Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives
Abstract
1. Introduction
2. Chronic CORT Administration-Induced Cognitive Impairment
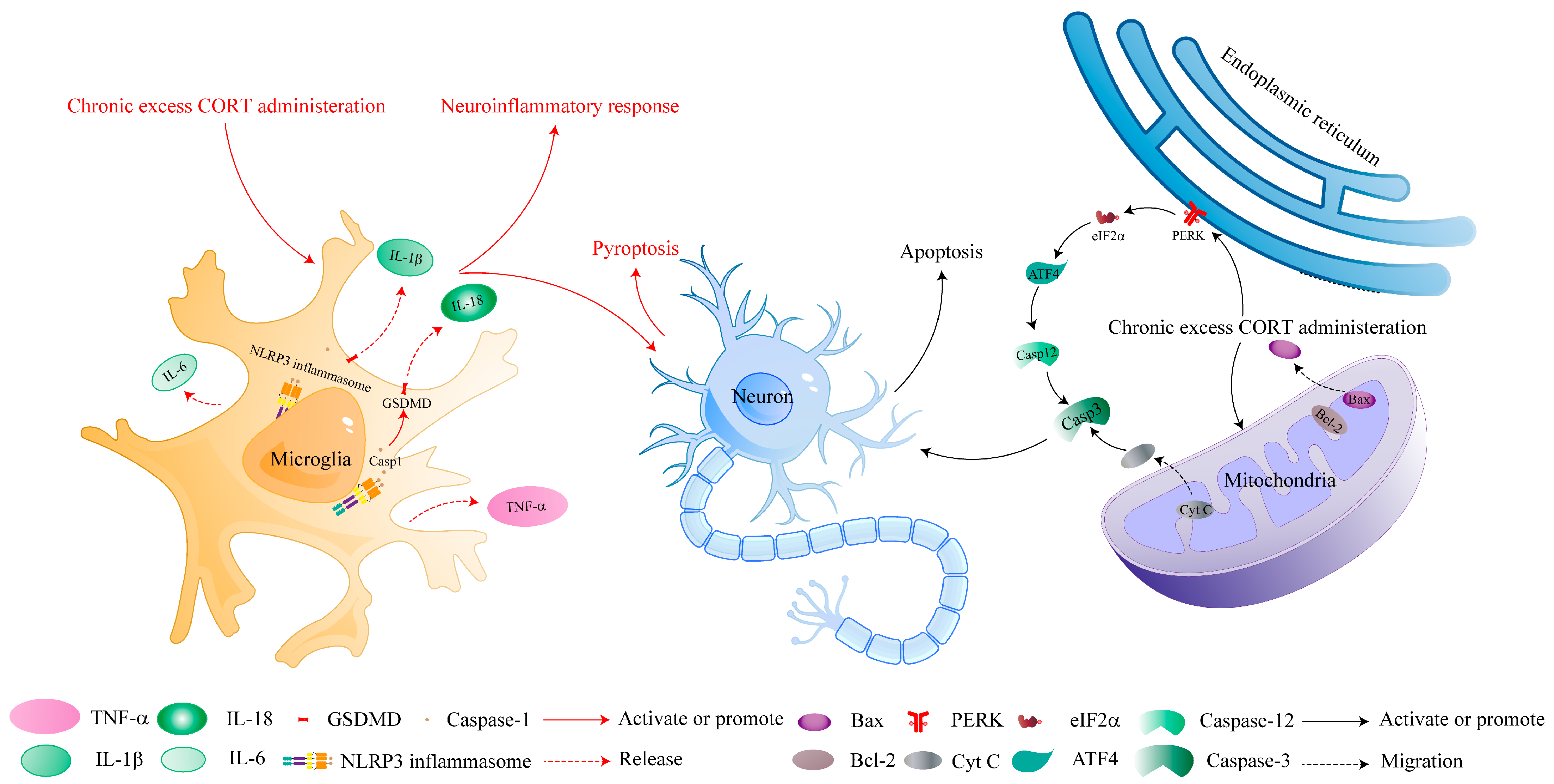
3. Chronic CORT Administration Is Detrimental to the Neuroinflammatory Response and Neural Cell Pyroptosis
4. Chronic Excess CORT-Induced Oxidative Stress Contributes to Cognitive Dysfunction
5. Chronic Excess CORT Administration Affects Neuroplasticity
6. Chronic CORT Administration Induces Apoptosis in the CNS
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AMPA | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ASC | Apoptosis-associated speck-like protein containing CARD |
| ATF4 | Activating transcription factor 4 |
| BDNF | Brain-derived neurotrophic factor |
| BLA | Basolateral amygdala |
| BMT | Barnes maze test |
| Capn2 | Calpain 2 |
| Cnp | C-type natriuretic peptide |
| CNS | Central nervous system |
| CORT | Corticosterone |
| Cox | Cytochrome oxidase |
| CPP | Conditioned place preference |
| DG | Dentate gyrus |
| eIF2α | Eukaryotic translation initiation factor-2α |
| EPM | Elevated plus maze |
| ER | Endoplasmic reticulum |
| EZM | Elevated zero maze |
| FC | Fear conditioning |
| FKBP5 | FK506-binding protein 5 |
| FST | Forced swim test |
| GC | Glucocorticoid |
| GDH | Glutamate dehydrogenase |
| GR | Glucocorticoid receptor |
| GRE | GC response elements |
| GSDMD | Pyroptosis executor gasdermin D |
| HBT | Hole-board test |
| HPA | Hypothalamus–pituitary–adrenal |
| HPC | Hippocampus |
| IDH2 | Isocitrate dehydrogenase 2 |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-18 | Interleukin-18 |
| i.p. | Intraperitoneal injection |
| i.v. | Intravenous injection |
| LDT | Light/dark test |
| MDA | Malondialdehyde |
| MERTK | Mer tyrosine kinase |
| MOMP | Mitochondrial outer membrane permeabilization |
| mPFC | Medial prefrontal cortex |
| MR | Mineralocorticoid receptor |
| ND | NADH-ubiquinone oxidoreductase |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NLR family, pyrin domain containing 3 |
| NOR | Novel object recognition test |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NSF | Novelty-suppressed feeding test |
| OFT | Open field test |
| OS | Oxidative stress |
| PAT | Passive avoidance test |
| PERK | Protein kinase RNA-like ER kinase |
| PPI | Prepulse inhibition test |
| PSD-95 | Postsynaptic density protein 95 |
| PVN | Paraventricular nucleus |
| ROS | Reactive oxygen species |
| s.c. | Subcutaneous injection |
| SOD | Superoxide dismutase |
| SDIT | Step-down avoidance test |
| SGK1 | Serum- and GC-inducible kinase 1 |
| SIRT | Sirtuin |
| SIT | Social interaction test |
| SLA | Spontaneous locomotor activity |
| SPT | Sucrose preference test |
| SST | Sucrose splash test |
| TNF-α | Tumor necrosis factor-α |
| TST | Tail suspension test |
| Vamp7 | Vesicle-associated membrane protein 7 |
| YMT | Y-maze test |
References
- Lin, Y.; Zhang, Z.; Wang, S.; Cai, J.; Guo, J. Hypothalamus-pituitary-adrenal Axis in Glucolipid metabolic disorders. Rev. Endocr. Metab. Disord. 2020, 21, 421–429. [Google Scholar] [CrossRef]
- Dunn, A.J. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–617. [Google Scholar] [CrossRef]
- Frodl, T.; O’Keane, V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013, 52, 24–37. [Google Scholar] [CrossRef]
- Son, G.H.; Chung, S.; Kim, K. The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front. Neuroendocrinol. 2011, 32, 451–465. [Google Scholar] [CrossRef]
- Droste, S.K.; de Groote, L.; Atkinson, H.C.; Lightman, S.L.; Reul, J.M.; Linthorst, A.C. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 2008, 149, 3244–3253. [Google Scholar] [CrossRef]
- Zajkowska, Z.; Gullett, N.; Walsh, A.; Zonca, V.; Pedersen, G.A.; Souza, L.; Kieling, C.; Fisher, H.L.; Kohrt, B.A.; Mondelli, V. Cortisol and development of depression in adolescence and young adulthood—A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 136, 105625. [Google Scholar] [CrossRef]
- Scarth, M.; Vonk, J.M.J.; Gerritsen, L.; Ggeerlings, M.I. Association of childhood maltreatment and cortisol with the severity and stability of depression symptoms. J. Affect. Disord. 2022, 299, 559–567. [Google Scholar] [CrossRef]
- Gerner, R.H.; Wilkins, J.N. CSF cortisol in patients with depression, mania, or anorexia nervosa and in normal subjects. Am. J. Psychiatry 1983, 140, 92–94. [Google Scholar]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress 2017, 6, 57–67. [Google Scholar] [CrossRef]
- Hartmann, J.; Bajaj, T.; Klengel, C.; Chatzinakos, C.; Ebert, T.; Dedic, N.; McCullough, K.M.; Lardenoije, R.; Joëls, M.; Meijer, O.C.; et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021, 35, 109185. [Google Scholar] [CrossRef]
- Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, Z.; Zhou, X.; He, W.B.; Yang, B.; Cui, L.Y.; He, H.Y.; Wang, Z.Z.; Chen, N.H. Low corticosterone levels attenuate late life depression and enhance glutamatergic neurotransmission in female rats. Acta Pharmacol. Sin. 2021, 42, 848–860. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Niu, F.; Mao, X.; Dong, J.; Yang, M.; Gao, F.; Liu, B. Corticosterone Replacement Alleviates Hippocampal Neuronal Apoptosis and Spatial Memory Impairment Induced by Dexamethasone via Promoting Brain Corticosteroid Receptor Rebalance after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 262–272. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, X.; Wang, F.; Ma, J.; Su, G.; Dong, Y.; Yang, J.; Wu, C. Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 2016, 6, 30951. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Chen, T.Y.; Yu, T.T.; Zhang, L.P.; Zhao, S.J.; Gu, X.Y.; Pan, Y.; Kong, L.D. Cinnamaldehyde prevents intergenerational effect of paternal depression in mice via regulating GR/miR-190b/BDNF pathway. Acta Pharmacol. Sin. 2022, 43, 1955–1969. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, M.; Yan, Q.; Xu, X.; Niu, F.; Dong, J.; Zhuang, Y.; Lu, S.; Ge, Q.; Liu, B. The Dual Dose-Dependent Effects of Corticosterone on Hippocampal Cell Apoptosis After Traumatic Brain Injury Depend on the Activation Ratio of Mineralocorticoid Receptors to Glucocorticoid Receptors. Front. Pharmacol. 2021, 12, 713715. [Google Scholar] [CrossRef]
- Samad, N.; Rafeeque, M.; Imran, I. Free-L-Cysteine improves corticosterone-induced behavioral deficits, oxidative stress and neurotransmission in rats. Metab. Brain Dis. 2023, 38, 983–997. [Google Scholar] [CrossRef]
- Wang, G.; Cao, L.; Li, S.; Zhang, M.; Li, Y.; Duan, J.; Li, Y.; Hu, Z.; Wu, J.; Li, T.; et al. Corticosterone Impairs Hippocampal Neurogenesis and Behaviors through p21-Mediated ROS Accumulation. Biomolecules 2024, 14, 268. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhang, T.; Xie, X.; Gong, J. Antidepressant effect of Jujuboside A on corticosterone-induced depression in mice. Biochem. Biophys. Res. Commun. 2022, 620, 56–62. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, W.; Zhou, H.; Li, Z.; Pi, T.; Wu, H.; Shi, H.; Huang, F.; Wu, X. Sex differences in a corticosterone-induced depression model in mice: Behavioral, neurochemical, and molecular insights. Brain Res. 2024, 1823, 148678. [Google Scholar] [CrossRef]
