Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization
Abstract
1. Introduction
2. Results and Discussion
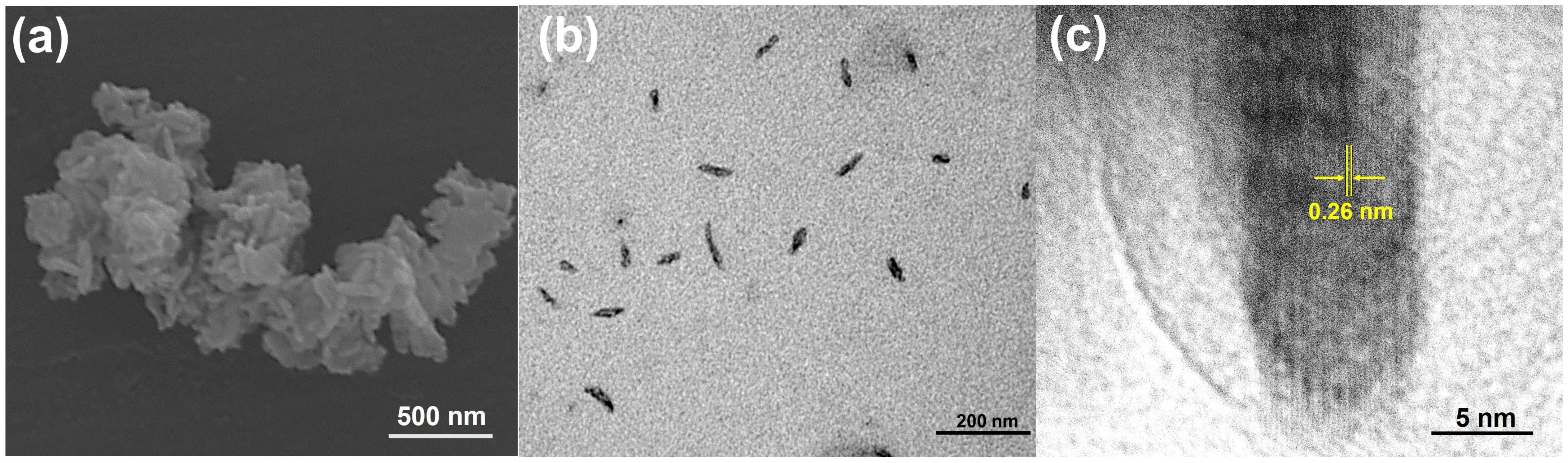
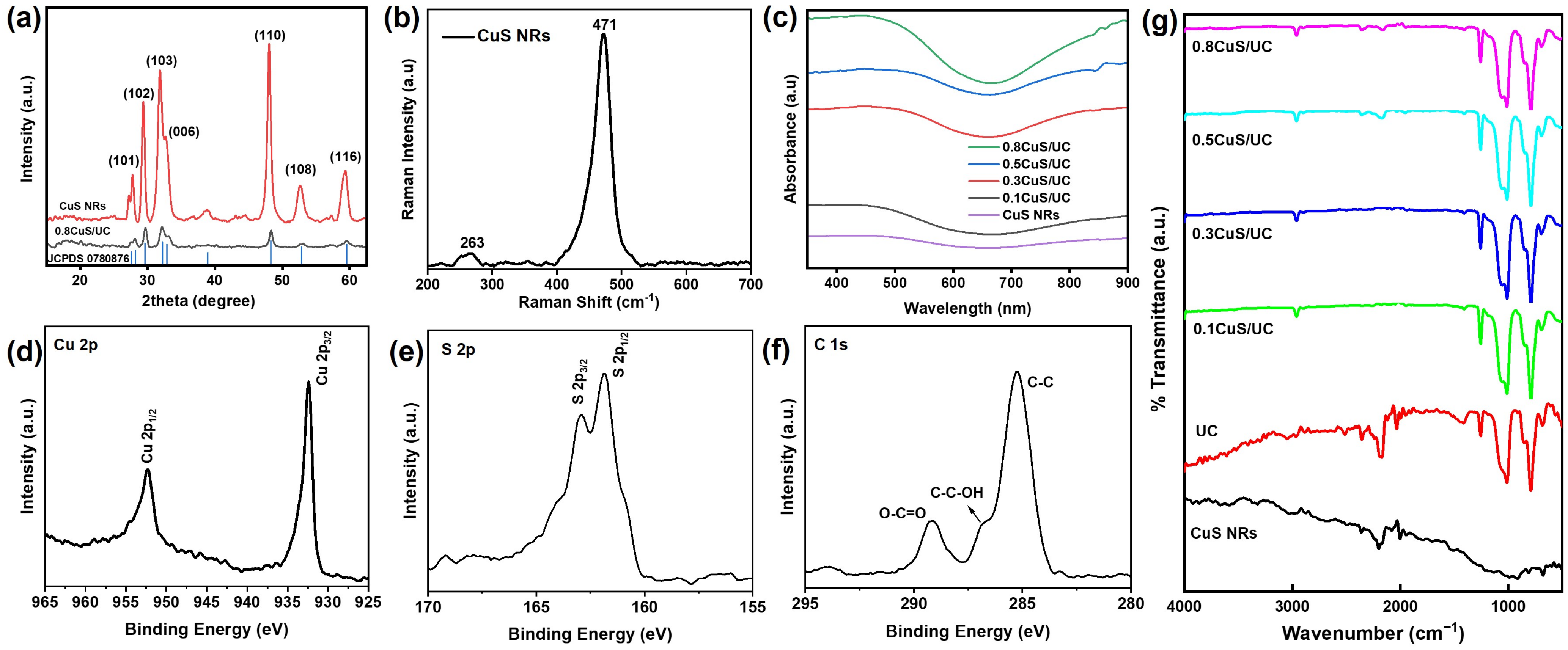
2.1. Structural and Optical Characterizations of CuS NRs
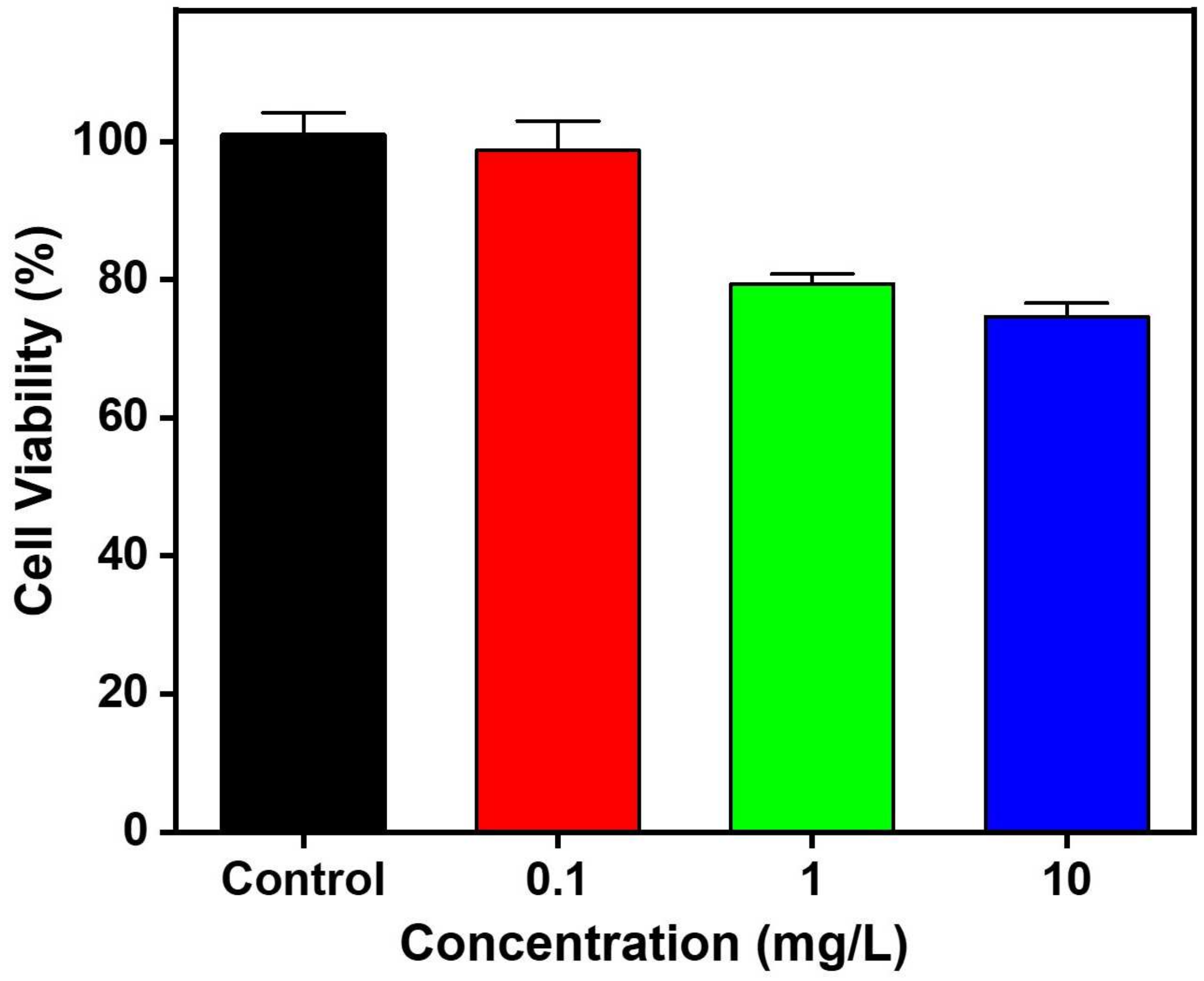
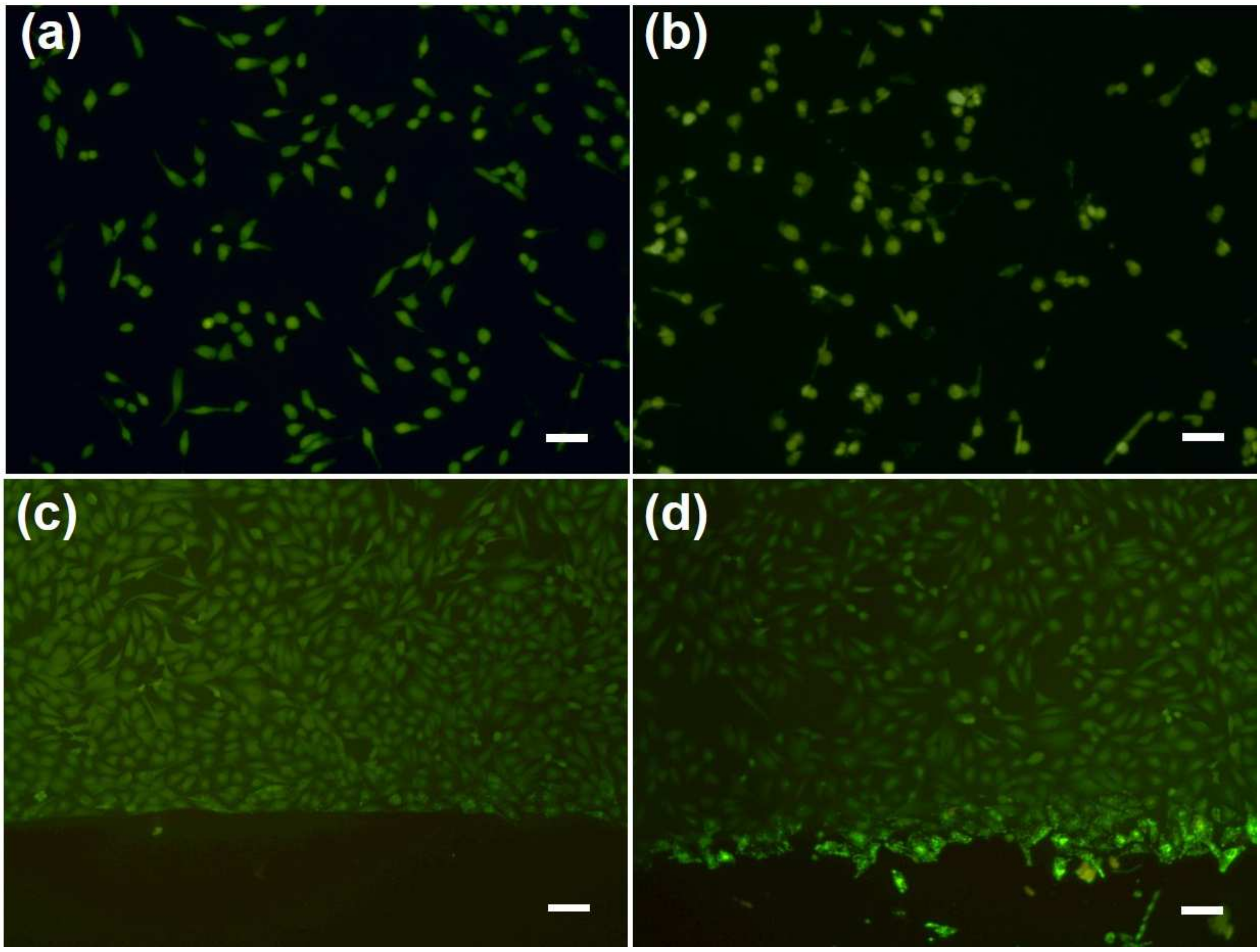
2.2. Cytotoxicity Assay of CuS NRs
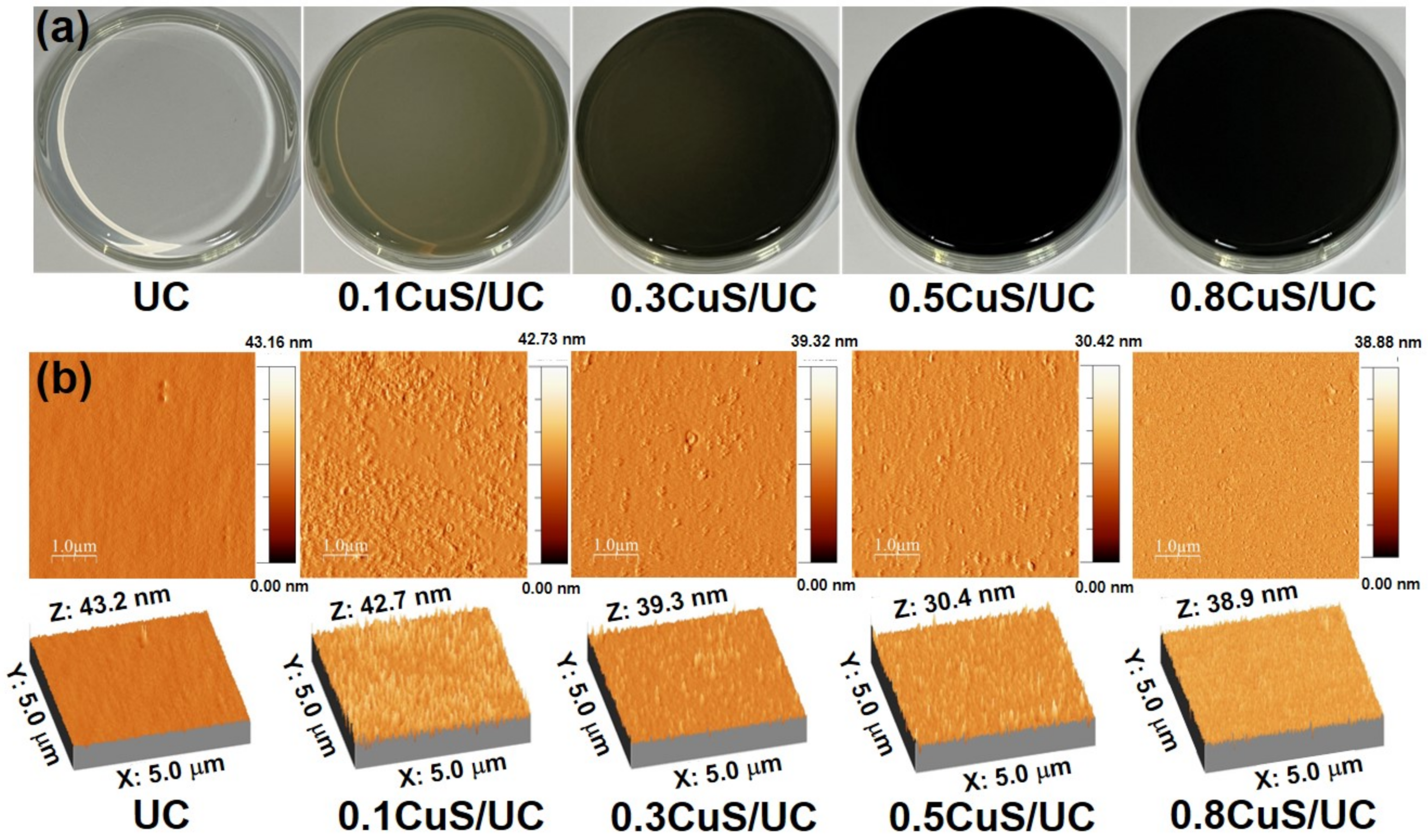
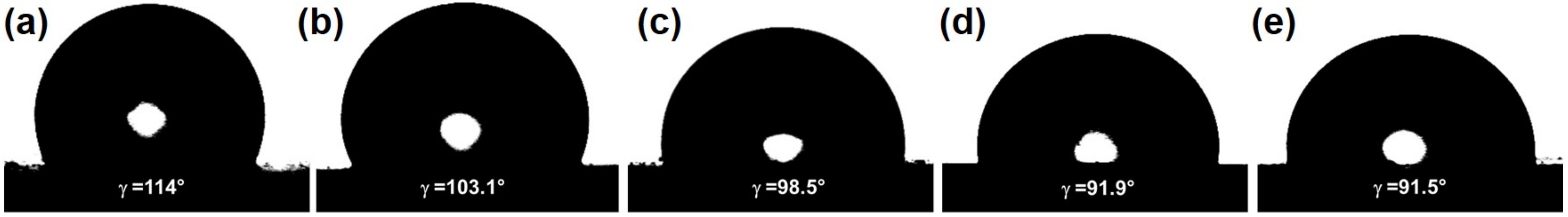
2.3. Surface Studies of CuS/UC
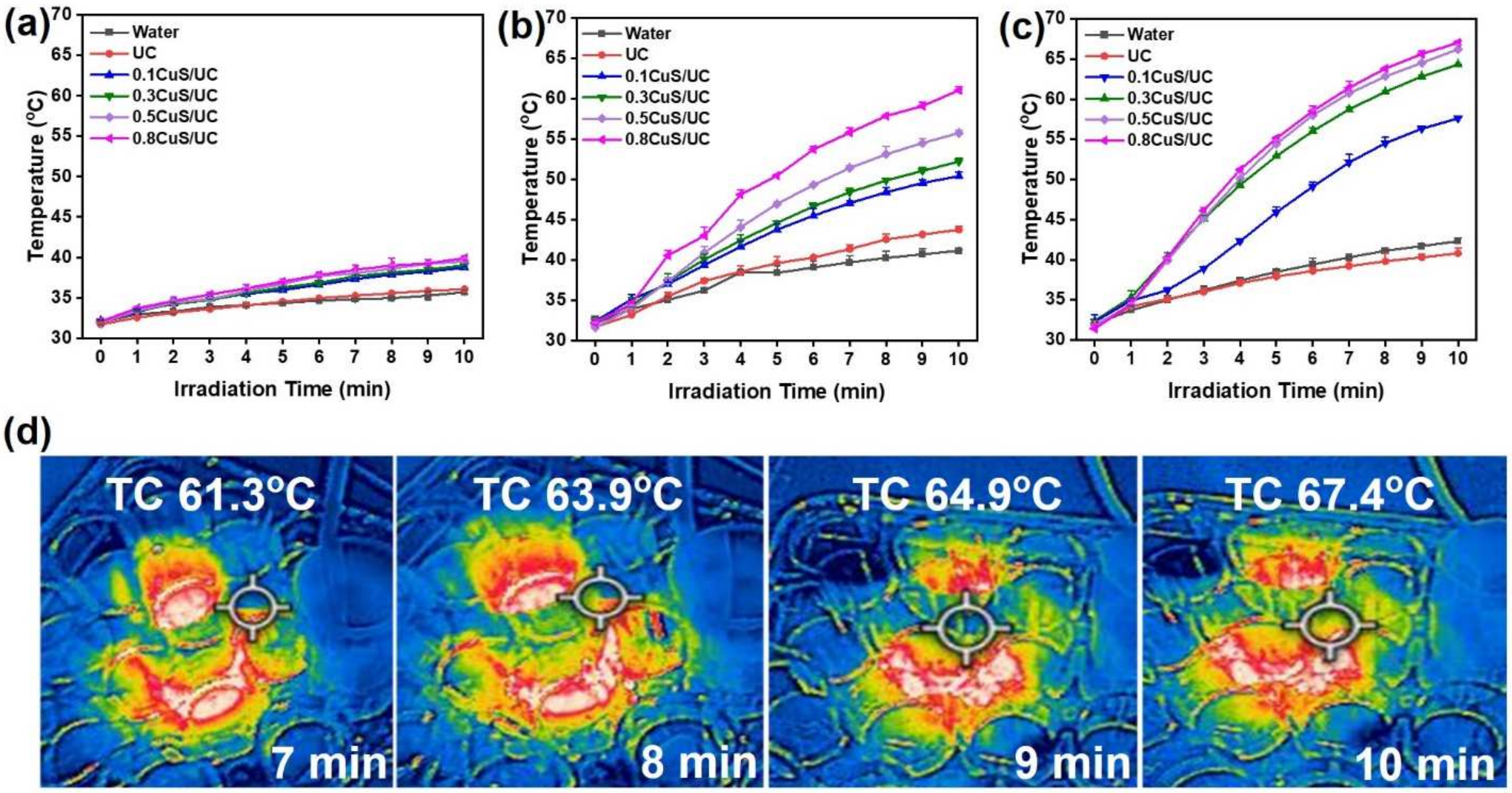
2.4. Photothermal Antibacterial Activity of CuS/UC
2.5. Evaluation of Photothermal Antibacterial Activity of CuS/UC by Agar Plate Test
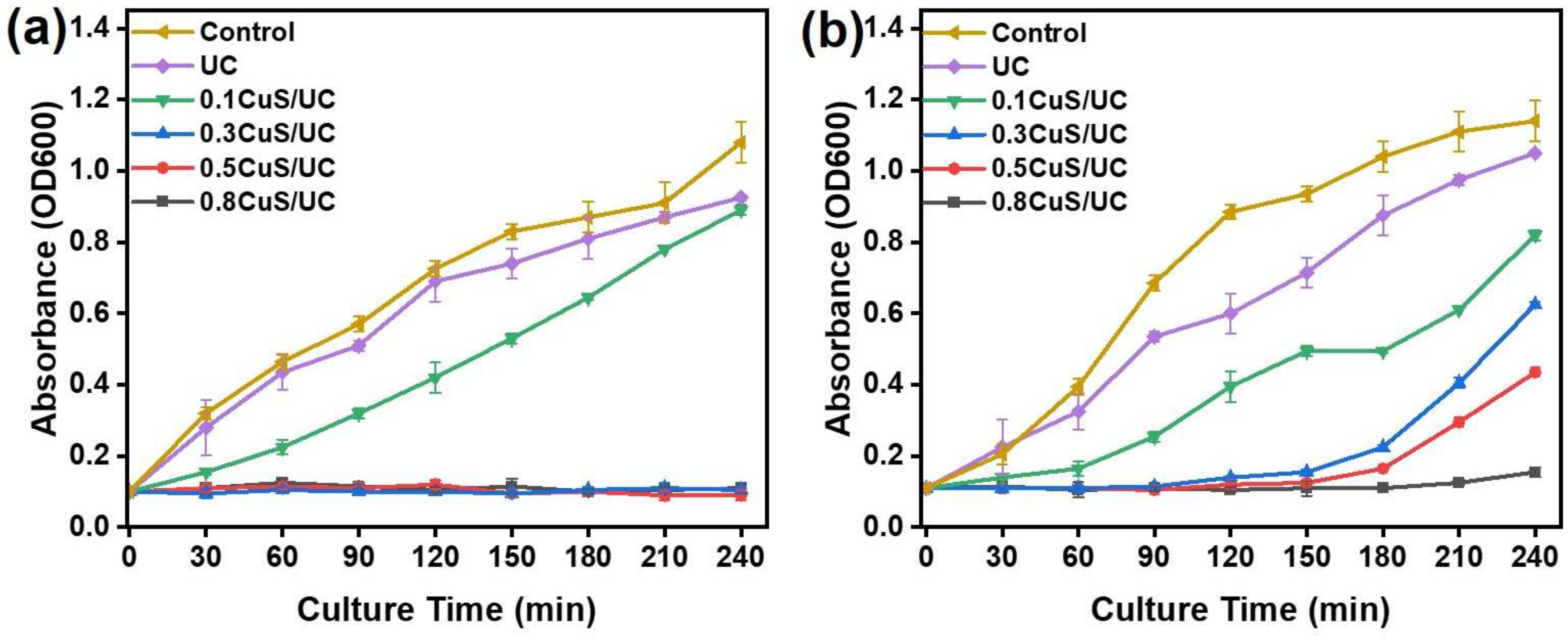
2.6. Biocompatibility and In Vitro Anti-Cell Adhesion of CuS/UC
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of CuS NRs
3.3. Preparation of CuS NR-Embedded Urinary Catheter
3.4. Material Characterization
3.5. Photothermal Properties of CuS/UC
3.6. Photothermal Antibacterial Assay
3.7. Cytotoxicity of CuS NRs
3.8. Biocompatibility Test of CuS/UC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Musco, S.; Giammò, A.; Savoca, F.; Gemma, L.; Geretto, P.; Soligo, M.; Sacco, E.; Del Popolo, G.; Li Marzi, V. How to Prevent Catheter-Associated Urinary Tract Infections: A Reappraisal of Vico’s Theory–Is History Repeating Itself? J. Clin. Med. 2022, 11, 3415. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.K.; Advani, S.D.; Kofman, A.D.; Lo, E.; Maragakis, L.L.; Pegues, D.A.; Pettis, A.M.; Saint, S.; Trautner, B.; Yokoe, D.S.; et al. Strategies to prevent catheter-associated urinary tract infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Bocquier, A.; Erkilic, B.; Babinet, M.; Pulcini, C.; Agrinier, N. Resident-, prescriber-, and facility-level factors associated with antibiotic use in long-term care facilities: A systematic review of quantitative studies. Antimicrob. Resist. Infect. Control 2024, 13, 29. [Google Scholar] [CrossRef]
