difference that implies primary and secondary roles for lepa and lepb in metabolic glucose utilization. This finding is consistent with the results reported by Londraville et al., who showed that leptin regulates the expression levels of pk and pklr, modulating glycolysis and thereby maintaining glucose homeostasis in the body [48,49]. Interestingly, for the first time in zebrafish leptin research, we found functional differences between lepa and lepb in terms of their involvement in glycolysis to maintain glucose homeostasis. Lepa may play a primary role in the maintenance of organismal glucose homeostasis by participating in glycolysis and gluconeogenesis, whereas lepb may play a secondary role in the maintenance of glucose homeostasis by participating in organismal glucose metabolism.
The liver plays an important role in the body’s energy metabolism, and adipose tissue plays a key role in energy storage [50]. When the amount of fat exceeds the liver’s ability to metabolize it, fat accumulates in the liver [51]. In mammals, fat deposition is associated with leptin, which mainly promotes fat hydrolysis and inhibits fat deposition, thereby maintaining energy metabolism and preventing obesity [52,53,54,55]. Intensive research on fish leptin in recent years has revealed that it is involved in processes related to lipid metabolism [56,57]. In this study, the biological functions of lepa and lepb in lipid metabolism induced by a high-carbohydrate diet were explored in zebrafish. The results revealed that triglyceride and cholesterol levels were reduced in lepa−/− zebrafish, either in blood or in liver and muscle tissues, while the opposite was true for lepb−/− zebrafish. These findings support the notion that leptin is involved in the process of lipid metabolism induced by a high-glucose diet. In molecular-level analyses of liver and muscle tissues, the expression levels of genes involved in adipogenesis, such as fas, acc, and foxo1a, were all significantly up-regulated in lepb−/− zebrafish, whereas fas and acc expression levels were significantly decreased in lepa−/− zebrafish. In combination with the above results, lepb−/− zebrafish also had high levels of body fat and fat deposition, while lepa−/− zebrafish had the lowest such levels. Our data suggest that liposynthesis is stimulated in lepb−/− zebrafish, while it is impaired in lepa−/− zebrafish. Combined with the process of glucose metabolism, it can be deduced that the conversion of glucose to lipids is blocked in zebrafish in the absence of lepa, whereas in the absence of lepb in zebrafish, lipids are accumulated due to the failure of lipolytic metabolism.
The expression of cpt1b, a sublipolysis-associated enzyme in the liver, was up-regulated in both mutant zebrafish but differed in liver and muscle tissues, possibly due to the negative feedback regulation of the organism caused by the higher liver fat content in lepb−/− zebrafish. This leads to an enhanced capacity for organismal fat oxidation. Similarly, different results were seen after leptin or receptor knockout in zebrafish [58,59] and medaka [18]. Phenotypic leptin or receptor knockout differences may be due to mutations in different alleles [60]. Induced by a high-glucose diet, our data support the hypothesis that the effect of leptin on lipid metabolism is highly conserved throughout the phylogeny [61]. The molecular mechanisms underlying the differences in lipid metabolism between lepa−/− and lepb−/− zebrafish were further explored, and it was found that lepb mainly promotes lipolytic metabolism, whereas lepa mainly promotes anabolic lipid effects.
In summary, purebred mutant lepa and lepb zebrafish lines were successfully constructed by CRISPR/Cas9 gene editing in this study. The role of leptin in feeding growth, as well as in glycolipid metabolism, was explored in zebrafish fed a high-glucose diet. The anorectic effects of lepa and lepb in zebrafish were confirmed to be similar to those in mammals. Lepa plays a major role in glucose metabolism, favoring the promotion of glucose utilization and lipogenesis, whereas lepb mainly promotes lipolytic oxidation and regulates protein production but plays a minor role in glucose metabolism.