- Kim, S.; Yang, S.; Kim, J.; Chung, K.W.; Jung, Y.S.; Chung, H.Y.; Lee, J. Glucocorticoid Receptor Down-Regulation Affects Neural Stem Cell Proliferation and Hippocampal Neurogenesis. Mol. Neurobiol. 2024, 61, 3198–3211. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.F.; Qiao, X.; Zhang, Y.; Hou, X.J.; Chang, H.X.; Chen, H.L.; Zhang, Y.; Li, Y.F. Sigma-1 receptor activation mediates the sustained antidepressant effect of ketamine in mice via increasing BDNF levels. Acta Pharmacol. Sin. 2024, 45, 704–713. [Google Scholar] [CrossRef]
- Du, Q.; Gao, C.; Tsoi, B.; Wu, M.; Shen, J. Niuhuang Qingxin Wan ameliorates depressive-like behaviors and improves hippocampal neurogenesis through modulating TrkB/ERK/CREB signaling pathway in chronic restraint stress or corticosterone challenge mice. Front. Pharmacol. 2023, 14, 1274343. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, Z.; Zhao, A.; Tang, Y.; Kuang, J.; Li, M.; Chen, T.; Wang, S.; Wang, J.; Zhang, H.; et al. Gut bacteria-driven homovanillic acid alleviates depression by modulating synaptic integrity. Cell Metab. 2024, 36, 1000–1012.e6. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, Z.; Chen, L.; Peng, X.; Luan, F.; Hu, J.; Xie, H.; Liu, R.; Zeng, N. Rosmarinic acid alleviate CORT-induced depressive-like behavior by promoting neurogenesis and regulating BDNF/TrkB/PI3K signaling axis. Biomed. Pharmacother. 2024, 170, 115994. [Google Scholar] [CrossRef]
- Xu, C.; Ye, J.; Sun, Y.; Sun, X.; Liu, J.G. The Antidepressant Effect of Magnolol on Depression-Like Behavior of CORT-Treated Mice. J. Mol. Neurosci. 2024, 74, 3. [Google Scholar] [CrossRef]
- Luo, S.; Wu, F.; Fang, Q.; Hu, Y.; Zhang, H.; Yuan, S.; Yang, C.; Shi, Y.; Luo, Y. Antidepressant effect of teriflunomide via oligodendrocyte protection in a mouse model. Heliyon 2024, 10, e29481. [Google Scholar] [CrossRef]
- Scheil, K.K.A.; Sánchez-Lafuente, C.L.; Reive, B.S.; Halvorson, C.S.; Floyd, J.; Reid, H.M.O.; Johnston, J.N.; Kalynchuk, L.E.; Caruncho, H.J. Time-dependent antidepressant-like effects of reelin and ketamine in the repeated-corticosterone model of chronic stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 132, 110998. [Google Scholar] [CrossRef]
- Bergosh, M.; Medvidovic, S.; Zepeda, N.; Crown, L.; Ipe, J.; Debattista, L.; Romero, L.; Amjadi, E.; Lam, T.; Hakopian, E.; et al. Immediate and long-term electrophysiological biomarkers of antidepressant-like behavioral effects after subanesthetic ketamine and medial prefrontal cortex deep brain stimulation treatment. Front. Neurosci. 2024, 18, 1389096. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, R.R.; Xu, R.H.; Wang, H.H.; Feng, W.S.; Zheng, X.K. Naringenin and apigenin ameliorates corticosterone-induced depressive behaviors. Heliyon 2023, 9, e15618. [Google Scholar] [CrossRef]
- He, J.; Han, D.; Jia, C.; Xie, J.; Zhu, F.; Wei, J.; Li, D.; Wei, D.; Li, Y.; Tang, L.; et al. Integrating Network Pharmacology, Molecular Docking and Pharmacological Evaluation for Exploring the Polyrhachis vicina Rogers in Ameliorating Depression. Drug Des. Dev. Ther. 2023, 17, 717–735. [Google Scholar] [CrossRef]
- Musaelyan, K.; Horowitz, M.A.; McHugh, S.; Szele, F.G. Fluoxetine Can Cause Epileptogenesis and Aberrant Neurogenesis in Male Wild Type Mice. Dev. Neurosci. 2023, 46, 158–166. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.T.; Lu, Y.; Sun, G.Q.; Pei, L. Baicalin Ameliorates Corticosterone-Induced Depression by Promoting Neurodevelopment of Hippocampal via mTOR/GSK3β Pathway. Chin. J. Integr. Med. 2023, 29, 405–412. [Google Scholar] [CrossRef]
- Wang, X.L.; Miao, C.; Su, Y.; Zhang, C.; Meng, X. MAD2B Blunts Chronic Unpredictable Stress and Corticosterone Stimulation-Induced Depression-Like Behaviors in Mice. Int. J. Neuropsychopharmacol. 2023, 26, 137–148. [Google Scholar] [CrossRef]
- Qin, Z.; Shi, D.D.; Li, W.; Cheng, D.; Zhang, Y.D.; Zhang, S.; Tsoi, B.; Zhao, J.; Wang, Z.; Zhang, Z.J. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflamm. 2023, 20, 54. [Google Scholar] [CrossRef]
- Głuch-Lutwin, M.; Sałaciak, K.; Pytka, K.; Gawalska, A.; Jamrozik, M.; Śniecikowska, J.; Kołaczkowski, M.; Depoortère, R.Y.; Newman-Tancredi, A. The 5-HT(1A) receptor biased agonist, NLX-204, shows rapid-acting antidepressant-like properties and neurochemical changes in two mouse models of depression. Behav. Brain Res. 2023, 438, 114207. [Google Scholar] [CrossRef]
- Ramadan, B.; Cabeza, L.; Cramoisy, S.; Houdayer, C.; Andrieu, P.; Millot, J.L.; Haffen, E.; Risold, P.Y.; Peterschmitt, Y. Beneficial effects of prolonged 2-phenylethyl alcohol inhalation on chronic distress-induced anxio-depressive-like phenotype in female mice. Biomed. Pharmacother. 2022, 151, 113100. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, D.; Lee, C. Pedicularis resupinata Extract Prevents Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice: A Preliminary Study. Molecules 2022, 27, 3434. [Google Scholar] [CrossRef]
- Su, B.; Cheng, S.; Wang, L.; Wang, B. MicroRNA-139-5p acts as a suppressor gene for depression by targeting nuclear receptor subfamily 3, group C, member 1. Bioengineered 2022, 13, 11856–11866. [Google Scholar] [CrossRef]
- Bai, G.; Jing, S.; Cao, H.; Qiao, Y.; Chen, G.; Duan, L.; Yang, Y.; Li, M.; Li, W.; Chang, X.; et al. Kai-Xin-San Protects Depression Mice Against CORT-Induced Neuronal Injury by Inhibiting Microglia Activation and Oxidative Stress. Evid. Based Complement. Altern. Med. 2022, 2022, 5845800. [Google Scholar] [CrossRef]
- Yang, Y.; Mouri, A.; Lu, Q.; Kunisawa, K.; Kubota, H.; Hasegawa, M.; Hirakawa, M.; Mori, Y.; Libo, Z.; Saito, K.; et al. Loureirin C and Xanthoceraside Prevent Abnormal Behaviors Associated with Downregulation of Brain Derived Neurotrophic Factor and AKT/mTOR/CREB Signaling in the Prefrontal Cortex Induced by Chronic Corticosterone Exposure in Mice. Neurochem. Res. 2022, 47, 2865–2879. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, Y.; Fu, Y.; Wang, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Miao, M.; Yan, T. Salidroside Ameliorates Depression by Suppressing NLRP3-Mediated Pyroptosis via P2X7/NF-κB/NLRP3 Signaling Pathway. Front. Pharmacol. 2022, 13, 812362. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, L.; Lu, S.; Li, M.; Bai, M.; Li, Y.; Xu, E. The antidepressant-like effect of formononetin on chronic corticosterone-treated mice. Brain Res. 2022, 1783, 147844. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Wang, H.; Hu, S.; Yu, X.; Reilly, J.; He, Z.; You, Y.; Shu, X. Dihydromyricetin Attenuates Depressive-like Behaviors in Mice by Inhibiting the AGE-RAGE Signaling Pathway. Cells 2022, 11, 3730. [Google Scholar] [CrossRef]
