Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes
Abstract
1. Introduction
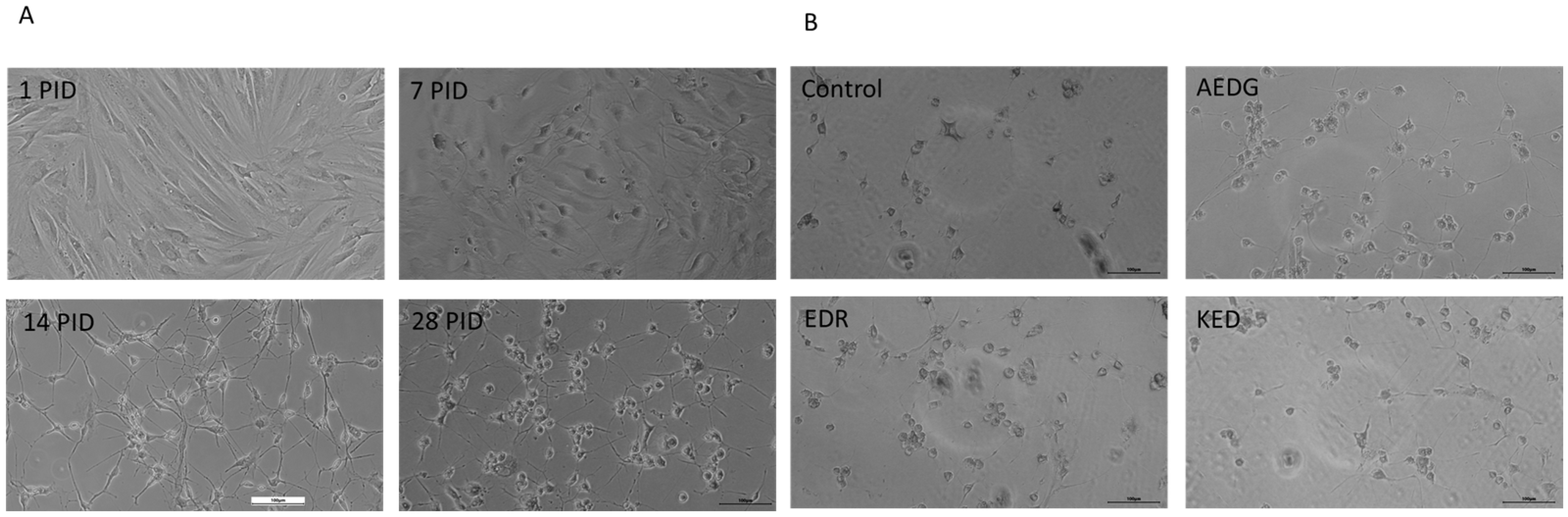
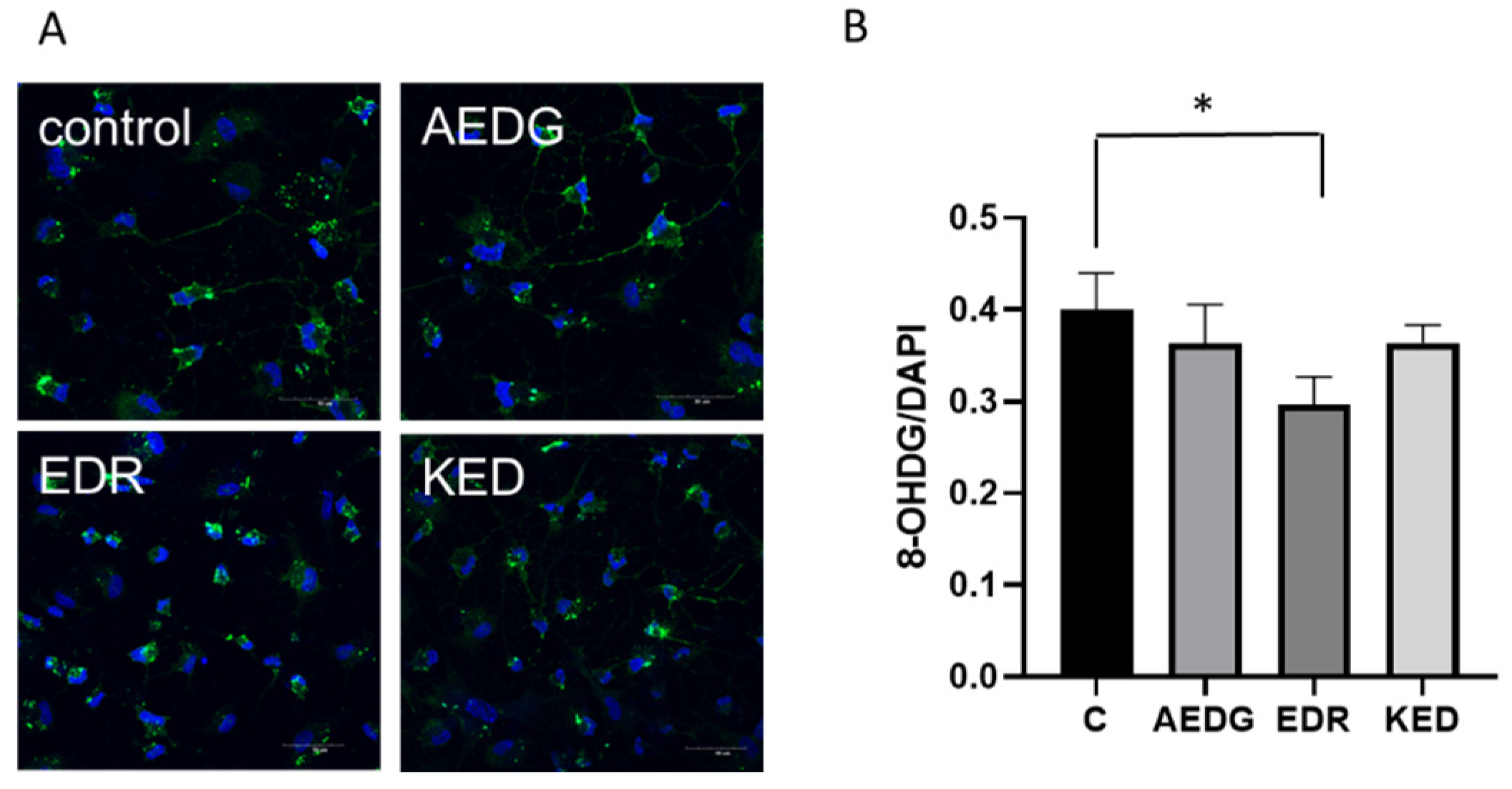
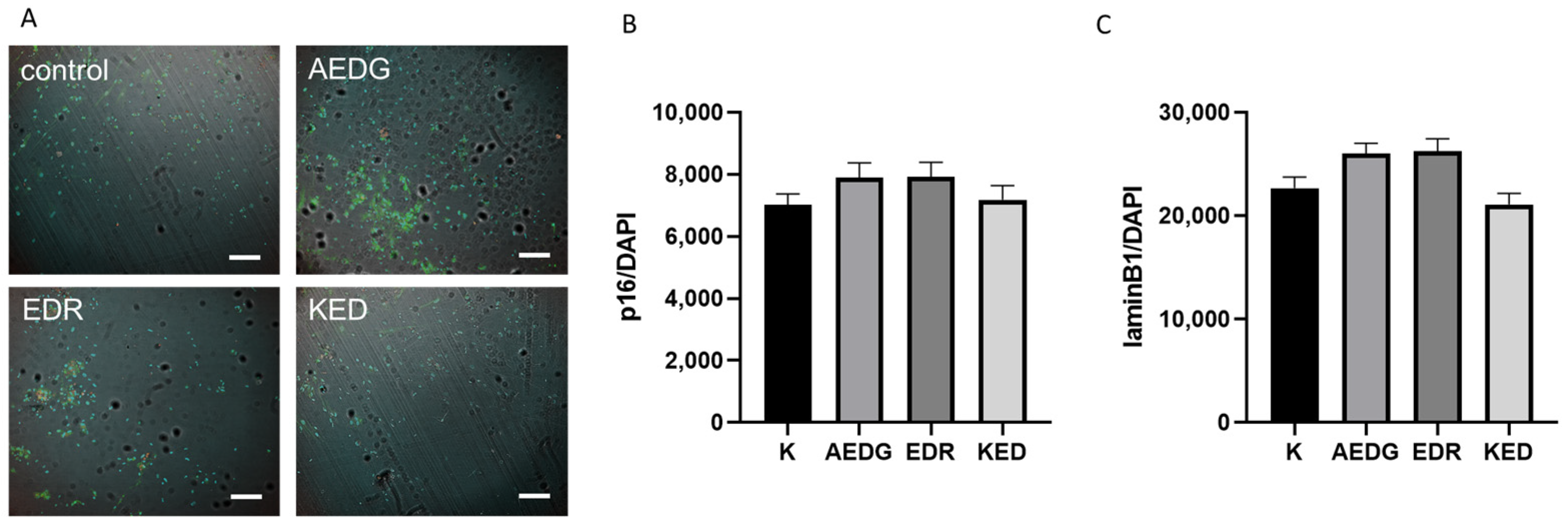
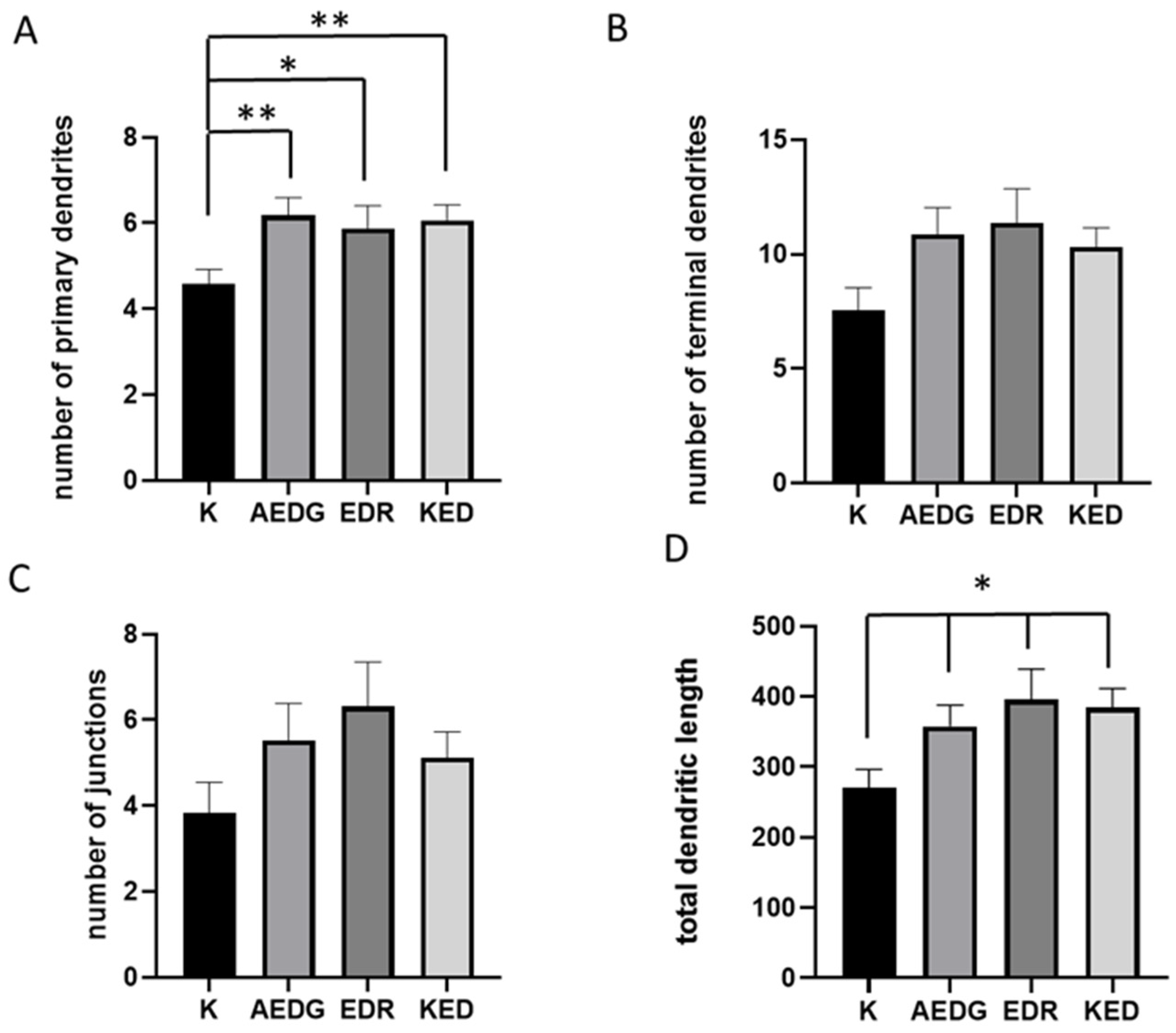
2. Results
3. Discussion
4. Materials and Methods
4.1. Cultivation of HEK293T Cells and Dermal Fibroblast Lines
4.2. Lentiviruses Production
4.3. Direct Reprogramming Technique
4.4. Immunofluorescence Staining
4.5. Neuronal Morphology Analysis
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattson, M.P.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef]
- Bhatia, S.; Rawal, R.; Sharma, P.; Singh, T.; Singh, M.; Singh, V. Mitochondrial Dysfunction in Alzheimer’s Disease: Opportunities for Drug Development. Curr. Neuropharmacol. 2022, 20, 675–692. [Google Scholar] [CrossRef]
- Udayar, V.; Chen, Y.; Sidransky, E.; Jagasia, R. Lysosomal dysfunction in neurodegeneration: Emerging concepts and methods. Trends Neurosci. 2022, 45, 184–199. [Google Scholar] [CrossRef]
- Zhang, H.; Bezprozvanny, I. “Dirty Dancing” of Calcium and Autophagy in Alzheimer’s Disease. Life 2023, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Galimberti, D.; Scarpini, E. Disease-modifying treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2011, 4, 203–216. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Scarpini, E.; Galimberti, D. Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies. Int. J. Mol. Sci. 2023, 24, 13900. [Google Scholar] [CrossRef]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Longo, F.M.; Massa, S.M. Neuroprotective strategies in Alzheimer’s disease. NeuroRx 2004, 1, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ilina, A.; Khavinson, V.; Linkova, N.; Petukhov, M. Neuroepigenetic Mechanisms of Action of Ultrashort Peptides in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4259. [Google Scholar] [CrossRef]