4. Materials and Methods
4.1. Acquisition of Mutants
The zebrafish used in the experiment were raised in an indoor recirculating water culture system at the Mandarin Fish Research Center of Huazhong Agricultural University (Wuhan, China). The temperature was maintained at approximately 26–28 °C year-round under a cycle of 14 h light and 10 h darkness. The ethics committee approval number for this experiment is HZAUFI-2020-0038. Wild-type zebrafish embryos were gene-edited using CRISPR/Cas9 technology, and knockout targets were designed on the second exon of lepa and lepb using an online tool (https://cctop.cos.uni-heidelberg.de/ (accessed on 22 October 2024)). pMD19-T plasmid was used as a template for PCR amplification, purified, and recovered, then transcribed in vitro with a TranscriptionAid T7 High Yield Transcription kit (Thermo Scientific, Waltham, MA, USA). sgRNA was recovered by purification with lithium chloride precipitation at the end of in vitro transcription. Equal volumes of 500 ng/µL Cas9 and 80 ng/µL sgRNA were mixed and injected into single-cell zebrafish embryos via microinjection. The mutated target fragment was detected by PCR amplification, and if a double peak appeared at the position of the sgRNA target sequence as a result of sequencing, the mutant F0 generation was raised to adulthood. The primers used for PCR detection of lepa and lepb are shown in Table S1. Mutant F0 parents were paired with wild-type fish to obtain the F1 generation. The F1 generation was raised with clipped caudal fins, and based on the sequencing results, males and females of the same mutation type were mated to obtain F2 pure lepa−/− and lepb−/− zebrafish haploids.
4.2. High-Glucose Diet Feeding
The high-glucose feed was made according to the experimental requirements and formulated based on the amino acid profile of zebrafish dorsal muscle. The formulation and composition of the high-carbohydrate diet are shown in Table S2. Crystalline L-amino acid premix was added to the experimental feed [62]. Wild-type and mutant zebrafish were fed Artemia nauplii reared to 60 days post fertilization (dpf). Males of uniform size for each genotype were randomly selected, including wild-type zebrafish, lepa−/− zebrafish, and lepb−/− zebrafish. Three parallel tanks were set up for each group, and 20 zebrafish were selected from each tank. During the 60-day experiment, fish were fed a high-glucose diet three times per day (08:30, 12:30, and 16:30) to apparent satiety.
4.3. Analysis of Growth Indices and Body Composition of Zebrafish
The weight and length of zebrafish were measured and recorded before and after they were fed a high-glucose diet. At the end of the high-glucose diet period, the liver and visceral mass were weighed separately. Mesenteric mucosal fat was collected to calculate the VSI, HSI, and MFI. The moisture content was determined using the drying method at 105 °C (GB/T5009.3-2016) [63]. The ash content of the sample was determined by constant temperature incineration at 550 °C (GB/T5009.4-2016) [64]. The protein content of the sample was determined by Kjeldahl nitrogen determination (GB/T5009.5-2016) [65], and the crude fat content was determined by the Soxhlet extraction method (GB/T5009.6-2016) [66].
4.4. Sample and Biochemical Analyses
After 60 d on a high-glucose diet, all the fish were anaesthetized with tricaine methanesulfonate (MS-222). The caudal fin was severed with scissors, and the whole blood was collected from the wound with a pipette tip treated with sodium heparin solution. The plasma was separated from the fish blood by refrigerated centrifuge (4 °C, 1500× g, 15 min).
Muscle and liver tissue were randomly taken from 6 fish of each genotype; the tissues from each group of fish were mixed together and divided equally into 3 portions, weighed, and packed in 2 mL test tubes. Glucose, triglyceride (TG), glycogen, protein, and total cholesterol levels were measured in plasma, liver, and muscle using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). [Glucose Assay Kit, Triglyceride Assay Kit, Liver/Muscle Glycogen Assay Kit, The Total Protein Assay Kit and Total Cholesterol Assay Kit].
4.5. Statistics on Food Intake
After the high-glucose diet experiment, 18 fish of each genotype were randomly selected and weighed after starvation for 24 h, and the food intake of each type was measured. Pre-weighed feed was placed in each tank. After 2 h, the remaining feed from each tank was carefully sucked up with a straw, oven-dried, and weighed.
4.6. Quantitative Real-Time PCR
At the end of the high-glucose diet experiment, liver and muscle tissue samples were randomly taken from 12 fish of each genotype, and total RNA of the samples was extracted using TRIzol Reagent (Takara, Tokyo, Japan). cDNA synthesis was carried out using a Reverse Transcription Kit (Vazyme, Nanjing, China), and samples were stored at −20 °C for later use. Zebrafish-specific primers were designed using Primer Premier 6.0 software (Table S1), and quantitative real-time PCR was used to detect gene expression. The RT-PCR experimental system is described as follows. Each reaction mixture (20 μL) contained 1 μL cDNA template, 10 μL SYBR (Vazyme Nanjing, China), 0.4 μL of each primer, and 8.2 μL ddH2O. The cycling parameters were 95 °C for 30 s, 40 cycles at 95 °C for 10 s, 58 °C for 30 s, and a melting curve ranging from 65 °C to 95 °C (gradually increasing 0.5 °C s−1), with data acquired every 6 s.
