Gender-Specific DNA Methylation Profiles Associated with Adult Weight in Hezuo Pigs
Abstract
1. Introduction
2. Results
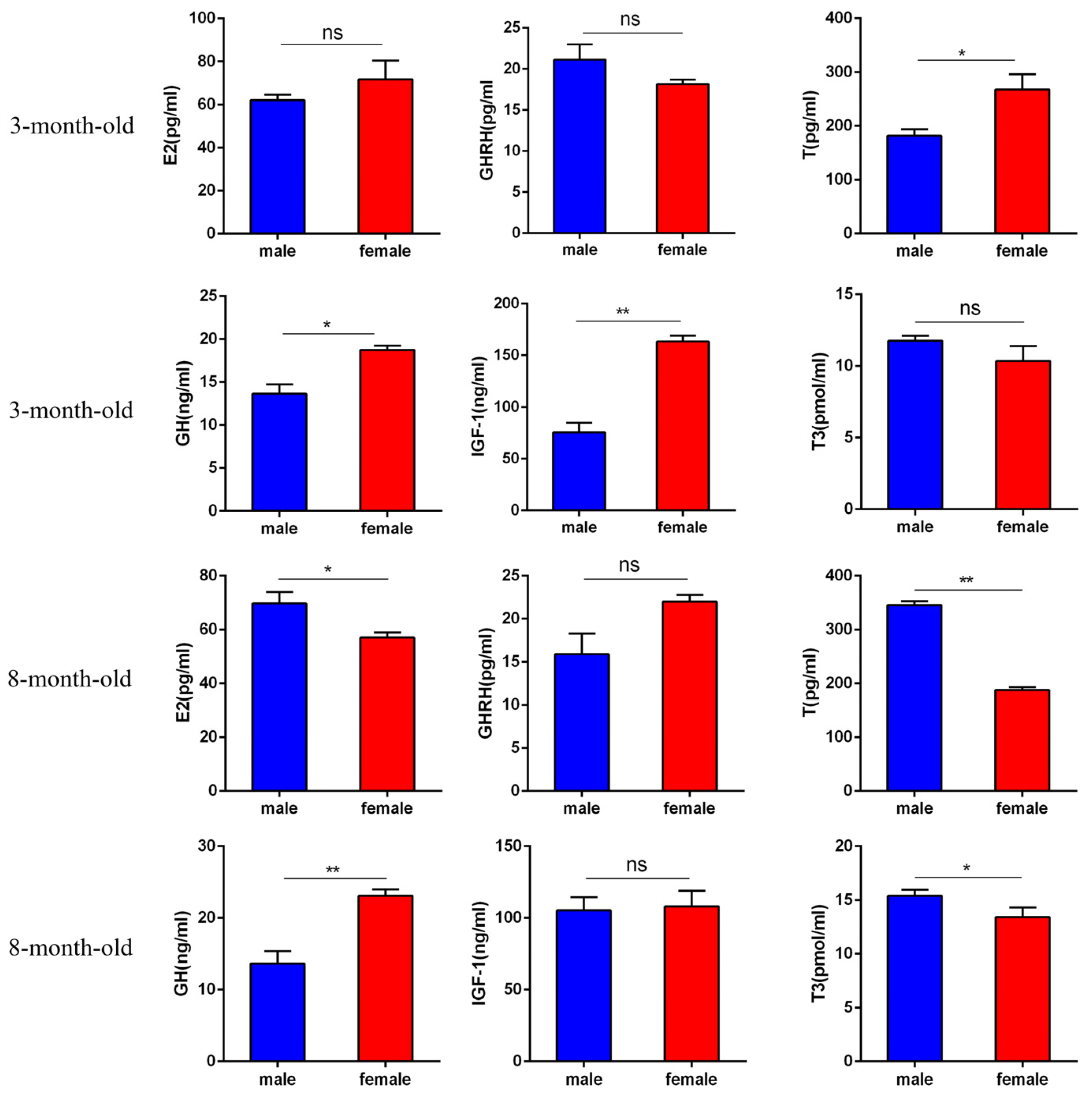
2.1. Changes in Serum Hormone Content in Male and Female Hezuo Pigs
2.2. Whole Genome Bisulfite Sequencing (WGBS) Sequencing of Pituitary Tissue in Male and Female Hezuo Pigs
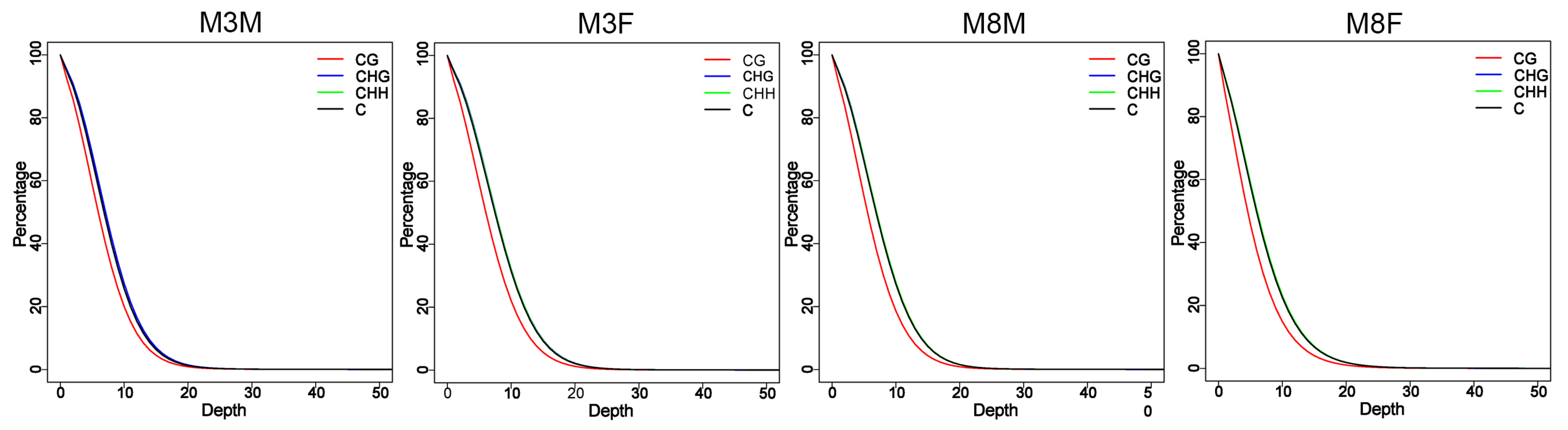
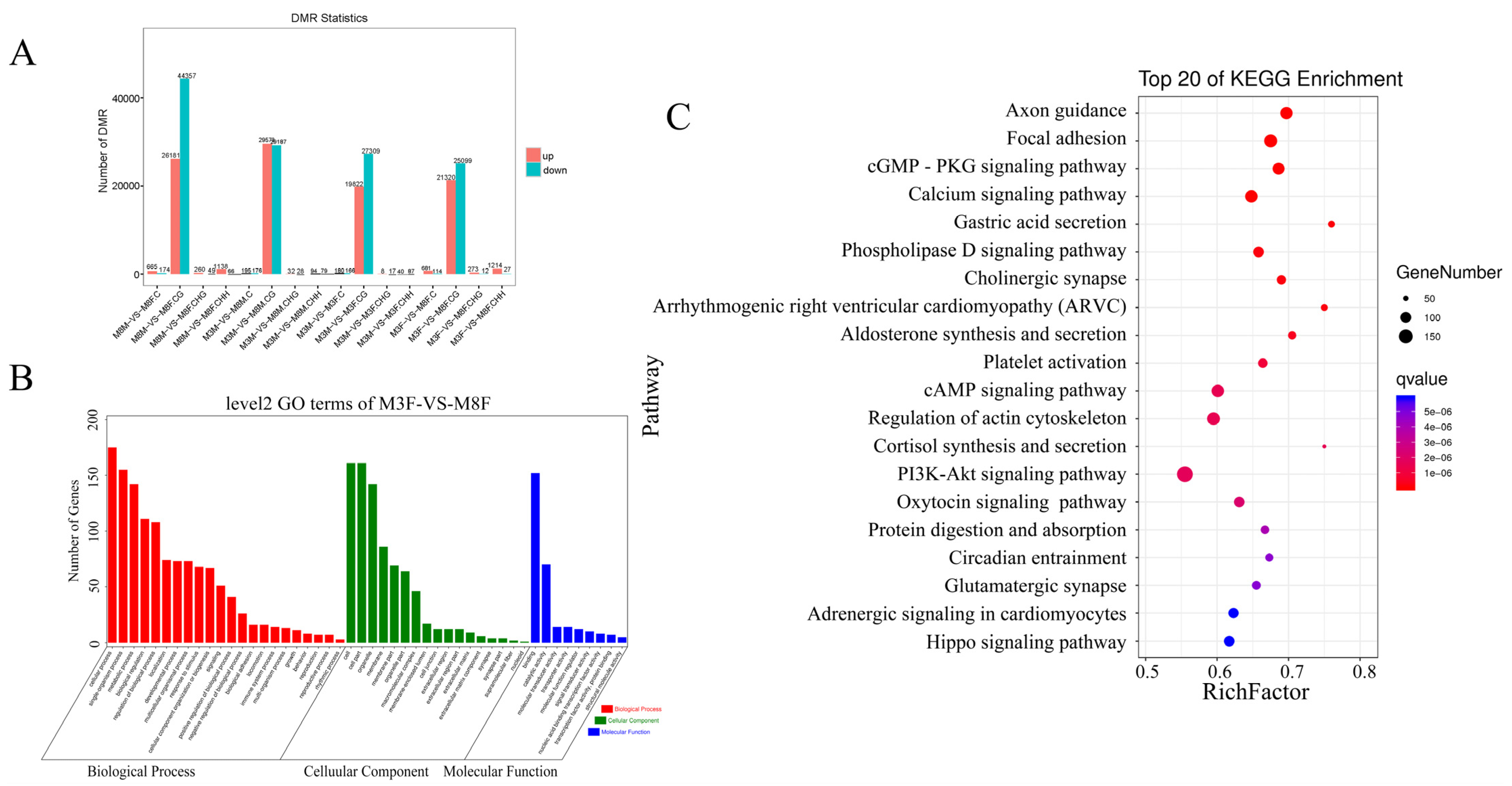
2.3. Sequencing Depth Statistics for Genome-Wide Methylated CG, CHG, and CHH
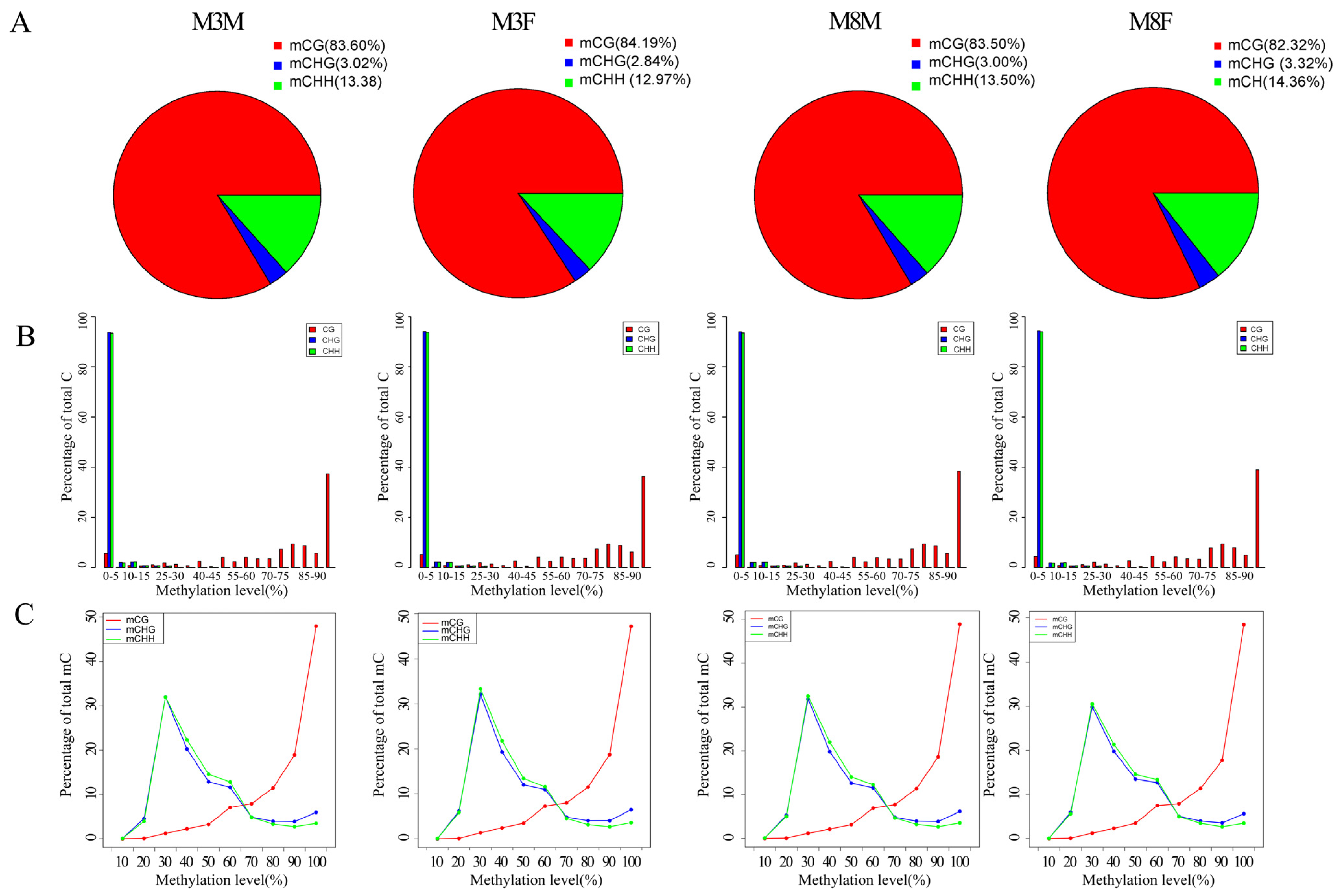
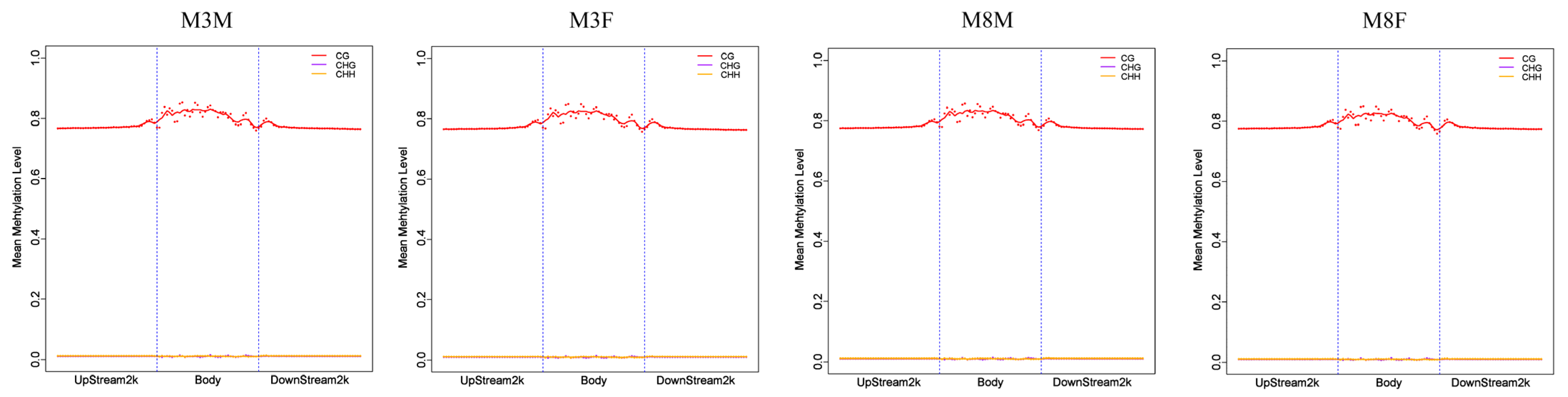
2.4. Analysis of C-Base Methylation Level in Pituitary Tissue of Hezuo Pig
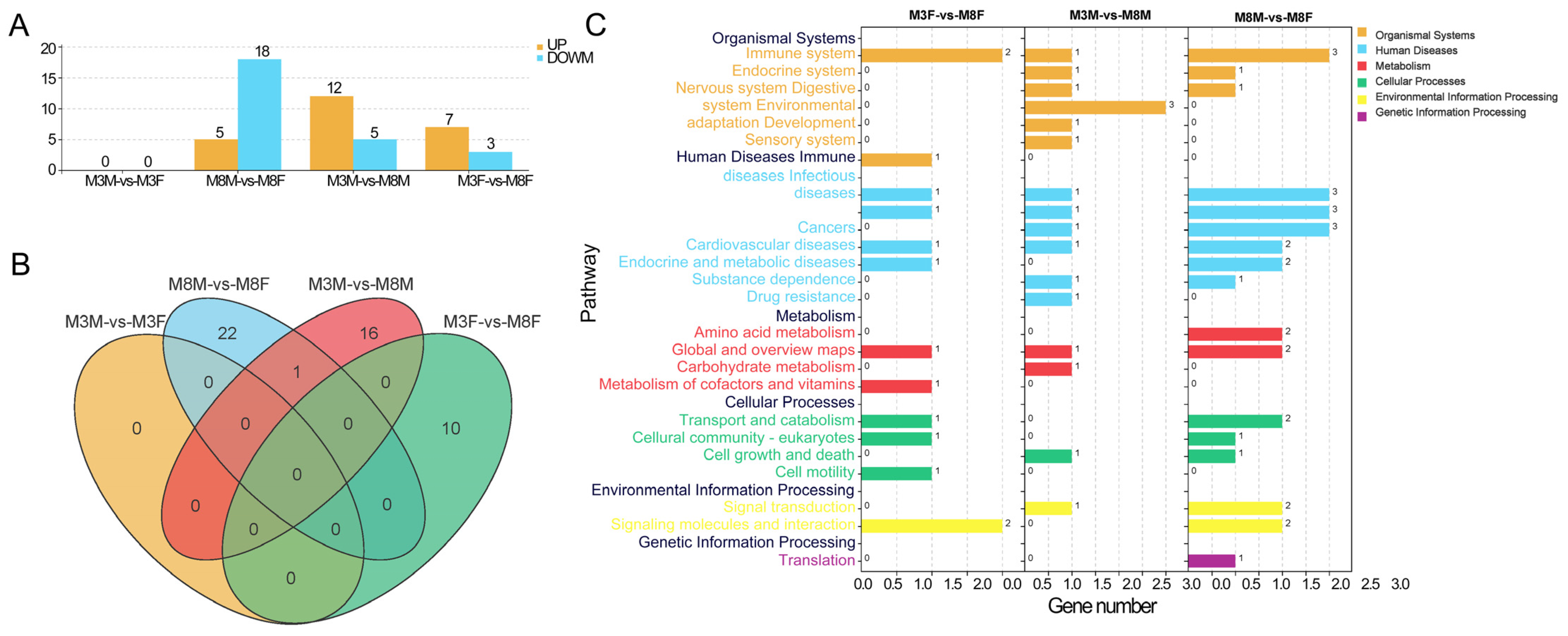
2.5. Enrichment Analysis of Differentially Methylated Sites (DMCs) and Associated Genes
2.6. Enrichment Analysis of DMR and Associated Genes
2.7. Transcriptome Sequencing Results
2.8. Screening for DMGs Associated with Adult Weight Development in Hezuo Pigs
2.9. Correlation Analysis of Different Hormones with Gene Expression
3. Discussion
4. Materials and Methods
4.1. Experimental Animal Selection
4.2. Detection of Serum Hormone Content
4.3. DNA Bisulfite Treatment and Sequencing
4.4. Methylation Level Analysis
4.5. Detection and Analysis of Methylation Sites
4.6. Analysis of DMRs
4.7. Functional Enrichment Analysis of Differentially Methylated Site (DMC)/DMR-Related Genes
4.8. Total RNA Extraction and RNA-Sequencing