- Sun, J.Y.; Liu, Y.T.; Jiang, S.N.; Guo, P.M.; Wu, X.Y.; Yu, J. Essential oil from the roots of Paeonia lactiflora pall. has protective effect against corticosterone-induced depression in mice via modulation of PI3K/Akt signaling pathway. Front. Pharmacol. 2022, 13, 999712. [Google Scholar] [CrossRef]
- Oliveira, T.Q.; Chaves Filho, A.J.M.; Jucá, P.M.; Soares, M.V.R.; Cunha, N.L.; Vieira, C.F.X.; Gadelha Filho, C.V.J.; Viana, G.A.; De Oliveira, G.M.F.; Macedo, D.S.; et al. Lipoic acid prevents mirtazapine-induced weight gain in mice without impairs its antidepressant-like action in a neuroendocrine model of depression. Behav. Brain Res. 2022, 419, 113667. [Google Scholar] [CrossRef]
- Shuster, A.L.; Rocha, F.E.; Wayszceyk, S.; de Lima, D.D.; Barauna, S.C.; Lopes, B.G.; Alberton, M.D.; Magro, D.D.D. Protective effect of Myrcia pubipetala Miq. against the alterations in oxidative stress parameters in an animal model of depression induced by corticosterone. Brain Res. 2022, 1774, 147725. [Google Scholar] [CrossRef]
- Maia Oliveira, I.C.; Vasconcelos Mallmann, A.S.; Adelvane de Paula Rodrigues, F.; Teodorio Vidal, L.M.; Lopes Sales, I.S.; Rodrigues, G.C.; Ferreira de Oliveira, N.; de Castro Chaves, R.; Cavalcanti Capibaribe, V.C.; Rodrigues de Carvalho, A.M.; et al. Neuroprotective and Antioxidant Effects of Riparin I in a Model of Depression Induced by Corticosterone in Female Mice. Neuropsychobiology 2022, 81, 28–38. [Google Scholar] [CrossRef]
- Chou, M.Y.; Ho, J.H.; Huang, M.J.; Chen, Y.J.; Yang, M.D.; Lin, L.H.; Chi, C.H.; Yeh, C.H.; Tsao, T.Y.; Tzeng, J.K.; et al. Potential antidepressant effects of a dietary supplement from the chlorella and lion’s mane mushroom complex in aged SAMP8 mice. Front. Nutr. 2022, 9, 977287. [Google Scholar] [CrossRef]
- Bai, G.; Qiao, Y.; Lo, P.C.; Song, L.; Yang, Y.; Duan, L.; Wei, S.; Li, M.; Huang, S.; Zhang, B.; et al. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 2022, 283, 114717. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Mitchell, M.A.; Brymer, K.J.; Johnston, J.; Sánchez-Lafuente, C.L.; Pinna, G.; Kalynchuk, L.E.; Caruncho, H.J. Reelin has antidepressant-like effects after repeated or singular peripheral injections. Neuropharmacology 2022, 211, 109043. [Google Scholar] [CrossRef]
- Araki, R.; Tachioka, H.; Kita, A.; Fujiwara, H.; Toume, K.; Matsumoto, K.; Yabe, T. Kihito prevents corticosterone-induced brain dysfunctions in mice. J. Tradit. Complement. Med. 2021, 11, 513–519. [Google Scholar] [CrossRef]
- Patel, S.D.; Cameron, L.P.; Olson, D.E. Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice. Life 2021, 11, 1327. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, Q.; Wu, X.; Xing, D. Photobiomodulation Therapy Ameliorates Glutamatergic Dysfunction in Mice with Chronic Unpredictable Mild Stress-Induced Depression. Oxidative Med. Cell. Longev. 2021, 2021, 6678276. [Google Scholar] [CrossRef]
- Luo, S.; Hou, Y.; Zhang, Y.; Feng, L.; Hunter, R.G.; Yuan, P.; Jia, Y.; Li, H.; Wang, G.; Manji, H.K.; et al. Bag-1 mediates glucocorticoid receptor trafficking to mitochondria after corticosterone stimulation: Potential role in regulating affective resilience. J. Neurochem. 2021, 158, 358–372. [Google Scholar] [CrossRef]
- Brymer, K.J.; Kulhaway, E.Y.; Howland, J.G.; Caruncho, H.J.; Kalynchuk, L.E. Altered acoustic startle, prepulse facilitation, and object recognition memory produced by corticosterone withdrawal in male rats. Behav. Brain Res. 2021, 408, 113291. [Google Scholar] [CrossRef]
- Hao, Y.; Tong, Y.; Guo, Y.; Lang, X.; Huang, X.; Xie, X.; Guan, Y.; Li, Z. Metformin Attenuates the Metabolic Disturbance and Depression-like Behaviors Induced by Corticosterone and Mediates the Glucose Metabolism Pathway. Pharmacopsychiatry 2021, 54, 131–141. [Google Scholar] [CrossRef]
- Chaves, R.C.; Mallmann, A.S.V.; de Oliveira, N.F.; Capibaribe, V.C.C.; da Silva, D.M.A.; Lopes, I.S.; Valentim, J.T.; Barbosa, G.R.; de Carvalho, A.M.R.; Fonteles, M.M.F.; et al. The neuroprotective effect of Riparin IV on oxidative stress and neuroinflammation related to chronic stress-induced cognitive impairment. Horm. Behav. 2020, 122, 104758. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Zeni, A.L.B.; Rodrigues, A.L.S. Guanosine potentiates the antidepressant-like effect of subthreshold doses of ketamine: Possible role of pro-synaptogenic signaling pathway. J. Affect. Disord. 2020, 271, 100–108. [Google Scholar] [CrossRef]
- V, A.K.; Madhana, R.M.; Bais, A.K.; Singh, V.B.; Malik, A.; Sinha, S.; Lahkar, M.; Kumar, P.; Samudrala, P.K. Cognitive Improvement by Vorinostat through Modulation of Endoplasmic Reticulum Stress in a Corticosterone-Induced Chronic Stress Model in Mice. ACS Chem. Neurosci. 2020, 11, 2649–2657. [Google Scholar]
- Zhang, S.Q.; Cao, L.L.; Liang, Y.Y.; Wang, P. The Molecular Mechanism of Chronic High-Dose Corticosterone-Induced Aggravation of Cognitive Impairment in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2020, 13, 613421. [Google Scholar] [CrossRef]
- Yokoyama, R.; Higuchi, M.; Tanabe, W.; Tsukada, S.; Naito, M.; Yamaguchi, T.; Chen, L.; Kasai, A.; Seiriki, K.; Nakazawa, T.; et al. (S)-norketamine and (2S,6S)-hydroxynorketamine exert potent antidepressant-like effects in a chronic corticosterone-induced mouse model of depression. Pharmacol. Biochem. Behav. 2020, 191, 172876. [Google Scholar] [CrossRef]
- Xie, X.; Shen, Q.; Yu, C.; Xiao, Q.; Zhou, J.; Xiong, Z.; Li, Z.; Fu, Z. Depression-like behaviors are accompanied by disrupted mitochondrial energy metabolism in chronic corticosterone-induced mice. J. Steroid Biochem. Mol. Biol. 2020, 200, 105607. [Google Scholar] [CrossRef]
- Zhao, F.; Tao, W.; Shang, Z.; Zhang, W.; Ruan, J.; Zhang, C.; Zhou, L.; Aiello, H.; Lai, H.; Qu, R. Facilitating Granule Cell Survival and Maturation in Dentate Gyrus With Baicalin for Antidepressant Therapeutics. Front. Pharmacol. 2020, 11, 556845. [Google Scholar] [CrossRef]
- Xie, X.; Yu, C.; Zhou, J.; Xiao, Q.; Shen, Q.; Xiong, Z.; Li, Z.; Fu, Z. Nicotinamide mononucleotide ameliorates the depression-like behaviors and is associated with attenuating the disruption of mitochondrial bioenergetics in depressed mice. J. Affect. Disord. 2020, 263, 166–174. [Google Scholar] [CrossRef]
- Notaras, M.J.; Vivian, B.; Wilson, C.; van den Buuse, M. Interaction of reelin and stress on immobility in the forced swim test but not dopamine-mediated locomotor hyperactivity or prepulse inhibition disruption: Relevance to psychotic and mood disorders. Schizophr. Res. 2020, 215, 485–492. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, G.; Zhao, Z.; Wang, C.; Duan, C.; Gao, L.; Li, S. Antidepressant-like effects of Lactobacillus plantarum DP189 in a corticosterone-induced rat model of chronic stress. Behav. Brain Res. 2020, 395, 112853. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhong, S.; Liao, X.; Lai, S.; Shan, Y.; Chen, J.; Zhang, L.; Lu, Q.; Shen, S.; et al. Alleviation of cognitive deficits and high copper levels by an NMDA receptor antagonist in a rat depression model. Compr. Psychiatry 2020, 102, 152200. [Google Scholar] [CrossRef]