- Zhurkovich, I.K.; Kovrov, N.G.; Ryzhak, G.A.; Mironova, E.S.; Khavinson, V.K. Identification of short peptides in polypeptide complexes isolated from animal organs. Adv. Mod. Biol. 2020, 140, 140–148. [Google Scholar] [CrossRef]
- Ilina, A.R.; Popovich, I.G.; Ryzhak, G.A.; Khavinson, V.K. Prospects for use of short peptides in pharmacotherapeutic correction of Alzheimer’s disease. Adv. Gerontol. 2024, 37, 10–20. [Google Scholar] [PubMed]
- Kraskovskaya, N.A.; Kukanova, E.O.; Lin’kova, N.S.; Popugaeva, E.A.; Khavinson, V.K. Tripeptides Restore the Number of Neuronal Spines under Conditions of In Vitro Modeled Alzheimer’s Disease. Bull. Exp. Biol. Med. 2017, 163, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.; Ilina, A.; Kraskovskaya, N.; Linkova, N.; Kolchina, N.; Mironova, E.; Erofeev, A.; Petukhov, M. Neuroprotective Effects of Tripeptides-Epigenetic Regulators in Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 515. [Google Scholar] [CrossRef]
- Chalisova, N.I.; Ryzhak, G.A.; Ivko, O.M. Protective Effect of Short Peptides on the Insect Nervous System. Adv. Mod. Biol. 2021, 141, 265–270. [Google Scholar] [CrossRef]
- Khavinson, V.; Diomede, F.; Mironova, E.; Linkova, N.; Trofimova, S.; Trubiani, O.; Caputi, S.; Sinjari, B. AEDG Peptide (Epitalon) Stimulates Gene Expression and Protein Synthesis during Neurogenesis: Possible Epigenetic Mechanism. Molecules 2020, 25, 609. [Google Scholar] [CrossRef]
- Sinjari, B.; Diomede, F.; Khavinson, V.; Mironova, E.; Linkova, N.; Trofimova, S.; Trubiani, O.; Caputi, S. Short Peptides Protect Oral Stem Cells from Ageing. Stem Cell Rev. Rep. 2020, 16, 159–166. [Google Scholar] [CrossRef]
- Huh, C.J.; Zhang, B.; Victor, M.B.; Dahiya, S.; Batista, L.F.; Horvath, S.; Yoo, A.S. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife 2016, 5, e18648. [Google Scholar] [CrossRef]