- Kranjec, C.; Mathew, J.P.; Ovchinnikov, K.; Fadayomi, I.; Yang, Y.; Kjos, M.; Li, W.W. A bacteriocin-based coating strategy to prevent vancomycin-resistant Enterococcus faecium biofilm formation on materials of interest for indwelling medical devices. Biofilm 2024, 8, 100211. [Google Scholar] [CrossRef]
- Nye, T.M.; Zou, Z.; Obernuefemann, C.L.P.; Pinkner, J.S.; Lowry, E.; Kleinschmidt, K.; Bergeron, K.; Klim, A.; Dodson, K.W.; Flores-Mireles, A.L.; et al. Microbial co-occurrences on catheters from long-term catheterized patients. Nat. Commun. 2024, 15, 61. [Google Scholar] [CrossRef]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F.-J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1802140. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Kottegoda, N.; Ratnaweera, D.R.; Weerasekera, M.M. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega 2021, 6, 11488–11496. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Yu, Q.; Chen, H. Photothermal bactericidal surfaces: Killing bacteria using light instead of biocides. Biomater. Sci. 2021, 9, 10–22. [Google Scholar] [CrossRef]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating Bacterial Biofilm Formation in Urinary Catheter by Green Silver Nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.; Balasubramanian, C.; Hans, S.; Patil, C.; Nema, S. Oxygen Plasma for Prevention of Biofilm Formation on Silicone Catheter Surfaces: Influence of Plasma Exposure Time. Plasma Chem. Plasma Process. 2022, 42, 815–831. [Google Scholar] [CrossRef]
- Khantamat, O.; Li, C.H.; Yu, F.; Jamison, A.C.; Shih, W.C.; Cai, C.; Lee, T.R. Gold nanoshell-decorated silicone surfaces for the near-infrared (NIR) photothermal destruction of the pathogenic bacterium E. faecalis. ACS Appl. Mater. Interfaces 2015, 7, 3981–3993. [Google Scholar] [CrossRef]
- Kumari, P.; Bharti, A.; Agarwal, V.; Kumar, N.; Saxena, K. ZnO coating on silicon rubber urinary catheter to reduce the biofilm infections. Adv. Mater. Process. Technol. 2022, 8, 423–433. [Google Scholar] [CrossRef]