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Q.; Li, C.W.; Xie, Z.G.; Fan, W.J.; Zhang, J.D. Studies on the Growth Difference of the Male and Female Siniperca chuatsi. Freshw. Fish. 2006, 3, 34–37. [Google Scholar]
- Pei, D.Z. Utilization Techniques for Small-Scale Pig Farming; China Braille Press: Beijing, China, 1999; pp. 2–11. [Google Scholar]
- Guang, H.M.; Liu, M.Z.; Gun, S.B. Analysis on Correlation Coefficient of Carcass Quality Traits about Hezuo Pig in Shelter Feeding. Swine Prod. 2016, 2, 27. [Google Scholar]
- Wang, L.; Wang, P.; Yan, Z.; Zhang, P.; Yin, X.; Jia, R.; Li, Y.; Yang, J.; Gun, S.; Yang, Q. Whole-plant silage maize to improve fiber digestive characteristics and intestinal microbiota of Hezuo pigs. Front. Microbiol. 2024, 15, 1360505. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z. Pig Line Breeding and Gansu Pig Breed Resources; Gansu Science & Technology Press: Lanzhou, China, 2007; p. 94. [Google Scholar]
- Guan, H.M.; Liu, M.Z. A study on the analysis and fitting of growth curves in Hezuo pigs. Guizhou J. Anim. Husb. Vet. Med. 2007, 2, 1–3. [Google Scholar]
- Willems, C.; Fu, Q.; Roose, H.; Mertens, F.; Cox, B.; Chen, J.; Vankelecom, H. Regeneration in the Pituitary After Cell-Ablation Injury: Time-Related Aspects and Molecular Analysis. Endocrinology 2016, 157, 705–721. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Rev. Aquac. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aïssi, D.; Wahl, S.; Meduri, E.; Morange, P.E.; Gagnon, F.; Grallert, H.; et al. DNA methylation and body-mass index: A genome-wide analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Sun, Y.; Li, Y.; Kang, L.; Jiang, Y. Dynamic transcriptome and DNA methylome analyses on longissimus dorsi to identify genes underlying intramuscular fat content in pigs. BMC Genom. 2017, 18, 780. [Google Scholar] [CrossRef]
- Singer, B.D. A practical guide to the measurement and Analysis of DNA Methylation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 417–428. [Google Scholar] [CrossRef]
- Rizzoti, K. Genetic regulation of murine pituitary development. J. Mol. Endocrinol. 2015, 54, R55–R73. [Google Scholar] [CrossRef]
- Zhao, Y.; Mailloux, C.M.; Hermesz, E.; Palkóvits, M.; Westphal, H. A role of the LIM-homeobox gene Lhx2 in the regulation of pituitary development. Dev. Biol. 2010, 337, 313–323. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, T.; Song, J.; Qi, Q.; Wang, C.; Xi, Q.; Liu, S.; Hao, L.; Zhang, Y. miR-709 inhibits GHRP6 induced GH synthesis by targeting PRKCA in pituitary. Mol. Cell. Endocrinol. 2020, 506, 110763. [Google Scholar] [CrossRef]
- Leone, S.; Chiavaroli, A.; Recinella, L.; Di Valerio, V.; Veschi, S.; Gasparo, I.; Bitto, A.; Ferrante, C.; Orlando, G.; Salvatori, R.; et al. Growth hormone-releasing hormone (GHRH) deficiency promotes inflammation-associated carcinogenesis. Pharmacol. Res. 2020, 152, 104614. [Google Scholar] [CrossRef]
- Aguiar-Oliveira, M.H.; Davalos, C.; Campos, V.C.; Oliveira Neto, L.A.; Marinho, C.G.; Oliveira, C.R.P. Hypothalamic abnormalities: Growth failure due to defects of the GHRH receptor. Growth Horm. IGF Res. 2018, 38, 14–18. [Google Scholar] [CrossRef]