- Yoo, A.S.; Sun, A.X.; Li, L.; Shcheglovitov, A.; Portmann, T.; Li, Y.; Lee-Messer, C.; Dolmetsch, R.E.; Tsien, R.W.; Crabtree, G.R. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011, 476, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Paquola, A.C.M.; Ku, M.; Hatch, E.; Bohnke, L.; Ladjevardi, S.; McGrath, S.; Campbell, B.; Lee, H.; Herdy, J.R.; et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 2015, 17, 705–718. [Google Scholar] [CrossRef]
- Chen, J.H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yoshimori, T. Autophagy and Longevity. Mol. Cells 2018, 41, 65–72. [Google Scholar] [CrossRef]
- Piras, A.; Collin, L.; Gruninger, F.; Graff, C.; Ronnback, A. Autophagic and lysosomal defects in human tauopathies: Analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. Commun. 2016, 4, 22. [Google Scholar] [CrossRef]
- Vazquez-Villasenor, I.; Garwood, C.J.; Heath, P.R.; Simpson, J.E.; Ince, P.G.; Wharton, S.B. Expression of p16 and p21 in the frontal association cortex of ALS/MND brains suggests neuronal cell cycle dysregulation and astrocyte senescence in early stages of the disease. Neuropathol. Appl. Neurobiol. 2020, 46, 171–185. [Google Scholar] [CrossRef]
- Ticli, G.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Revisiting the Function of p21(CDKN1A) in DNA Repair: The Influence of Protein Interactions and Stability. Int. J. Mol. Sci. 2022, 23, 7058. [Google Scholar] [CrossRef]
- Herdy, J.R.; Traxler, L.; Agarwal, R.K.; Karbacher, L.; Schlachetzki, J.C.M.; Boehnke, L.; Zangwill, D.; Galasko, D.; Glass, C.K.; Mertens, J.; et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell 2022, 29, 1637–1652.e1636. [Google Scholar] [CrossRef]
- Takamori, Y.; Tamura, Y.; Kataoka, Y.; Cui, Y.; Seo, S.; Kanazawa, T.; Kurokawa, K.; Yamada, H. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur. J. Neurosci. 2007, 25, 1653–1662. [Google Scholar] [CrossRef]