- Lebedeva, K.A.; Allen, J.; Kulhawy, E.Y.; Caruncho, H.J.; Kalynchuk, L.E. Cyclical administration of corticosterone results in aggravation of depression-like behaviors and accompanying downregulations in reelin in an animal model of chronic stress relevant to human recurrent depression. Physiol. Behav. 2020, 224, 113070. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety evaluation and protective effects of ethanolic extract from maca (Lepidium meyenii Walp.) against corticosterone and H2O2 induced neurotoxicity. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef]
- Capibaribe, V.C.C.; Vasconcelos Mallmann, A.S.; Lopes, I.S.; Oliveira, I.C.M.; de Oliveira, N.F.; Chaves, R.C.; Fernandes, M.L.; de Araujo, M.A.; da Silva, D.M.A.; Valentim, J.T.; et al. Thymol reverses depression-like behaviour and upregulates hippocampal BDNF levels in chronic corticosterone-induced depression model in female mice. J. Pharm. Pharmacol. 2019, 71, 1774–1783. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Q.; Zhang, S.; Hu, K.; Xiong, W.; Xiao, L.; Cong, R.; Liu, Q.; Wang, Z. The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice. Nutrients 2019, 11, 859. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Wang, F.; Zhang, H.; Li, Y.; Yang, J.; Wu, C. Revealing Antidepressant Mechanisms of Baicalin in Hypothalamus Through Systems Approaches in Corticosterone- Induced Depressed Mice. Front. Neurosci. 2019, 13, 834. [Google Scholar] [CrossRef]
- Chaves, R.C.; Mallmann, A.S.V.; Oliveira, N.F.; Oliveira, I.C.M.; Capibaribe, V.C.C.; da Silva, D.M.A.; Lopes, I.S.; Valentim, J.T.; de Carvalho, A.M.R.; Macêdo, D.S.; et al. Reversal effect of Riparin IV in depression and anxiety caused by corticosterone chronic administration in mice. Pharmacol. Biochem. Behav. 2019, 180, 44–51. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, J.; Ni, Y.; Xie, X.; Yu, C.; Xiao, Q.; Zhou, J.; Wang, X.; Fu, Z. Exposure to jet lag aggravates depression-like behaviors and age-related phenotypes in rats subject to chronic corticosterone. Acta Biochim. Biophys. Sin. 2019, 51, 834–844. [Google Scholar] [CrossRef]
- Murata, K.; Fujita, N.; Takahashi, R.; Inui, A. Ninjinyoeito Improves Behavioral Abnormalities and Hippocampal Neurogenesis in the Corticosterone Model of Depression. Front. Pharmacol. 2018, 9, 1216. [Google Scholar] [CrossRef]
- Lopes, I.S.; Oliveira, I.C.M.; Capibaribe, V.C.C.; Valentim, J.T.; da Silva, D.M.A.; de Souza, A.G.; de Araújo, M.A.; Chaves, R.C.; Gutierrez, S.J.C.; Barbosa Filho, J.M.; et al. Riparin II ameliorates corticosterone-induced depressive-like behavior in mice: Role of antioxidant and neurotrophic mechanisms. Neurochem. Int. 2018, 120, 33–42. [Google Scholar] [CrossRef]
- de Sousa, C.N.S.; Meneses, L.N.; Vasconcelos, G.S.; da Silva Medeiros, I.; Silva, M.C.C.; Mouaffak, F.; Kebir, O.; da Silva Leite, C.M.G.; Patrocinio, M.C.A.; Macedo, D.; et al. Neuroprotective evidence of alpha-lipoic acid and desvenlafaxine on memory deficit in a neuroendocrine model of depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 803–817. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simão da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef]
- Kv, A.; Madhana, R.M.; Js, I.C.; Lahkar, M.; Sinha, S.; Naidu, V.G.M. Antidepressant activity of vorinostat is associated with amelioration of oxidative stress and inflammation in a corticosterone-induced chronic stress model in mice. Behav. Brain Res. 2018, 344, 73–84. [Google Scholar] [CrossRef]
- Bai, Y.; Song, L.; Dai, G.; Xu, M.; Zhu, L.; Zhang, W.; Jing, W.; Ju, W. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids 2018, 135, 73–78. [Google Scholar] [CrossRef]
- Kott, J.M.; Mooney-Leber, S.M.; Li, J.; Brummelte, S. Elevated stress hormone levels and antidepressant treatment starting before pregnancy affect maternal care and litter characteristics in an animal model of depression. Behav. Brain Res. 2018, 348, 101–114. [Google Scholar] [CrossRef]
- Wang, S.S.; Mu, R.H.; Li, C.F.; Dong, S.Q.; Geng, D.; Liu, Q.; Yi, L.T. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79 Pt B, 417–425. [Google Scholar] [CrossRef]
- Mendez-David, I.; Guilloux, J.P.; Papp, M.; Tritschler, L.; Mocaer, E.; Gardier, A.M.; Bretin, S.; David, D.J. S 47445 Produces Antidepressant- and Anxiolytic-Like Effects through Neurogenesis Dependent and Independent Mechanisms. Front. Pharmacol. 2017, 8, 462. [Google Scholar] [CrossRef]
- Oliveira, T.Q.; de Sousa, C.N.S.; Vasconcelos, G.S.; de Sousa, L.C.; de Oliveira, A.A.; Patrocínio, C.F.V.; Medeiros, I.D.S.; Honório Júnior, J.E.R.; Maes, M.; Macedo, D.; et al. Brain antioxidant effect of mirtazapine and reversal of sedation by its combination with alpha-lipoic acid in a model of depression induced by corticosterone. J. Affect. Disord. 2017, 219, 49–57. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Azevedo, D.; Rosa, J.M.; Colla, A.; de Oliveira, J.; Ramos-Hryb, A.B.; Brocardo, P.S.; Gil-Mohapel, J.; Rodrigues, A.L.S. Creatine Prevents Corticosterone-Induced Reduction in Hippocampal Proliferation and Differentiation: Possible Implication for Its Antidepressant Effect. Mol. Neurobiol. 2017, 54, 6245–6260. [Google Scholar] [CrossRef]
- Lebedeva, K.A.; Caruncho, H.J.; Kalynchuk, L.E. Cyclical corticosterone administration sensitizes depression-like behavior in rats. Neurosci. Lett. 2017, 650, 45–51. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Li, Y.; Liu, X.; Liao, X.; Su, Y.A.; Si, T. Differential Behavioral and Neurobiological Effects of Chronic Corticosterone Treatment in Adolescent and Adult Rats. Front. Mol. Neurosci. 2017, 10, 25. [Google Scholar] [CrossRef]
- Brachman, R.A.; McGowan, J.C.; Perusini, J.N.; Lim, S.C.; Pham, T.H.; Faye, C.; Gardier, A.M.; Mendez-David, I.; David, D.J.; Hen, R.; et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol. Psychiatry 2016, 79, 776–786. [Google Scholar] [CrossRef]
- Siopi, E.; Denizet, M.; Gabellec, M.M.; de Chaumont, F.; Olivo-Marin, J.C.; Guilloux, J.P.; Lledo, P.M.; Lazarini, F. Anxiety- and Depression-Like States Lead to Pronounced Olfactory Deficits and Impaired Adult Neurogenesis in Mice. J. Neurosci. 2016, 36, 518–531. [Google Scholar] [CrossRef]
- Darcet, F.; Gardier, A.M.; David, D.J.; Guilloux, J.P. Chronic 5-HT4 receptor agonist treatment restores learning and memory deficits in a neuroendocrine mouse model of anxiety/depression. Neurosci. Lett. 2016, 616, 197–203. [Google Scholar] [CrossRef]
- Nashed, M.G.; Linher-Melville, K.; Frey, B.N.; Singh, G. RNA-sequencing profiles hippocampal gene expression in a validated model of cancer-induced depression. Genes Brain Behav. 2016, 15, 711–721. [Google Scholar] [CrossRef]
- Govic, A.; Penman, J.; Tammer, A.H.; Paolini, A.G. Paternal calorie restriction prior to conception alters anxiety-like behavior of the adult rat progeny. Psychoneuroendocrinology 2016, 64, 1–11. [Google Scholar] [CrossRef]