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013, 93, 571–598. [Google Scholar] [CrossRef]
- Icyuz, M.; Fitch, M.; Zhang, F.; Challa, A.; Sun, L.Y. Physiological and metabolic features of mice with CRISPR/Cas9-mediated loss-of-function in growth hormone-releasing hormone. Aging 2020, 12, 9761–9780. [Google Scholar] [CrossRef]
- Sun, L.Y.; Spong, A.; Swindell, W.R.; Fang, Y.; Hill, C.; Huber, J.A.; Boehm, J.D.; Westbrook, R.; Salvatori, R.; Bartke, A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife 2013, 2, e01098. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Batistuzzo, A.; Salas-Lucia, F.; Gereben, B.; Ribeiro, M.O.; Bianco, A.C. Sustained Pituitary T3 Production Explains the T4-mediated TSH Feedback Mechanism. Endocrinology 2023, 64, bqad155. [Google Scholar] [CrossRef]
- Burch, H.B. Drug Effects on the Thyroid. N. Engl. J. Med. 2019, 381, 749–761. [Google Scholar] [CrossRef]
- Alrabadi, N.; Al-Rabadi, G.J.; Maraqa, R.; Sarayrah, H.; Alzoubi, K.H.; Alqudah, M.; Al-U’datt, D.G. Androgen effect on body weight and behaviour of male and female rats: Novel insight on the clinical value. Androloga 2020, 52, e13730. [Google Scholar] [CrossRef]
- Ding, C.L. Functiongal Study of Rcan2 Gene on Body Weight Regulatory Mechanisms in Females. Master’s Thesis, Shandong University, Jinan, China, 2015. [Google Scholar]