4.7. Histological Analysis
Dorsal muscle and liver tissue samples from each genotype of zebrafish (n = 3) were randomly taken and fixed overnight by adding 4% paraformaldehyde (PFA); then, frozen sections of muscle and liver tissue with a thickness of 4 μm were made. The frozen sections of liver and muscle tissue were stained with Oil Red O and Alcian Blue-Phosphoric Acid Schiff (AB-PAS), respectively, to observe the changes in fat and glycogen contents of the samples.
4.8. Data Analysis
All data collected this experiment were expressed as the mean ± standard error (mean ± S.E.M.) and analyzed using IBM SPSS Statistics 25 software. The normality of the data was first tested by the Shapiro–Wilk test. A one-Sample T test was used to exclude sample data that deviated from the overall mean, and an independent T test was used to compare two groups of data, with p < 0.05 indicating a significant difference. Comparisons between multiple datasets were performed using one-way analysis of variance (ANOVA), and Duncan’s multiple range test was used for significant differences, with p < 0.05 indicating statistical significance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms252111647/s1.
Author Contributions
J.W., W.Z. and X.-F.L. designed the experiments and drafted the manuscript. J.W. and W.Z. performed the experiments. K.L., L.Z., Y.W. and F.C. were involved in experimental sampling and the feeding of zebrafish. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Research and Development Program of Jiangxi Province (20232BBF60011) and the Fishery Seed Industry Joint Breeding Project of Jiangxi Province (2023yyzygg-02).
Institutional Review Board Statement
All experimental protocols were approved by the Institutional Animal Care and Use Ethics Committee of Huazhong Agricultural University (an approval reference number HZAUFI-2020-0038).
Informed Consent Statement
Not applicable
Data Availability Statement
All data are available from the corresponding author by request.
Conflicts of Interest
The authors declare no competing interests.
References
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, Y.; Yu, L. Natural Selection and Adaptive Evolution of Leptin. Chin. Sci. Bull. 2013, 58, 2104–2112. [Google Scholar] [CrossRef]
- Gorissen, M.; Bernier, N.J.; Nabuurs, S.B.; Flik, G.; Huising, M.O. Two Divergent Leptin Paralogues in Zebrafish (Danio rerio) That Originate Early in Teleostean Evolution. J. Endocrinol. 2009, 201, 329–339. [Google Scholar] [CrossRef]
- Kurokawa, T.; Murashita, K. Genomic Characterization of Multiple Leptin Genes and a Leptin Receptor Gene in the Japanese Medaka, Oryzias Latipes. Gen. Comp. Endocrinol. 2009, 161, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Angotzi, A.R.; Stefansson, S.O.; Nilsen, T.O.; Øvrebø, J.I.; Andersson, E.; Taranger, G.L.; Rønnestad, I. Identification of a Novel Leptin Receptor Duplicate in Atlantic Salmon: Expression Analyses in Different Life Stages and in Response to Feeding Status. Gen. Comp. Endocrinol. 2016, 235, 108–119. [Google Scholar] [CrossRef]
- Yuan, X.; Li, A.; Liang, X.-F.; Huang, W.; Song, Y.; He, S.; Cai, W.; Tao, Y. Leptin Expression in Mandarin Fish Siniperca Chuatsi (Basilewsky): Regulation by Postprandial and Short-Term Fasting Treatment. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 194, 8–18. [Google Scholar] [CrossRef]
- On the Molecular Evolution of Leptin, Leptin Receptor, and Endospanin—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28443063/ (accessed on 21 September 2024).