- Matias, I.; Diniz, L.P.; Damico, I.V.; Araujo, A.P.B.; Neves, L.D.S.; Vargas, G.; Leite, R.E.P.; Suemoto, C.K.; Nitrini, R.; Jacob-Filho, W.; et al. Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus. Aging Cell 2022, 21, e13521. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Blas, D.; Gorostieta-Salas, E.; Pommer-Alba, A.; Mucino-Hernandez, G.; Geronimo-Olvera, C.; Maciel-Baron, L.A.; Konigsberg, M.; Massieu, L.; Castro-Obregon, S. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging 2019, 11, 6175–6198. [Google Scholar] [CrossRef]
- Pannese, E. Morphological changes in nerve cells during normal aging. Brain Struct. Funct. 2011, 216, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Oh, Y.M.; Victor, M.B.; Yang, Y.; Chen, S.; Strunilin, I.; Dahiya, S.; Dolle, R.E.; Pak, S.C.; Silverman, G.A.; et al. Longitudinal modeling of human neuronal aging reveals the contribution of the RCAN1-TFEB pathway to Huntington’s disease neurodegeneration. Nat. Aging 2024, 4, 95–109. [Google Scholar] [CrossRef]
- Dragunow, M. The adult human brain in preclinical drug development. Nat. Rev. Drug Discov. 2008, 7, 659–666. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Studer, L.; Vera, E.; Cornacchia, D. Programming and Reprogramming Cellular Age in the Era of Induced Pluripotency. Cell Stem Cell 2015, 16, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Ganat, Y.M.; Kishinevsky, S.; Bowman, R.L.; Liu, B.; Tu, E.Y.; Mandal, P.K.; Vera, E.; Shim, J.W.; Kriks, S.; et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 2013, 13, 691–705. [Google Scholar] [CrossRef]
- Dong, C.M.; Wang, X.L.; Wang, G.M.; Zhang, W.J.; Zhu, L.; Gao, S.; Yang, D.J.; Qin, Y.; Liang, Q.J.; Chen, Y.L.; et al. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis. 2014, 5, e1116. [Google Scholar] [CrossRef]
- Vera, E.; Bosco, N.; Studer, L. Generating Late-Onset Human iPSC-Based Disease Models by Inducing Neuronal Age-Related Phenotypes through Telomerase Manipulation. Cell Rep. 2016, 17, 1184–1192. [Google Scholar] [CrossRef]