- Zhao, N.; Gao, X.; Chen, Z.; Feng, Y.; Liu, G.; Zhou, F.; Liu, W. Super-lubricating hybrid elastomer with rapid photothermal sterilization and strong anti-cell adhesion. Chem. Eng. J. 2022, 434, 134763. [Google Scholar] [CrossRef]
- Mukherjee, S.G.; O’Claonadh, N.; Casey, A.; Chambers, G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol. Vitr. 2012, 26, 238–251. [Google Scholar] [CrossRef]
- Liu, C.; Feng, S.; Ma, L.; Sun, M.; Wei, Z.; Wang, J.; Chen, Z.; Guo, Y.; Shi, J.; Wu, Q. An Amphiphilic Carbonaceous/Nanosilver Composite-Incorporated Urinary Catheter for Long-Term Combating Bacteria and Biofilms. ACS Appl. Mater. Interfaces 2021, 13, 38029–38039. [Google Scholar] [CrossRef]
- Draviana, H.T.; Fitriannisa, I.; Khafid, M.; Krisnawati, D.I.; Lai, C.H.; Fan, Y.J.; Kuo, T.R. Size and charge effects of metal nanoclusters on antibacterial mechanisms. J. Nanobiotechnology 2023, 21, 428. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.T.; Liu, H.Y. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. Int. Ed. 2020, 59, 5890–5900. [Google Scholar] [CrossRef]
- Hu, R.Z.; Fang, Y.; Huo, M.F.; Yao, H.L.; Wang, C.M.; Chen, Y.; Wu, R. Ultrasmall Cu2-XS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II biowindow. Biomaterials 2019, 206, 101–114. [Google Scholar] [CrossRef]
- Liu, Y.J.; Bhattarai, P.; Dai, Z.F.; Chen, X.Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-K.; Cheng, T.-M.; Chu, H.-L.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Chen, H.-M.; Lee, C.-M.; Kuo, T.-R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.C.; Wang, D.Y.; Kuo, S.R. Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Sun, Y.; Zhang, Y.; Ding, X.; Zhao, N.; Yu, B.; Zhao, H.; Duan, S.; Xu, F.-J. Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia. ACS Nano 2020, 14, 2265–2275. [Google Scholar] [CrossRef]
- Yang, X.; Xia, P.; Zhang, Y.; Lian, S.; Li, H.; Zhu, G.; Wang, P. Photothermal Nano-antibiotic for Effective Treatment of Multidrug-Resistant Bacterial Infection. ACS Appl. Bio Mater. 2020, 3, 5395–5406. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, E.B.; Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Light controllable surface coating for effective photothermal killing of bacteria. ACS Appl. Mater. Interfaces 2015, 7, 15600–15606. [Google Scholar] [CrossRef]