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Hotta, K.; Gustafson, T.A.; Ortmeyer, H.K.; Bodkin, N.L.; Nicolson, M.A.; Hansen, B.C. Regulation of Obese (Ob) mRNA and Plasma Leptin Levels in Rhesus Monkeys. Effects of Insulin, Body Weight, and Non-Insulin-Dependent Diabetes Mellitus. J. Biol. Chem. 1996, 271, 25327–25331. [Google Scholar] [CrossRef][Green Version]
- Hope, P.J.; Webb, G.C.; Lok, S.; Hope, R.M.; Turnbull, H.; Jelmberg, A.C.; Wittert, G.A. Cloning of Leptin cDNA and Assignment to the Long Arm of Chromosome 5 in the Marsupial Sminthopsis crassicaudata. Cytogenet. Cell Genet. 2000, 90, 22–29. [Google Scholar] [CrossRef]
- Paolucci, M.; Buono, S.; Sciarrillo, R.; Putti, R. Effects of Leptin Administration on the Endocrine Pancreas and Liver in the Lizard Podarcis Sicula. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA Coding for a Homologue to Mammalian Leptin from Pufferfish, Takifugu Rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef]
- Murashita, K.; Uji, S.; Yamamoto, T.; Rønnestad, I.; Kurokawa, T. Production of Recombinant Leptin and Its Effects on Food Intake in Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 377–384. [Google Scholar] [CrossRef]
- Rønnestad, I.; Nilsen, T.O.; Murashita, K.; Angotzi, A.R.; Gamst Moen, A.-G.; Stefansson, S.O.; Kling, P.; Thrandur Björnsson, B.; Kurokawa, T. Leptin and Leptin Receptor Genes in Atlantic Salmon: Cloning, Phylogeny, Tissue Distribution and Expression Correlated to Long-Term Feeding Status. Gen. Comp. Endocrinol. 2010, 168, 55–70. [Google Scholar] [CrossRef]
- Gong, N.; Einarsdottir, I.E.; Johansson, M.; Björnsson, B.T. Alternative Splice Variants of the Rainbow Trout Leptin Receptor Encode Multiple Circulating Leptin-Binding Proteins. Endocrinology 2013, 154, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Zhang, Y.; Li, S.; Lu, D.; Zhang, H.; Meng, Z.; Liu, X.; Lin, H. Molecular Cloning, Characterization and Expression Profiles of Multiple Leptin Genes and a Leptin Receptor Gene in Orange-Spotted Grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2013, 181, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Shpilman, M.; Hollander-Cohen, L.; Ventura, T.; Gertler, A.; Levavi-Sivan, B. Production, Gene Structure and Characterization of Two Orthologs of Leptin and a Leptin Receptor in Tilapia. Gen. Comp. Endocrinol. 2014, 207, 74–85. [Google Scholar] [CrossRef]
- Ohga, H.; Matsumori, K.; Kodama, R.; Kitano, H.; Nagano, N.; Yamaguchi, A.; Matsuyama, M. Two Leptin Genes and a Leptin Receptor Gene of Female Chub Mackerel (Scomber japonicus): Molecular Cloning, Tissue Distribution and Expression in Different Obesity Indices and Pubertal Stages. Gen. Comp. Endocrinol. 2015, 222, 88–98. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M.S. Leptin Modulates the Intrinsic Excitability of AgRP/NPY Neurons in the Arcuate Nucleus of the Hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Soengas, J.L. Leptin Signalling in Teleost Fish with Emphasis in Food Intake Regulation. Mol. Cell. Endocrinol. 2021, 526, 111209. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, I.; Flik, G.; Gorissen, M. Comparative Physiology of Energy Metabolism: Fishing for Endocrine Signals in the Early Vertebrate Pool. Front. Endocrinol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Duan, J.; Choi, Y.-H.; Hartzell, D.; Della-Fera, M.A.; Hamrick, M.; Baile, C.A. Effects of Subcutaneous Leptin Injections on Hypothalamic Gene Profiles in Lean and ob/ob Mice. Obesity 2007, 15, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Singh, R.; Rai, U. Trajectory of Leptin and Leptin Receptor in Vertebrates: Structure, Function and Their Regulation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110652. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, X.; Gonzales, F.; Mangelsdorf, D.J.; Wang, M.-Y.; Richardson, C.; Witters, L.A.; Unger, R.H. PPAR Alpha Is Necessary for the Lipopenic Action of Hyperleptinemia on White Adipose and Liver Tissue. Proc. Natl. Acad. Sci. USA 2002, 99, 11848–11853. [Google Scholar] [CrossRef]