- Kim, Y.; Zheng, X.; Ansari, Z.; Bunnell, M.C.; Herdy, J.R.; Traxler, L.; Lee, H.; Paquola, A.C.M.; Blithikioti, C.; Ku, M.; et al. Mitochondrial Aging Defects Emerge in Directly Reprogrammed Human Neurons due to Their Metabolic Profile. Cell Rep. 2018, 23, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, M.L.; Zang, T.; Zhang, C.L. Direct Reprogramming Rather than iPSC-Based Reprogramming Maintains Aging Hallmarks in Human Motor Neurons. Front. Mol. Neurosci. 2017, 10, 359. [Google Scholar] [CrossRef]
- Capano, L.S.; Sato, C.; Ficulle, E.; Yu, A.; Horie, K.; Kwon, J.S.; Burbach, K.F.; Barthelemy, N.R.; Fox, S.G.; Karch, C.M.; et al. Recapitulation of endogenous 4R tau expression and formation of insoluble tau in directly reprogrammed human neurons. Cell Stem Cell 2022, 29, 918–932.e918. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Herdy, J.R.; Traxler, L.; Schafer, S.T.; Schlachetzki, J.C.M.; Bohnke, L.; Reid, D.A.; Lee, H.; Zangwill, D.; Fernandes, D.P.; et al. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 2021, 28, 1533–1548.e1536. [Google Scholar] [CrossRef]
- Sun, Z.; Kwon, J.S.; Ren, Y.; Chen, S.; Walker, C.K.; Lu, X.; Cates, K.; Karahan, H.; Sviben, S.; Fitzpatrick, J.A.J.; et al. Modeling late-onset Alzheimer’s disease neuropathology via direct neuronal reprogramming. Science 2024, 385, adl2992. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Lee, S.W.; Kim, W.K.; Chen, S.; Church, V.A.; Cates, K.; Li, T.; Zhang, B.; Dolle, R.E.; Dahiya, S.; et al. Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy. Nat. Neurosci. 2022, 25, 1420–1433. [Google Scholar] [CrossRef]
- Oh, Y.M.; Lee, S.W.; Yoo, A.S. Modeling Huntington disease through microRNA-mediated neuronal reprogramming identifies age-associated autophagy dysfunction driving the onset of neurodegeneration. Autophagy 2023, 19, 2613–2615. [Google Scholar] [CrossRef] [PubMed]
- Storozhevykh, T.P.; Tukhbatova, G.R.; Senilova, Y.E.; Pinelis, V.G.; Andreeva, L.A.; Myasoyedov, N.F. Effects of semax and its Pro-Gly-Pro fragment on calcium homeostasis of neurons and their survival under conditions of glutamate toxicity. Bull. Exp. Biol. Med. 2007, 143, 601–604. [Google Scholar] [CrossRef]