- Lu, K.Y.; Jheng, P.R.; Lu, L.S.; Rethi, L.; Mi, F.L.; Chuang, E.Y. Enhanced anticancer effect of ROS-boosted photothermal therapy by using fucoidan-coated polypyrrole nanoparticles. Int. J. Biol. Macromol. 2021, 166, 98–107. [Google Scholar] [CrossRef]
- Lu, H.T.; Huang, G.Y.; Chang, W.J.; Lu, T.W.; Huang, T.W.; Ho, M.H.; Mi, F.L. Modification of chitosan nanofibers with CuS and fucoidan for antibacterial and bone tissue engineering applications. Carbohydr. Polym. 2022, 281, 119035. [Google Scholar] [CrossRef]
- Chen, C.A.; Lee, C.L.; Yang, P.K.; Tsai, D.S.; Lee, C.P. Active site engineering on two-dimensional-layered transition metal dichalcogenides for electrochemical energy applications: A mini-review. Catalysts 2021, 11, 151. [Google Scholar] [CrossRef]
- Patil, S.B.; Chou, H.-L.; Chen, Y.-M.; Hsieh, S.-H.; Chen, C.-H.; Chang, C.-C.; Li, S.-R.; Lee, Y.-C.; Lin, Y.-S.; Li, H.; et al. Enhanced N2 affinity of 1T-MoS2 with a unique pseudo-six-membered ring consisting of N–Li–S–Mo–S–Mo for high ambient ammonia electrosynthesis performance. J. Mater. Chem. A 2021, 9, 1230–1239. [Google Scholar] [CrossRef]
- Liang, J.W.; Liu, C.H.; Wu, K.C.-W.; Jheng, P.R.; Chuang, E.Y. Plasma-treated nano-enabled multimodal coatings made of phototherapeutic molybdenum disulfide and fucoidan prevent catheter-associated urinary tract issues. Chem. Eng. J. 2023, 468, 143749. [Google Scholar] [CrossRef]
- Kurahashi, H.; Umezawa, M.; Okubo, K.; Soga, K. Pixel Screening in Lifetime-Based Temperature Mapping Using β-NaYF4:Nd3+,Yb3+ by Time-Gated Near-Infrared Fluorescence Imaging on Deep Tissue in Live Mice. ACS Appl. Bio Mater. 2024, 7, 3821–3827. [Google Scholar] [CrossRef]
- Chia, Z.C.; Chen, Y.L.; Chuang, C.H.; Hsieh, C.H.; Chen, Y.J.; Chen, K.H.; Huang, T.C.; Chen, M.C.; Huang, C.C. Polyphenol-assisted assembly of Au-deposited polylactic acid microneedles for SERS sensing and antibacterial photodynamic therapy. Chem. Commun. 2023, 59, 6339–6342. [Google Scholar] [CrossRef]