- Gallardo, N.; Bonzón-Kulichenko, E.; Fernández-Agulló, T.; Moltó, E.; Gómez-Alonso, S.; Blanco, P.; Carrascosa, J.M.; Ros, M.; Andrés, A. Tissue-Specific Effects of Central Leptin on the Expression of Genes Involved in Lipid Metabolism in Liver and White Adipose Tissue. Endocrinology 2007, 148, 5604–5610. [Google Scholar] [CrossRef]
- Buettner, C.; Muse, E.D.; Cheng, A.; Chen, L.; Scherer, T.; Pocai, A.; Su, K.; Cheng, B.; Li, X.; Harvey-White, J.; et al. Leptin Controls Adipose Tissue Lipogenesis via Central, STAT3-Independent Mechanisms. Nat. Med. 2008, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the Obese Gene Product on Body Weight Regulation in Ob/Ob Mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Murashita, K.; Verri, T.; Gomes, A.S.; Rønnestad, I. Leptin Receptor-Deficient (Knockout) Zebrafish: Effects on Nutrient Acquisition. Gen. Comp. Endocrinol. 2021, 310, 113832. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, N.; Martínez-Alvarez, R.; Delgado, M.J. Acute and Chronic Leptin Reduces Food Intake and Body Weight in Goldfish (Carassius auratus). J. Endocrinol. 2006, 188, 513–520. [Google Scholar] [CrossRef]
- Aguilar, A.J.; Conde-Sieira, M.; Polakof, S.; Míguez, J.M.; Soengas, J.L. Central Leptin Treatment Modulates Brain Glucosensing Function and Peripheral Energy Metabolism of Rainbow Trout. Peptides 2010, 31, 1044–1054. [Google Scholar] [CrossRef]
- Yuan, X.-C.; Liang, X.-F.; Cai, W.-J.; Li, A.-X.; Huang, D.; He, S. Differential Roles of Two Leptin Gene Paralogues on Food Intake and Hepatic Metabolism Regulation in Mandarin Fish. Front. Endocrinol. 2020, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.T.; Kalra, P.S.; Crowley, W.R.; Kalra, S.P. Neuropeptide Y and Human Pancreatic Polypeptide Stimulate Feeding Behavior in Rats. Endocrinology 1984, 115, 427–429. [Google Scholar] [CrossRef]
- Stanley, B.G.; Leibowitz, S.F. Neuropeptide Y Injected in the Paraventricular Hypothalamus: A Powerful Stimulant of Feeding Behavior. Proc. Natl. Acad. Sci. USA 1985, 82, 3940–3943. [Google Scholar] [CrossRef]
- Shutter, J.R.; Graham, M.; Kinsey, A.C.; Scully, S.; Lüthy, R.; Stark, K.L. Hypothalamic Expression of ART, a Novel Gene Related to Agouti, Is up-Regulated in Obese and Diabetic Mutant Mice. Genes Dev. 1997, 11, 593–602. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Beck, B. Neuropeptide Y in Normal Eating and in Genetic and Dietary-Induced Obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1159–1185. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Physiology of Leptin: Energy Homeostasis, Neuroendocrine Function and Metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Singh, P.; Heymsfield, S.B.; Yu, S.; Morrison, C.D. Recent Advances in Understanding the Role of Leptin in Energy Homeostasis. F1000Research 2020, 9, F1000 Faculty Rev-451. [Google Scholar] [CrossRef]
- Song, Y.; Golling, G.; Thacker, T.L.; Cone, R.D. Agouti-Related Protein (AGRP) Is Conserved and Regulated by Metabolic State in the Zebrafish, Danio rerio. Endocrine 2003, 22, 257–265. [Google Scholar] [CrossRef]
- Yokobori, E.; Azuma, M.; Nishiguchi, R.; Kang, K.S.; Kamijo, M.; Uchiyama, M.; Matsuda, K. Neuropeptide Y Stimulates Food Intake in the Zebrafish, Danio rerio. J. Neuroendocrinol. 2012, 24, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Opazo, R.; Plaza-Parrochia, F.; Cardoso Dos Santos, G.R.; Carneiro, G.R.A.; Sardela, V.F.; Romero, J.; Valladares, L. Fasting Upregulates Npy, Agrp, and Ghsr Without Increasing Ghrelin Levels in Zebrafish (Danio rerio) Larvae. Front. Physiol. 2018, 9, 1901. [Google Scholar] [CrossRef]