- Kott, J.M.; Mooney-Leber, S.M.; Shoubah, F.A.; Brummelte, S. Effectiveness of different corticosterone administration methods to elevate corticosterone serum levels, induce depressive-like behavior, and affect neurogenesis levels in female rats. Neuroscience 2016, 312, 201–214. [Google Scholar] [CrossRef]
- Quesseveur, G.; Portal, B.; Basile, J.A.; Ezan, P.; Mathou, A.; Halley, H.; Leloup, C.; Fioramonti, X.; Déglon, N.; Giaume, C.; et al. Attenuated Levels of Hippocampal Connexin 43 and its Phosphorylation Correlate with Antidepressant- and Anxiolytic-Like Activities in Mice. Front. Cell. Neurosci. 2015, 9, 490. [Google Scholar] [CrossRef]
- Mendez-David, I.; Tritschler, L.; Ali, Z.E.; Damiens, M.H.; Pallardy, M.; David, D.J.; Kerdine-Römer, S.; Gardier, A.M. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef]
- Nashed, M.G.; Seidlitz, E.P.; Frey, B.N.; Singh, G. Depressive-like behaviours and decreased dendritic branching in the medial prefrontal cortex of mice with tumors: A novel validated model of cancer-induced depression. Behav. Brain Res. 2015, 294, 25–35. [Google Scholar] [CrossRef]
- Hill, A.S.; Sahay, A.; Hen, R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 2015, 40, 2368–2378. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Orvoen, S.; Jimenez, D.V.; Hardy, N.F.; Maynard, K.R.; Sukumar, M.; Manji, H.K.; Gardier, A.M.; David, D.J.; Martinowich, K. Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression. Brain Stimul. 2015, 8, 862–867. [Google Scholar] [CrossRef]
- Schroeder, A.; Buret, L.; Hill, R.A.; van den Buuse, M. Gene-environment interaction of reelin and stress in cognitive behaviours in mice: Implications for schizophrenia. Behav. Brain Res. 2015, 287, 304–314. [Google Scholar] [CrossRef]
- de Sousa, C.N.; Meneses, L.N.; Vasconcelos, G.S.; Silva, M.C.; da Silva, J.C.; Macêdo, D.; de Lucena, D.F.; Vasconcelos, S.M. Reversal of corticosterone-induced BDNF alterations by the natural antioxidant alpha-lipoic acid alone and combined with desvenlafaxine: Emphasis on the neurotrophic hypothesis of depression. Psychiatry Res. 2015, 230, 211–219. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; Athira, K.V.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Kurhe, Y. Effect of a novel 5-HT3 receptor antagonist 4i, in corticosterone-induced depression-like behavior and oxidative stress in mice. Steroids 2015, 96, 95–102. [Google Scholar] [CrossRef]
- Tran, L.; Schulkin, J.; Ligon, C.O.; Greenwood-Van Meerveld, B. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol. Psychiatry 2015, 20, 1219–1231. [Google Scholar] [CrossRef]
- Kvarta, M.D.; Bradbrook, K.E.; Dantrassy, H.M.; Bailey, A.M.; Thompson, S.M. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J. Neurophysiol. 2015, 114, 1713–1724. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Roy, B.; Lugli, G.; Rizavi, H.; Zhang, H.; Smalheiser, N.R. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: Relevance to depression pathophysiology. Transl. Psychiatry 2015, 5, e682. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Yang, D.; Wu, C.; Ma, C.; Liu, X.; Moynagh, P.N.; Wang, B.; Hu, G.; Yang, S. Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2019, 216, 2562–2581. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.C.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. The Inflammasome in Times of COVID-19. Front. Immunol. 2020, 11, 583373. [Google Scholar] [CrossRef]
- Alam, M.M.; Okazaki, K.; Nguyen, L.T.T.; Ota, N.; Kitamura, H.; Murakami, S.; Shima, H.; Igarashi, K.; Sekine, H.; Motohashi, H. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem. 2017, 292, 7519–7530. [Google Scholar] [CrossRef]
- Ahlbom, E.; Gogvadze, V.; Chen, M.; Celsi, G.; Ceccatelli, S. Prenatal exposure to high levels of glucocorticoids increases the susceptibility of cerebellar granule cells to oxidative stress-induced cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14726–14730. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Cho, W.H.; Noh, K.; Lee, B.H.; Barcelon, E.; Jun, S.B.; Park, H.Y.; Lee, S.J. Hippocampal astrocytes modulate anxiety-like behavior. Nat. Commun. 2022, 13, 6536. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Zhu, L.J.; Shi, H.J.; Chang, L.; Zhang, C.C.; Si, M.; Li, N.; Zhu, D.Y. nNOS-CAPON blockers produce anxiolytic effects by promoting synaptogenesis in chronic stress-induced animal models of anxiety. Br. J. Pharmacol. 2020, 177, 3674–3690. [Google Scholar] [CrossRef]
- van Calker, D.; Serchov, T.; Normann, C.; Biber, K. Recent insights into antidepressant therapy: Distinct pathways and potential common mechanisms in the treatment of depressive syndromes. Neurosci. Biobehav. Rev. 2018, 88, 63–72. [Google Scholar] [CrossRef]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef]
- Chen, H.; Lombès, M.; Le Menuet, D. Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol. Brain 2017, 10, 12. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Um, M.Y.; Yoon, M.; Kim, T.E.; Kim, Y.T.; Han, D.; Lee, J.; Lee, C.H. Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Nutrients 2019, 11, 2841. [Google Scholar] [CrossRef]
- Furuse, K.; Ukai, W.; Hashimoto, E.; Hashiguchi, H.; Kigawa, Y.; Ishii, T.; Tayama, M.; Deriha, K.; Shiraishi, M.; Kawanishi, C. Antidepressant activities of escitalopram and blonanserin on prenatal and adolescent combined stress-induced depression model: Possible role of neurotrophic mechanism change in serum and nucleus accumbens. J. Affect. Disord. 2019, 247, 97–104. [Google Scholar] [CrossRef]
- Fraga, D.B.; Camargo, A.; Olescowicz, G.; Azevedo Padilha, D.; Mina, F.; Budni, J.; Brocardo, P.S.; Rodrigues, A.L.S. A single administration of ascorbic acid rapidly reverses depressive-like behavior and hippocampal synaptic dysfunction induced by corticosterone in mice. Chem. Biol. Interact. 2021, 342, 109476. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; de Souza, M.M.; Zeni, A.L.B.; Rodrigues, A.L.S. Ketamine, but not guanosine, as a prophylactic agent against corticosterone-induced depressive-like behavior: Possible role of long-lasting pro-synaptogenic signaling pathway. Exp. Neurol. 2020, 334, 113459. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Wolin, I.A.V.; Siteneski, A.; Zeni, A.L.B.; Rodrigues, A.L.S. A low-dose combination of ketamine and guanosine counteracts corticosterone-induced depressive-like behavior and hippocampal synaptic impairments via mTORC1 signaling. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110371. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Adams, T.G.; Kelmendi, B.; Esterlis, I.; Sanacora, G.; Krystal, J.H. Ketamine’s mechanism of action: A path to rapid-acting antidepressants. Depress. Anxiety 2016, 33, 689–697. [Google Scholar] [CrossRef]
- Li, M.; Teng, H.; Sun, G.; Zhao, J.; Fan, M.; Zhao, Z.; Zhou, J.; Zhao, M. Transcriptome profiles of corticosterone-induced cytotoxicity reveals the involvement of neurite growth-related genes in depression. Psychiatry Res. 2019, 276, 79–86. [Google Scholar] [CrossRef]