- Chin, Y.C.; Yang, L.X.; Hsu, F.T.; Hsu, C.W.; Chang, T.W.; Chen, H.Y.; Chen, L.Y.C.; Chia, Z.C.; Hung, C.H.; Su, W.C.; et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J. Nanobiotechnol. 2022, 20, 373. [Google Scholar] [CrossRef]
- Chang, T.-W.; Ko, H.; Huang, W.-S.; Chiu, Y.-C.; Yang, L.-X.; Chia, Z.-C.; Chin, Y.-C.; Chen, Y.-J.; Tsai, Y.-T.; Hsu, C.-W. Tannic acid-induced interfacial ligand-to-metal charge transfer and the phase transformation of Fe3O4 nanoparticles for the photothermal bacteria destruction. Chem. Eng. J. 2022, 428, 131237. [Google Scholar] [CrossRef]
- Quiñones, E.D.; Lu, T.Y.; Liu, K.T.; Fan, Y.J.; Chuang, E.Y.; Yu, J. Glycol chitosan/iron oxide/polypyrrole nanoclusters for precise chemodynamic/photothermal synergistic therapy. Int. J. Biol. Macromol. 2022, 203, 268–279. [Google Scholar] [CrossRef]
- Huang, G.Y.; Chang, W.J.; Lu, T.W.; Tsai, I.L.; Wu, S.J.; Ho, M.H.; Mi, F.L. Electrospun CuS nanoparticles/chitosan nanofiber composites for visible and near-infrared light-driven catalytic degradation of antibiotic pollutants. Chem. Eng. J. 2022, 431, 134059. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Chou, H.-L.; Lin, I.H.; Yougbaré, S.; Chang, C.-C.; Kuo, T.-R. Phase-Dependent 1T/2H-MoS2 Nanosheets for Effective Photothermal Killing of Bacteria. ACS Sustain. Chem. Eng. 2022, 10, 8949–8957. [Google Scholar] [CrossRef]
- Dong, X.; Ye, J.; Chen, Y.; Tanziela, T.; Jiang, H.; Wang, X. Intelligent peptide-nanorods against drug-resistant bacterial infection and promote wound healing by mild-temperature photothermal therapy. Chem. Eng. J. 2022, 432, 134061. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.T.; Kuo, T.R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Cheng, Z.; Kang, L.; Xu, X.; Zhang, F.; Yang, H. Controllable synthesis of CuS decorated TiO2 nanofibers for enhanced photocatalysis. CrystEngComm 2015, 17, 5496–5501. [Google Scholar] [CrossRef]