- Londraville, R.L.; Duvall, C.S. Murine Leptin Injections Increase Intracellular Fatty Acid-Binding Protein in Green Sunfish (Lepomis cyanellus). Gen. Comp. Endocrinol. 2002, 129, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Baltzegar, D.A.; Reading, B.J.; Seale, A.P.; Lerner, D.T.; Grau, E.G.; Borski, R.J. Leptin Stimulates Cellular Glycolysis Through a STAT3 Dependent Mechanism in Tilapia. Front. Endocrinol. 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Lebovics, E.; Rubin, J. Non-Alcoholic Fatty Liver Disease (NAFLD): Why You Should Care, When You Should Worry, What You Should Do. Diabetes Metab. Res. Rev. 2011, 27, 419–424. [Google Scholar] [CrossRef]
- Chen, G.; Koyama, K.; Yuan, X.; Lee, Y.; Zhou, Y.T.; O’Doherty, R.; Newgard, C.B.; Unger, R.H. Disappearance of Body Fat in Normal Rats Induced by Adenovirus-Mediated Leptin Gene Therapy. Proc. Natl. Acad. Sci. USA 1996, 93, 14795–14799. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, U.; Benson, B.; Kaufman, S.; Ross, L.; Qi, M.; Scully, S.; DiPalma, C. Morphologic and Molecular Changes Induced by Recombinant Human Leptin in the White and Brown Adipose Tissues of C57BL/6 Mice. Lab. Investig. 1997, 77, 243–256. [Google Scholar] [PubMed]
- Frühbeck, G.; Aguado, M.; Martínez, J.A. In Vitro Lipolytic Effect of Leptin on Mouse Adipocytes: Evidence for a Possible Autocrine/Paracrine Role of Leptin. Biochem. Biophys. Res. Commun. 1997, 240, 590–594. [Google Scholar] [CrossRef]
- Siegrist-Kaiser, C.A.; Pauli, V.; Juge-Aubry, C.E.; Boss, O.; Pernin, A.; Chin, W.W.; Cusin, I.; Rohner-Jeanrenaud, F.; Burger, A.G.; Zapf, J.; et al. Direct Effects of Leptin on Brown and White Adipose Tissue. J. Clin. Investig. 1997, 100, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Tan, X.-Y.; Xu, Y.-H.; Chen, Q.-L.; Pan, Y.-X. JAK and STAT Members of Yellow Catfish Pelteobagrus Fulvidraco and Their Roles in Leptin Affecting Lipid Metabolism. Gen. Comp. Endocrinol. 2016, 226, 14–26. [Google Scholar] [CrossRef]
- Liu, C.-Z.; He, A.-Y.; Ning, L.-J.; Luo, Y.; Li, D.-L.; Zhang, M.-L.; Chen, L.-Q.; Du, Z.-Y. Leptin Selectively Regulates Nutrients Metabolism in Nile Tilapia Fed on High Carbohydrate or High Fat Diet. Front. Endocrinol. 2018, 9, 574. [Google Scholar] [CrossRef]
- Michel, M.; Page-McCaw, P.S.; Chen, W.; Cone, R.D. Leptin Signaling Regulates Glucose Homeostasis, but Not Adipostasis, in the Zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, 3084–3089. [Google Scholar] [CrossRef]
- Audira, G.; Sarasamma, S.; Chen, J.-R.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Hsieh, M.-C.; Hsiao, C.-D. Zebrafish Mutants Carrying Leptin a (Lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation. Int. J. Mol. Sci. 2018, 19, 4038. [Google Scholar] [CrossRef]
- Fischer-Posovszky, P.; von Schnurbein, J.; Moepps, B.; Lahr, G.; Strauss, G.; Barth, T.F.; Kassubek, J.; Mühleder, H.; Möller, P.; Debatin, K.-M.; et al. A New Missense Mutation in the Leptin Gene Causes Mild Obesity and Hypogonadism without Affecting T Cell Responsiveness. J. Clin. Endocrinol. Metab. 2010, 95, 2836–2840. [Google Scholar] [CrossRef]
- Ramsay, T.G.; Bush, J.A.; McMurtry, J.P.; Thivierge, M.C.; Davis, T.A. Peripheral Leptin Administration Alters Hormone and Metabolite Levels in the Young Pig. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 17–25. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Zhai, G.; Gong, Y.-L.; Su, J.-Z.; Peng, X.-Y.; Shang, G.-H.; Han, D.; Jin, J.-Y.; Liu, H.-K.; Du, Z.-Y.; et al. Different Physiological Roles of Insulin Receptors in Mediating Nutrient Metabolism in Zebrafish. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E38–E51. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; National Food Safety Standard, Determination of Moisture in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard, Determination of Ash Content in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.5-2016; National Food Safety Standard, Determination of Protein in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard, Determination of Fat in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).