- Weinhard, L.; di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Byun, Y.G.; Kim, N.S.; Kim, G.; Jeon, Y.S.; Choi, J.B.; Park, C.W.; Kim, K.; Jang, H.; Kim, J.; Kim, E.; et al. Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis. Immunity 2023, 56, 2105–2120.e13. [Google Scholar] [CrossRef]
- Krugers, H.J.; Hoogenraad, C.C.; Groc, L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat. Rev. Neurosci. 2010, 11, 675–681. [Google Scholar] [CrossRef]
- Nair, S.M.; Werkman, T.R.; Craig, J.; Finnell, R.; Joëls, M.; Eberwine, J.H. Corticosteroid regulation of ion channel conductances and mRNA levels in individual hippocampal CA1 neurons. J. Neurosci. 1998, 18, 2685–2696. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Li, Y.; Sun, Y.; Wang, H.; Liu, X.; Zhao, Y.; Wang, H.; Su, Y.; Si, T. Chronic mild corticosterone exposure during adolescence enhances behaviors and upregulates neuroplasticity-related proteins in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 400–411. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, J.T.; Xie, X.M.; Gu, Y.; Si, T.M.; Schmidt, M.V.; Wang, X.D. Nectin-3 modulates the structural plasticity of dentate granule cells and long-term memory. Transl. Psychiatry 2017, 7, e1228. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2022, 29, 272–284. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Soni, K.K.; Hwang, J.; Ramalingam, M.; Kim, C.; Kim, B.C.; Jeong, H.S.; Jang, S. Endoplasmic Reticulum Stress Causing Apoptosis in a Mouse Model of an Ischemic Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 1307. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chen, A.W.; Varner, J.D. A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 2011, 108, 2777–2793. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Rui, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Chen, R.; Chen, Y.; Wang, Y.; Li, S.; et al. GSDMD enhances cisplatin-induced apoptosis by promoting the phosphorylation of eIF2α and activating the ER-stress response. Cell Death Discov. 2022, 8, 114. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Chen, F.; Zhang, H.; Zhu, Z.; Xu, S.; Cheng, Y.; Zhao, Y. A possible mechanism to the antidepressant-like effects of 20 (S)-protopanaxadiol based on its target protein 14-3-3 ζ. J. Ginseng Res. 2022, 46, 666–674. [Google Scholar] [CrossRef]
- Joëls, M.; Karst, H.; Krugers, H.J.; Lucassen, P.J. Chronic stress: Implications for neuronal morphology, function and neurogenesis. Front. Neuroendocrinol. 2007, 28, 72–96. [Google Scholar] [CrossRef]
- Cabeza, L.; Ramadan, B.; Giustiniani, J.; Houdayer, C.; Pellequer, Y.; Gabriel, D.; Fauconnet, S.; Haffen, E.; Risold, P.Y.; Fellmann, D.; et al. Chronic exposure to glucocorticoids induces suboptimal decision-making in mice. Eur. Neuropsychopharmacol. 2021, 46, 56–67. [Google Scholar] [CrossRef]
- Vouimba, R.M.; Yaniv, D.; Richter-Levin, G. Glucocorticoid receptors and beta-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology 2007, 52, 244–252. [Google Scholar] [CrossRef]
- Piechota, M.; Skupio, U.; Borczyk, M.; Ziółkowska, B.; Gołda, S.; Szumiec, Ł.; Szklarczyk-Smolana, K.; Bilecki, W.; Rodriguez Parkitna, J.M.; Korostyński, M. Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice. Int. J. Mol. Sci. 2022, 23, 12328. [Google Scholar] [CrossRef]
- Sacedón, R.; Vicente, A.; Varas, A.; Jiménez, E.; Muñoz, J.J.; Zapata, A.G. Early maturation of T-cell progenitors in the absence of glucocorticoids. Blood 1999, 94, 2819–2826. [Google Scholar] [CrossRef]
- Caradonna, S.G.; Zhang, T.Y.; O’Toole, N.; Shen, M.J.; Khalil, H.; Einhorn, N.R.; Wen, X.; Parent, C.; Lee, F.S.; Akil, H.; et al. Genomic modules and intramodular network concordance in susceptible and resilient male mice across models of stress. Neuropsychopharmacology 2022, 47, 987–999. [Google Scholar] [CrossRef]
- Hill, R.A.; Grech, A.M.; Notaras, M.J.; Sepulveda, M.; van den Buuse, M. Brain-Derived Neurotrophic Factor Val66Met polymorphism interacts with adolescent stress to alter hippocampal interneuron density and dendritic morphology in mice. Neurobiol. Stress. 2020, 13, 100253. [Google Scholar] [CrossRef]
- Lebeau, R.H.; Mendez-David, I.; Kucynski-Noyau, L.; Henry, C.; Attali, D.; Plaze, M.; Colle, R.; Corruble, E.; Gardier, A.M.; Gaillard, R.; et al. Peripheral proteomic changes after electroconvulsive seizures in a rodent model of non-response to chronic fluoxetine. Front. Pharmacol. 2022, 13, 993449. [Google Scholar] [CrossRef]
- Qiu, W.; Duarte-Guterman, P.; Eid, R.S.; Go, K.A.; Lamers, Y.; Galea, L.A.M. Postpartum fluoxetine increased maternal inflammatory signalling and decreased tryptophan metabolism: Clues for efficacy. Neuropharmacology 2020, 175, 108174. [Google Scholar] [CrossRef]
- Morgan, A.; Kondev, V.; Bedse, G.; Baldi, R.; Marcus, D.; Patel, S. Cyclooxygenase-2 inhibition reduces anxiety-like behavior and normalizes enhanced amygdala glutamatergic transmission following chronic oral corticosterone treatment. Neurobiol. Stress 2019, 11, 100190. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Rosa, J.M.; Zeni, A.L.B.; Kaster, M.P.; Tasca, C.I.; Rodrigues, A.L.S. Subthreshold doses of guanosine plus ketamine elicit antidepressant-like effect in a mouse model of depression induced by corticosterone: Role of GR/NF-κB/IDO-1 signaling. Neurochem. Int. 2020, 139, 104797. [Google Scholar] [CrossRef]
- Wang, H.; Xing, X.; Liang, J.; Bai, Y.; Lui, Z.; Zheng, X. High-dose corticosterone after fear conditioning selectively suppresses fear renewal by reducing anxiety-like response. Pharmacol. Biochem. Behav. 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Shi, H.J.; Wu, D.L.; Chen, R.; Li, N.; Zhu, L.J. Requirement of hippocampal DG nNOS-CAPON dissociation for the anxiolytic and antidepressant effects of fluoxetine. Theranostics 2022, 12, 3656–3675. [Google Scholar] [CrossRef]
- Michalovicz, L.T.; Kelly, K.A.; Miller, D.B.; Sullivan, K.; O’Callaghan, J.P. The β-adrenergic receptor blocker and anti-inflammatory drug propranolol mitigates brain cytokine expression in a long-term model of Gulf War Illness. Life Sci. 2021, 285, 119962. [Google Scholar] [CrossRef]
- Bachmann, C.G.; Linthorst, A.C.; Holsboer, F.; Reul, J.M. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology 2003, 28, 1056–1067. [Google Scholar] [CrossRef][Green Version]
| Species | Gender | Weight | Age | Intervention | Behavior Tests | Results | References |
|---|---|---|---|---|---|---|---|
| C57BL/6 mice | Male | 7–8 weeks old | CORT (40 mg/kg, s.c.) for 36 days | OFT, EMP, FST, TST | Anxiety↑ Depression↑ | Wang, G. et al. 2024 [19] | |
| C57BL/6 mice | Male and female | 5 weeks old | CORT (40 mg/kg, s.c.) for 5 weeks | OFT, EMP, SPT, FST, TST | Depression↑ | Tao, Y. et al. 2024 [21] | |
| C57BL/6 mice | Male | 4 weeks old | CORT (35 μg/mL) in drinking water for 4 weeks | FST, TST | Depression↑ | Kim, S. et al. 2024 [22] | |
| C57BL/6 mice | Male | 6–8 weeks old | CORT (25 μg/mL) in drinking water for 21 days | TST | Depression↑ | Ma, H. et al. 2024 [23] | |