- Ma, J.; Du, Q.; Ge, H.; Zhang, Q. Fabrication of core–shell TiO2@CuS nanocomposite via a bifunctional linker-assisted synthesis and its photocatalytic performance. J. Mater. Sci. 2019, 54, 2928–2939. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Li, S.; Ma, X.; Ji, Y.; Xu, J.; Que, D. Controllable growth of ZnO nanostructures by citric acid assisted hydrothermal process. Mater. Lett. 2005, 59, 1696–1700. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Zobel, M.; Neder, R.B.; Kimber, S.A. Universal solvent restructuring induced by colloidal nanoparticles. Science 2015, 347, 292–294. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of various size gold nanoparticles by chemical reduction method with different solvent polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W.; Jaiswal, L. Bioactive agar-based functional composite film incorporated with copper sulfide nanoparticles. Food Hydrocoll. 2019, 93, 156–166. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Wang, H.; Lei, Z. High-performance supercapacitor based on multi-structural CuS@ polypyrrole composites prepared by in situ oxidative polymerization. J. Mater. Chem. A 2014, 2, 3303–3307. [Google Scholar] [CrossRef]
- Hurma, T.; Kose, S. XRD Raman analysis and optical properties of CuS nanostructured film. Optik 2016, 127, 6000–6006. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Fan, Y. One-step solvothermal synthesis of different morphologies CuS nanosheets compared as supercapacitor electrode materials. J. Alloys Compd. 2015, 625, 158–163. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, K.; Nozaki, H. Anion distributions and phase transitions in CuS1-xSex (x = 0-1) studied by Raman spectroscopy. J. Solid State Chem. 1993, 105, 504–511. [Google Scholar] [CrossRef]