- Veinbergs, I.; Mante, M.; Mallory, M.; Masliah, E. Neurotrophic effects of Cerebrolysin in animal models of excitotoxicity. J. Neural Transm. Suppl. 2000, 59, 273–280. [Google Scholar] [CrossRef]
- Pourmemar, E.; Majdi, A.; Haramshahi, M.; Talebi, M.; Karimi, P.; Sadigh-Eteghad, S. Intranasal Cerebrolysin Attenuates Learning and Memory Impairments in D-galactose-Induced Senescence in Mice. Exp. Gerontol. 2017, 87, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hartbauer, M.; Hutter-Paie, B.; Windisch, M. Effects of Cerebrolysin on the outgrowth and protection of processes of cultured brain neurons. J. Neural Transm. 2001, 108, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Shypshyna, M.S.; Veselovsky, N.S.; Myasoedov, N.F.; Shram, S.I.; Fedulova, S.A. Effect of Peptide Semax on Synaptic Activity and Short-Term Plasticity of Glutamatergic Synapses of Co-Cultured Dorsal Root Ganglion and Dorsal Horn Neurons. Fiziol. Zhurnal 2015, 61, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Church, V.A.; Cates, K.; Capano, L.; Aryal, S.; Kim, W.K.; Yoo, A.S. Generation of Human Neurons by microRNA-Mediated Direct Conversion of Dermal Fibroblasts. Methods Mol. Biol. 2021, 2239, 77–100. [Google Scholar] [CrossRef]
- Kraskovskaya, N.; Bolshakova, A.; Khotin, M.; Bezprozvanny, I.; Mikhailova, N. Protocol Optimization for Direct Reprogramming of Primary Human Fibroblast into Induced Striatal Neurons. Int. J. Mol. Sci. 2023, 24, 6799. [Google Scholar] [CrossRef]


| Peptide | 2D Structure | Characteristics |
|---|---|---|
| AEDG peptide (Ala-Glu-Asp-Gly) | 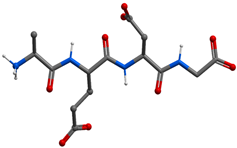 | Gross formula without counterion: C14H22N4O9 Molecular weight without counterion: 390, 35 Da Counterion: trifluoroacetic acid (TFA) Polar molecule |