| C57BL/6N mice | Male | 6–8 weeks old | CORT (70 μg/mL equivalent to 5 mg/kg/day) in drinking water for 25 days | OFT, NSF, SST, FST, TST | Depression↑ Memory loss↑ | Du, Q. et al. 2024 [24] | |
| C57BL/6J mice | Male | 6–8 weeks old | CORT (40 mg/kg, s.c.) for 5 weeks | SPT, FST, TST | Depression↑ | Zhao, M. et al. 2024 [25] | |
| C57BL/6J mice | Male | 8 weeks old | CORT (10 mg/kg, i.p.) between the hours of 09:30 and 11:00 a.m. daily for 21 days | SPT, NSF, SST, FST, TST | Depression↑ | Zeng, J. et al. 2024 [26] | |
| C57BL/6J mice | Male | 20–25 g | 7–8 weeks old | CORT (70 μg/mL equivalent to 10 mg/kg/day) in drinking water for 21 days | OFT, SPT, FST, TST | Depression↑ | Xu, C. et al. 2024 [27] |
| C57BL/6J mice | Male | 8 weeks old | CORT (20 mg/kg, s.c.) for 21 days | OFT, NSF, SPT, FST, TST | Depression↑ | Luo, S. et al. 2024 [28] | |
| Long Evans rats | Female | 150–250 g | CORT (40 mg/kg, s.c.) between the hours of 08:00 and 11:00 a.m. daily for 21 days | FST | Depression↑ | Scheil, K. K. A. et al. 2024 [29] | |
| Sprague Dawley rats | Male | 350 ± 50 g | 6–8 weeks old | CORT (40 mg/kg, s.c.) for 21 days | OFT, NOR, EPM, FST, Groom test | Anxiety↑ Depression↑ | Bergosh, M. et al. 2024 [30] |
| C57BL/6 mice | Male | 18–22 g | CORT (20 mg/kg, s.c.) every day for 3 weeks | OFT, SPT, TST | Depression↑ | Zhang, L. et al. 2023 [31] | |
| C57BL/6J mice | Male | 18–22 g | CORT (20 mg/kg, i.p.) for 21 consecutive days | FST, TST | Depression↑ | He, J. et al. 2023 [32] | |
| C57BL/6J mice | Male | 7 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 10 weeks | OFT, NSF, SST | Depression↑ | MUSAEL Musaelyan, K. et al. 2023 [33] | |
| ICR mice | Male | 26 ± 2 g | 7 weeks old | CORT (40 mg/kg, s.c.) for 6 weeks | EPM, SPT, NSF, FST, TST | Anxiety↑ Depression↑ | Wang, Z. et al. 2023 [34] |
| C57BL/6J mice | Male | CORT (20 mg/kg, s.c.) for 21 consecutive days | SPT, FST, TST | Depression↑ | Wang, X. L. et al. 2023 [35] | ||
| C57BL/6N mice | Male | 8–10 weeks old | CORT (20 mg/kg, oral gavage) every day for 28 days | OFT, SPT, FST, TST | Depression↑ | Qin, Z. et al. 2023 [36] | |
| Rats | Male | 190–200 g | 7–8 weeks old | CORT (20 mg/kg, i.v.) for 28 days | LDT, EPM, FST, MWM | Anxiety↑ Depression↑ Memory loss↑ | Samad, N. et al. 2023 [18] |
| Albino Swiss CD-1 mice | Male | 21 ± 2 g | 6 weeks old | CORT (20 mg/kg, s.c.) for 21 days | SPT, FST | Depression↑ | Głuch-Lutwin, M. et al. 2023 [37] |
| C57BL/6J mice | Female | 18.85 ± 0.16 g | 6–8 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 7 weeks | OFT, NSF, FST | Anxiety↑ Depression↑ | Ramadan, B. et al. 2022 [38] |
| ICR mice | Male | 25–28 g | 7 weeks old | CORT (40 mg/kg, i.p.) once daily for 21 consecutive days | OFT, PAT, SPT, FST, TST | Depression↑ Memory loss↑ | Lim, D. W. et al. 2022 [39] |
| C57BL/6 mice | Male | 24.38 ± 2.05 g | 5 weeks old | CORT (40 mg/kg, s.c.) once a day for 14 days | SPT, FST, TST | Depression↑ | Su, B. et al. 2022 [40] |
| Swiss mice | Male | 23–26 g | CORT (20 mg/kg, s.c.) for 3 consecutive weeks | OFT, EPM, TST, FST | Depression↑ | Bai, G. et al. 2022 [41] | |
| C57BL/6J mice | Male | 6 weeks old | CORT (20 mg/kg, s.c.) for 3 weeks | OFT, SIT, NSF | Anxiety↑ Depression↑ | Yang, Y. et al. 2022 [42] | |
| C57BL/6 mice | Male | 20–22 g | 8 weeks old | CORT (20 mg/kg, s.c.) for 3 weeks | OFT, SPT, FST | Depression↑ | Chai, Y. et al. 2022 [43] |
| C57BL/6 mice | CORT (40 mg/kg, i.p.) for 3 weeks | OFT, FST, TST, MWM | Depression↑ Memory loss↑ | LI H et al. 2022 [20] | |||
| C57BL/6 mice | Male | 7 weeks old | CORT (20 mg/kg, s.c.) for 6 weeks | OFT, SPT, FST | Depression↑ | Gao ZY et al. 2022 [16] | |
| ICR mice | Male | 18–22 g | 6–8 weeks old | CORT (40 mg/kg, s.c.) for 3 weeks | SPT, FST | Depression↑ | Zhang, C. et al. 2022 [44] |
| ICR mice | Male | 30 ± 2 g | 7 weeks old | CORT (20 mg/kg, s.c.) for 3 weeks | OFT, SPT, FST, TST | Depression↑ | Huang, J. et al. 2022 [45] |
| ICR mice | Male | 18–22 g | 6–8 weeks old | CORT (20 mg/kg, i.p.) for 4 weeks | OFT, SPT, FST, TST | Anxiety↑ Depression↑ | Sun, J. Y. et al. 2022 [46] |
| Swiss mice | Male | 25–30 g | CORT (20 mg/kg, s.c.) between 09:00 and 11:30 a.m. for 21 consecutive days | NOR, YMT, SST, SPT, FST, TST, | Depression↑ Memory loss↑ | Oliveira, T. Q. et al. 2022 [47] | |
| Swiss mice | Male | CORT (20 mg/kg, s.c.) for 21 days | OFT, FST | Depression↑ | Shuster, A. L. et al. 2022 [48] | ||
| Swiss mice | Female | 19–23 g | 8–10 weeks old | CORT (20 mg/kg, s.c.) for 21 days | OFT, SDA Test, YMT, SPT, FST | Depression↑ Memory loss↑ | MAIA Maia Oliveira, I. C. et al. 2022 [49] |
| SAMP8 mice | Male | 6 months old | CORT (40 mg/kg, s.c.) for 21 days | OFT, FST | Depression↑ | Chou, M. Y. et al. 2022 [50] | |
| Kunming mice | Male | 18–22 g | CORT (20 mg/kg, s.c.) for 21 days | OFT, EPM, FST, TST | Depression↑ | Bai, G. et al. 2022 [51] | |
| Long Evans rats | Male | CORT (40 mg/kg, s.c.) for 21 days | OFT, FST | Depression↑ | Allen, J. et al. 2022 [52] | ||
| ddY mice | Male | 7 weeks old | CORT (40 mg/kg, s.c.) for 14 days | SLA, BMT, FST | Depression↑ | Araki, R. et al. 2021 [53] | |
| C57BL/6J mice | Male and female | 9–10 weeks old | CORT (20 mg/kg, i.p.) for 10 days | SPT, FST | Depression↑ | Patel, S. D. et al. 2021 [54] | |
| C57BL/6J mice | Male | 5 weeks old | CORT (20 mg/kg, s.c.) between 09:00 and 09:30 a.m. daily for 28 days | SPT, FST, TST | Depression↑ | Zhang, D. et al. 2021 [55] | |
| FVB wild-type mice | Male | 20–25 g | 6–8 weeks old | CORT (400 μg/mL) in drinking water for 21 days | SPT, FST | Depression↑ | Luo, S. et al. 2021 [56] |
| Long Evans rats | Male | 225–250 g | CORT (40 mg/kg, s.c.) for 21 days | NOR, FST, PPI | Depression↑ Memory loss↑ | Brymer, K. J. et al. 2021 [57] | |
| Wistar rats | Male | 6 weeks old | CORT (40 mg/kg, s.c.) for 4 weeks | SPT, FST, | Depression↑ | Hao, Y. et al. 2021 [58] | |
| Swiss mice | Female | 22–25 g | 8–10 weeks old | CORT (20 mg/kg, s.c.) for 22 consecutive days | YMT, SDIT, SIT, PPI | Memory loss↑ | Chaves, R. C. et al. 2020 [59] |
| Swiss mice | Male | 30–40 g | 45–60 days old | CORT (20 mg/kg) in drinking water for 21 days | OFT, TST | Depression↑ | Camargo, A. et al. 2020 [60] |
| Swiss albino mice | Male | 25–30 g | CORT (20 mg/kg, s.c.) for 21 days | NOR, MWM, object in place test | Memory loss↑ | K, V. A. et al. 2020 [61] | |
| APP/PS1 Tg mice, C57BL/6J mice | 5 months old | CORT (10 mg/kg, s.c.) for 3 months | MWM, nest construction | Memory loss↑ | Zhang, S. Q. et al. 2020 [62] | ||
| C57BL/6J mice | Male | 5 weeks old | CORT (20 mg/kg, s.c.) for 14 days | SLA, FST, female encounter test | Depression↑ | YOKO Yokoyama, R. et al. 2020 [63] | |
| C57BL/6J mice | Male | CORT (20 mg/kg, s.c.) for 6 weeks | OFT, FST, TST | Depression↑ | Xie, X. et al. 2020 [64] | ||
| C57BL/6J mice | Female | 18–22 g | 6–8 weeks old | CORT (40 mg/kg, s.c.) for 6 weeks | OFT, SPT, FST, TST, NSF | Depression↑ | Zhao, F. et al. 2020 [65] |