- Sciortino, G.; Maréchal, J.-D.; Fábián, I.; Lihi, N.; Garribba, E. Quantitative prediction of electronic absorption spectra of copper(II)–bioligand systems: Validation and applications. J. Inorg. Biochem. 2020, 204, 110953. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Li, H.; Wang, L.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Biomolecule-assisted, environmentally friendly, one-pot synthesis of CuS/reduced graphene oxide nanocomposites with enhanced photocatalytic performance. Langmuir 2012, 28, 12893–12900. [Google Scholar] [CrossRef]
- Li, B.; Xie, Y.; Xue, Y. Controllable synthesis of CuS nanostructures from self-assembled precursors with biomolecule assistance. J. Phys. Chem. C 2007, 111, 12181–12187. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef]
- Gautam, U.K.; Mukherjee, B. A simple synthesis and characterization of CuS nanocrystals. Bull. Mater. Sci. 2006, 29, 1–5. [Google Scholar] [CrossRef]
- Peng, S.; He, Y.; Er, M.; Sheng, Y.; Gu, Y.; Chen, H. Biocompatible CuS-based nanoplatforms for efficient photothermal therapy and chemotherapy in vivo. Biomater. Sci. 2017, 5, 475–484. [Google Scholar] [CrossRef]
- Cannella, V.; Altomare, R.; Chiaramonte, G.; Di Bella, S.; Mira, F.; Russotto, L.; Pisano, P.; Guercio, A. Cytotoxicity Evaluation of Endodontic Pins on L929 Cell Line. BioMed Res. Int. 2019, 2019, 3469525. [Google Scholar] [CrossRef]
- Markad, G.; Padma, N.; Chadha, R.; Gupta, K.; Rajarajan, A.; Deb, P.; Kapoor, S. Mutual influence on aggregation and magnetic properties of graphene oxide and copper phthalocyanine through non-covalent, charge transfer interaction. Appl. Surf. Sci. 2020, 505, 144624. [Google Scholar] [CrossRef]
- Nikam, A.N.; Pandey, A.; Fernandes, G.; Kulkarni, S.; Mutalik, S.P.; Padya, B.S.; George, S.D.; Mutalik, S. Copper sulphide based heterogeneous nanoplatforms for multimodal therapy and imaging of cancer: Recent advances and toxicological perspectives. Coord. Chem. Rev. 2020, 419, 213356. [Google Scholar] [CrossRef]
- Park, E.; Selvaraj, R.; Kim, Y. High-efficiency photothermal sterilization on PDMS film with Au@ CuS yolk-shell nanoparticles. J. Ind. Eng. Chem. 2022, 113, 522–529. [Google Scholar] [CrossRef]