- Voegtline, K.M.; Costigan, K.A.; DiPietro, J.A. Maternal salivary testosterone in pregnancy and fetal neuromaturation. Dev. Psychobiol. 2017, 59, 822–831. [Google Scholar] [CrossRef]
- Lathi, R.B.; Moayeri, S.E.; Reddy, C.D.; Gebhardt, J.; Behr, B.; Westphal, L.M. Testosterone concentrations in early pregnancy: Relation to method of conception in an infertile population. Reprod. Biomed. Online 2012, 24, 360–363. [Google Scholar] [CrossRef][Green Version]
- Sin, T.K.; Yung, B.Y.; Siu, P.M. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: Implications in skeletal muscle aging and insulin resistance. Cell Physiol. Biochem. 2015, 35, 541–552. [Google Scholar] [CrossRef]
- Hovestadt, V.; Jones, D.T.; Picelli, S.; Wang, W.; Kool, M.; Northcott, P.A.; Sultan, M.; Stachurski, K.; Ryzhova, M.; Warnatz, H.J.; et al. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature 2014, 510, 537–541. [Google Scholar] [CrossRef]
- Fagan, A.M.; Vos, S.J. Preclinical Alzheimer’s disease criteria. Lancet Neurol. 2013, 12, 1134. [Google Scholar] [CrossRef]
- Therriault, J.; Vermeiren, M.; Servaes, S.; Tissot, C.; Ashton, N.J.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Lussier, F.Z.; et al. Association of Phosphorylated Tau Biomarkers with Amyloid Positron Emission Tomography vs Tau Positron Emission Tomography. JAMA Neurol. 2023, 80, 188–199. [Google Scholar] [CrossRef]
- Therriault, J.; Vermeiren, M.; Servaes, S.; Tissot, C.; Ashton, N.J.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Lussier, F.Z.; et al. Tau Kinetics in neurons and the human central nervous System. Neurology 2018, 97, 1284–1298.e7. [Google Scholar]