| EDR peptide (Glu-Asp-Arg) | 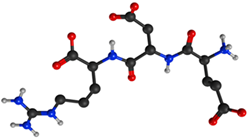 | Gross formula without counterion: C15H26N6O8 Molecular weight without counterion: 418, 40 Da Counterion: acetate Polar molecule |
| KED peptide (Lys-Glu-Asp) | 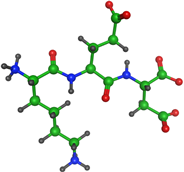 | Gross formula without counterion: C15H26N4O8 Molecular weight without counterion: 390, 39 Da Counterion: acetate Polar molecule |
| Title | Vendor | Catalog Number | Dilution |
|---|---|---|---|
| Monoclonal Anti-DNA/RNA Damage antibody | Sigma-Aldrich | SAB5200010 | 1/1000 |
| P16 | Cell Signaling | 18769 | 1/800 |
| TUJ-1 | R&D Systems (Minneapolis, MN, USA) | MAB1195 | 1/2000 |
| MAP2 | Abcam (Cambridge, UK) | ab5392 | 1/1000 |
| laminB1 | Novus (Chesterfield, MO, USA) | NBP2-59783 | 1/100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraskovskaya, N.; Linkova, N.; Sakhenberg, E.; Krieger, D.; Polyakova, V.; Medvedev, D.; Krasichkov, A.; Khotin, M.; Ryzhak, G. Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes. Int. J. Mol. Sci. 2024, 25, 11363. https://doi.org/10.3390/ijms252111363
Kraskovskaya N, Linkova N, Sakhenberg E, Krieger D, Polyakova V, Medvedev D, Krasichkov A, Khotin M, Ryzhak G. Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes. International Journal of Molecular Sciences. 2024; 25(21):11363. https://doi.org/10.3390/ijms252111363
Chicago/Turabian StyleKraskovskaya, Nina, Natalia Linkova, Elena Sakhenberg, Daria Krieger, Victoria Polyakova, Dmitrii Medvedev, Alexander Krasichkov, Mikhail Khotin, and Galina Ryzhak. 2024. "Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes" International Journal of Molecular Sciences 25, no. 21: 11363. https://doi.org/10.3390/ijms252111363
APA StyleKraskovskaya, N., Linkova, N., Sakhenberg, E., Krieger, D., Polyakova, V., Medvedev, D., Krasichkov, A., Khotin, M., & Ryzhak, G. (2024). Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes. International Journal of Molecular Sciences, 25(21), 11363. https://doi.org/10.3390/ijms252111363