- Ibelli, T.; Templeton, S.; Levi-Polyachenko, N. Progress on utilizing hyperthermia for mitigating bacterial infections. Int. J. Hyperth. 2018, 34, 144–156. [Google Scholar] [CrossRef]
- Guan, G.; Win, K.Y.; Yao, X.; Yang, W.; Han, M.-Y. Plasmonically Modulated Gold Nanostructures for Photothermal Ablation of Bacteria. Adv. Healthc. Mater. 2021, 10, 2001158. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the Photothermal Conversion Efficiency of Gold Nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef]
- Marin, R.; Skripka, A.; Besteiro, L.V.; Benayas, A.; Wang, Z.; Govorov, A.O.; Canton, P.; Vetrone, F. Highly Efficient Copper Sulfide-Based Near-Infrared Photothermal Agents: Exploring the Limits of Macroscopic Heat Conversion. Small 2018, 14, 1803282. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
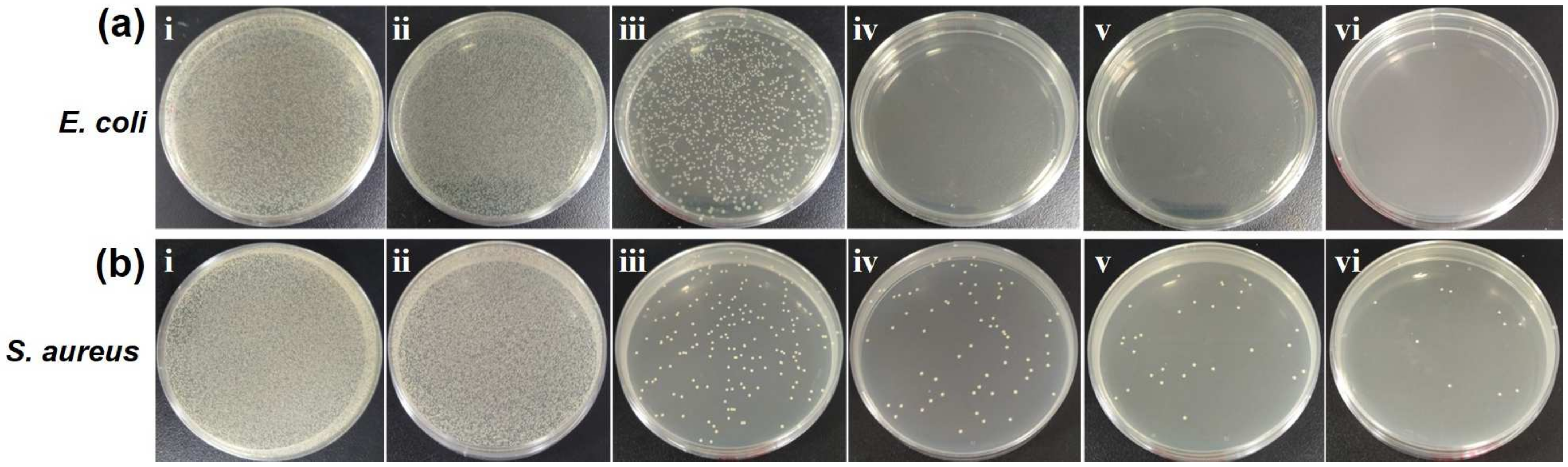
| Bacteria | Control | UC | 0.1CuS/UC | 0.3CuS/UC | 0.5CuS/UC | 0.8CuS/UC |
|---|---|---|---|---|---|---|
| E. coli | 100 | 80.40 | 5.55 | 0.00 | 0.00 | 0.00 |
| S. aureus | 100 | 87.92 | 0.35 | 0.14 | 0.06 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saukani, M.; Lai, C.-H.; Mutalik, C.; Krisnawati, D.I.; Chu, H.-Y.; Kuo, T.-R. Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization. Int. J. Mol. Sci. 2024, 25, 11440. https://doi.org/10.3390/ijms252111440
Saukani M, Lai C-H, Mutalik C, Krisnawati DI, Chu H-Y, Kuo T-R. Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization. International Journal of Molecular Sciences. 2024; 25(21):11440. https://doi.org/10.3390/ijms252111440
Chicago/Turabian StyleSaukani, Muhammad, Chien-Hung Lai, Chinmaya Mutalik, Dyah Ika Krisnawati, Hsiu-Yi Chu, and Tsung-Rong Kuo. 2024. "Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization" International Journal of Molecular Sciences 25, no. 21: 11440. https://doi.org/10.3390/ijms252111440
APA StyleSaukani, M., Lai, C.-H., Mutalik, C., Krisnawati, D. I., Chu, H.-Y., & Kuo, T.-R. (2024). Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization. International Journal of Molecular Sciences, 25(21), 11440. https://doi.org/10.3390/ijms252111440