- Huang, W.; Liang, J.; Yuan, C.C.; Kazmierczak, K.; Zhou, Z.; Morales, A.; McBride, K.L.; Fitzgerald-Butt, S.M.; Hershberger, R.E.; Szczesna-Cordary, D. Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain. FEBS J. 2015, 282, 2379–2393. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef]
- Weterman, M.A.; Barth, P.G.; van Spaendonck-Zwarts, K.Y.; Aronica, E.; Poll-The, B.T.; Brouwer, O.F.; van Tintelen, J.P.; Qahar, Z.; Bradley, E.J.; de Wissel, M.; et al. Recessive MYL2 mutations cause infantile type I muscle fiber disease and cardiomyopathy. Brain 2013, 136 Pt 1, 282–293. [Google Scholar] [CrossRef]
- Wu, M.T.; Pan, C.H.; Chen, C.Y.; Chen, C.J.; Huang, L.H.; Tsai, L.Y.; Huang, C.T.; Ho, C.K. Lack of modulating influence of GSTM1 and GSTT1 polymorphisms on urinary biomonitoring markers in coke-oven workers. Am. J. Ind. Med. 2004, 46, 112–119. [Google Scholar] [CrossRef]
- Garcia-Cattaneo, A.; Gobert, F.X.; Müller, M.; Toscano, F.; Flores, M.; Lescure, A.; Del Nery, E.; Benaroch, P. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 9053–9058. [Google Scholar] [CrossRef]
- Macneil, D.J. The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front. Endocrinol. 2013, 4, 49. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef]
- Yao, X.; Li, F.; Wei, Z.; Ei-Samahy, M.A.; Feng, X.; Yang, F.; Wang, F. Integrative Genome-Wide DNA Methylome and Transcriptome Analysis of Ovaries from Hu Sheep with High and Low Prolific. Front. Cell Dev. Biol. 2022, 10, 820558. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, W.F.; Pan, M.H.; Xiao, J.S.; Feng, Y.J.; Dong, Z.Q.; Zou, B.X.; Zhou, L.; Zhang, Y.H.; Lu, C. Comparative genome-wide DNA methylation analysis reveals epigenomic differences in response to heat-humidity stress in Bombyx mori. Int. J. Biol. Macromol. 2020, 164, 3771–3779. [Google Scholar] [CrossRef]
- Xi, Y.; Li, W. BSMAP: Whole genome bisulfite sequence MAPping program. BMC Bioinform. 2009, 10, 232. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Akalin, A.; Kormaksson, M.; Li, S.; Garrett-Bakelman, F.E.; Figueroa, M.E.; Melnick, A.; Mason, C.E. methylKit: A comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012, 13, R87. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]




| Sample | Total Reads | Mapped Reads | Mapped Ratio (%) | Genome Size | Sequence Depth |
|---|---|---|---|---|---|
| M3M | 455,956,276 | 378,242,478 | 82.96 | 2,485,970,876 | 22.82 |
| M3F | 481,350,952 | 401,708,691 | 83.45 | 2,485,970,876 | 24.24 |
| M8M | 465,351,434 | 386,271,639 | 83.01 | 2,485,970,876 | 23.31 |
| M8F | 468,237,436 | 390,204,927 | 83.33 | 2,485,970,876 | 23.54 |
| Sample | C (%) | mCG (%) | mCHG (%) | mCHH (%) |
|---|---|---|---|---|
| M3M | 5.15 | 76.25 | 1.03 | 1.15 |
| M3F | 4.92 | 76.16 | 0.94 | 1.05 |
| M8M | 4.97 | 77.04 | 0.99 | 1.10 |
| M8F | 4.76 | 77.13 | 0.98 | 1.08 |
| Gene ID | Symbol | Chromosome | Genomic Context |
|---|---|---|---|
| LOC397422 | CCL2 | NC_010454.4 | promoter |
| LOC396969 | CTSH | NC_010449.5 | promoter |
| LOC733682 | MCH2 | NC_010443.5 | promoter |
| LOC396690 | MYL2 | NC_010456.5 | promoter |
| LOC100152951 | GST | NC_010449.5 | promoter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Huang, X.; Yang, J.; Wang, L.; Li, J.; Li, Y.; Gun, S.; Yan, Z.; Wang, P.; Yang, Q. Gender-Specific DNA Methylation Profiles Associated with Adult Weight in Hezuo Pigs. Int. J. Mol. Sci. 2024, 25, 11488. https://doi.org/10.3390/ijms252111488
Jia R, Huang X, Yang J, Wang L, Li J, Li Y, Gun S, Yan Z, Wang P, Yang Q. Gender-Specific DNA Methylation Profiles Associated with Adult Weight in Hezuo Pigs. International Journal of Molecular Sciences. 2024; 25(21):11488. https://doi.org/10.3390/ijms252111488
Chicago/Turabian StyleJia, Rui, Xiaoyu Huang, Jiaojiao Yang, Longlong Wang, Jie Li, Yao Li, Shuangbao Gun, Zunqiang Yan, Pengfei Wang, and Qiaoli Yang. 2024. "Gender-Specific DNA Methylation Profiles Associated with Adult Weight in Hezuo Pigs" International Journal of Molecular Sciences 25, no. 21: 11488. https://doi.org/10.3390/ijms252111488
APA StyleJia, R., Huang, X., Yang, J., Wang, L., Li, J., Li, Y., Gun, S., Yan, Z., Wang, P., & Yang, Q. (2024). Gender-Specific DNA Methylation Profiles Associated with Adult Weight in Hezuo Pigs. International Journal of Molecular Sciences, 25(21), 11488. https://doi.org/10.3390/ijms252111488