| C57BL/6 mice | Male | 6 weeks old | CORT (20 mg/kg, s.c.) for 6 weeks | FST, TST | Depression↑ | Xie, X. et al. 2020 [66] | |
| Heterozygous Reeler mice and wild-type mice | Male and female | 6 weeks old | CORT (25 mg/L) in drinking water for 3 weeks | YMT, FST, PPI and startle reactivity, drug-induced hyper-locomotor activity | Depression↑ Memory loss↑ | Notaras, M. J.et al. 2020 [67] | |
| Sprague Dawley rats | Male | 220–250 g | CORT (40 mg/kg, s.c.) for 21 days | SPT, FST, MWM | Depression↑ Memory loss↑ | Zhao, Y. et al. 2020 [68] | |
| Sprague Dawley rats | Male | 180–220 g | CORT (20 mg/kg, s.c.) for 6 weeks | OFT, SPT, MWM | Anxiety↑ Depression↑ Memory loss↑ | Li, Z. et al. 2020 [69] | |
| Long Evans rats | Male | 225–250 g | 7 weeks old | repeated and cyclic CORT (20 mg/kg, s.c.) between 9 and 11 a.m. for 21 days | OFT, SPT, FST | Depression↑ | Lebedeva, K. A. et al. 2020 [70] |
| Wistar rats | Male | 180 ± 20 g | CORT (40 mg/kg, s.c.) for 21 days | OFT, SPT, FST | Depression↑ | Yu, Z. et al. 2020 [71] | |
| Swiss mice | Female | 21–25 g | 8–10 weeks old | CORT (20 mg/kg, s.c.) for 23 days | OFT, LDT, HBT, EPM, YMT, SIT, SPT, FST, TST | Anxiety↑ Depression↑ Memory loss↑ | Capibaribe, V. C. C. et al. 2019 [72] |
| C57BL/6J mice | Male | 18–22 g | CORT (40 mg/kg, s.c.) for 21 days | OFT, NOR, MWM, TST | Depression↑ Memory loss↑ | Chen, H. et al. 2019 [73] | |
| C57BL/6 mice | Male | 18–22 g | 8–10 weeks old | CORT (40 mg/kg, s.c.) between 8:00 and 10:00 a.m. for 8 weeks | EPM, FST, TST | Anxiety↑ Depression↑ | Zhang, K. et al. 2019 [74] |
| Swiss mice | Female | 22–25 g | 8–10 weeks old | CORT (20 mg/kg, s.c.) daily for 21 days | OFT, EPM, SPT, FST, TST | Anxiety↑ Depression↑ | Chaves, R. C. et al. 2019 [75] |
| Wistar rats | Male | ~200 g | 6 weeks old | CORT (40 mg/kg, s.c.) for 21 consecutive days | OFT, SPT, FST | Depression↑ | Shen, Q. et al. 2019 [76] |
| C57BL/6 mice | Male | 5 weeks old | CORT (100 μg /mL) in drinking water for 14 days, CORT (50 and 25 μg /mL) in drinking water for 3 days | OFT, YMT, NOR, SPT, FST, TST | Depression↑ Memory loss↑ | Murata, K. et al. 2018 [77] | |
| Swiss mice | Female | 22–25 g | CORT (20 mg/kg, s.c.) between 9:00 and 10:30 a.m. for consecutive 21 days | OFT, YMT, EPM, SPT, FST, TST | Anxiety↑ Depression↑ Memory loss↑ | Lopes, I. S. et al. 2018 [78] | |
| Swiss mice | Female | 30–32 g | CORT (20 mg/kg, s.c.) between 9:00 and 11:30 a.m. for 21 days | YMT, NOR, SIT, TST | Depression↑ Memory loss↑ | de Sousa, C. N. S. et al. 2018 [79] | |
| Swiss mice | Male | 30–40 g | CORT (20 mg/kg, orally administration) for 21 days | OFT, SST, FST, TST | Depression↑ | Camargo, A.et al. 2018 [80] | |
| Swiss albino mice | Male | 26–32 g | CORT (20 mg/kg, s.c.) for 21 days | OFT, EPM, SPT, SST, NSF, FST, TST | Anxiety↑ Depression↑ | Kv, A. et al. 2018 [81] | |
| ICR mice | Male | 18–22 g | CORT (20 mg/kg, s.c.) for 21 days | OFT, SPT, FST, TST | Depression↑ | Bai, Y. et al. 2018 [82] | |
| CD Sprague Dawley rats | Female | 3 months old | CORT (40 mg/kg, s.c.) between 9:30 and 11:30 a.m. for 21 days | OFT, FST, maternal care | Depression↑ | Kott, J. M. et al. 2018 [83] | |
| C57BL/6 mice | Male | 24 ± 2 g | 5 weeks old | CORT (40 mg/kg, s.c.) between 9:00 and 11:00 a.m. for 21 consecutive days | SPT, TST | Depression↑ | Wang, S. S. et al. 2017 [84] |
| C57BL/6NTac mice | Male | 25–30 g | 7–8 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 8 weeks | OFT, EPM, SPT, NSF, FST, TST | Anxiety↑ Depression↑ | Mendez-David, I. et al. 2017 [85] |
| Swiss mice | Male | 25–30 g | CORT (20 mg/kg, s.c.) between 9:00 and 11:30 a.m. for 21 days | OFT, EPM, SPT, TST, sleeping time test, rota rod test | Anxiety↑ Depression↑ | Oliveira, T. Q. et al. 2017 [86] | |
| Swiss mice | Female | 30–40 g | 40–45 days old | CORT (20 mg/kg, oral gavage) for 21 days | OFT, FST, TST | Depression↑ | Pazini, F. L. et al. 2017 [87] |
| Long Evans rats | Male | 200–250 g | CORT (20 and 40 mg/kg, s.c.) for 21 consecutive days | FST | Depression↑ | Lebedeva, K. A. et al. 2017 [88] | |
| Sprague Dawley rats | Male | Adolescent (90 ± 5 g), adult (350 ± 20 g) | Adolescent (28–29 days), adult (70–71 days) | CORT (40 mg/kg, i.p.) between 9:00 and 11:00 a.m. for 21 days | OFT, MWM, SLA, SPT, PPI | Anxiety↑ Depression↑ Memory loss↑ | Li, J. et al. 2017 [89] |
| C57BL/6NTac mice | Male | 8 weeks old | CORT (10 mg/kg) for 3 weeks | EPM, NSF, SST, FST | Anxiety↑ Depression↑ | Brachman, R. A. et al. 2016 [90] | |
| C57BL/6NTac mice | Male | 20–25 g | 8 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 4 weeks | OFT, EPM, NSF, SST | Anxiety↑ Depression↑ | Siopi, E. et al. 2016 [91] |
| C57BL/6JRj mice | Male | 8–10 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 4 weeks | OFT, NOR, FC, BMT, SST | Anxiety↑ Depression↑ Memory loss↑ | Darcet, F. et al. 2016 [92] | |
| BALB/c mice | Female | CORT (35 μg/mL) in drinking water for 21 days | SPT, FST | Depression↑ | Nashed, M. G. et al. 2016 [93] | ||
| Wistar rats | Male | 8 weeks old | CORT (200 μg/mL equivalent to ∼27 ± 1.6 mg/kg/day) in drinking water for 21 days | OFT, EPM, predator odor test | Anxiety↑ | Govic, A. et al. 2016 [94] | |
| Sprague Dawley rats | Female | 77–84 days old | CORT (200 mg, pellet implantation); CORT (40 mg/kg, s.c.) between 8:00 and 10:00 a.m. for 23 days; CORT (200 μg /mL) in drinking water for 20 days, CORT (150 μg /mL) in drinking water for 1 day, CORT (100 μg /mL) in drinking water for 1 day, CORT (50 μg /mL) in drinking water for 1 day | OFT, FST | Depression↑ | Kott, J. M. et al. 2016 [95] | |
| C57BL/6J mice | 25–35 g | 7 weeks old | CORT (35 μg/mL equivalent to 5 mg/kg/day) in drinking water for 21 days | OFT, NOR, FC, object location test, EPM, NSF, SST, SPT, TST, | Anxiety↑ Depression↑ Memory loss↑ | Quesseveur, G. et al. 2015 [96] | |
| C57BL/6NTac mice | Male | 23–25 g | 7–8 weeks old | CORT (35 mg/mL) in drinking water for 4 weeks | OFT, EPM, NSF, SST | Anxiety↑ Depression↑ | Mendez-David, I. et al. 2015 [97] |
| BALB/c mice | Female | 4–6 weeks old | CORT (35 μg/mL equivalent to 6.53 ± 0.16 mg/kg/day) in drinking water for 21 days | SPT, FST, TST | Depression↑ | Nashed, M. G. et al. 2015 [98] | |
| iBax mice | Male | 8–10 weeks old | CORT (70 μg/mL equivalent to 10 mg/kg/day) in drinking water for 21 days | OFT, EPM, NSF, FST, TST | Anxiety↑ Depression↑ | Hill, A. S. et al. 2015 [99] | |
| Wild-type mice | Male | 8 weeks old | CORT (35 μg/mL) in drinking water for 6 weeks | NSF, splash grooming test | Anxiety↑ Depression↑ | Schloesser, R. J. et al. 2015 [100] | |
| HRM and wild-type mice | Male and female | 6 weeks old | CORT (50 mg/L) in drinking water for 21 days | YMT, NOR, SIT, PPI | Memory loss↑ | Schroeder, A. et al. 2015 [101] | |
| Swiss mice | Female | 30–32 g | CORT (20 mg/kg, s.c.) between 9:00 and 11:30 a.m. for 21 days | SPT, FST | Depression↑ | de Sousa, C. N. et al. 2015 [102] | |
| Swiss albino mice | Male | 25–30 g | 4–6 weeks old | CORT (40 mg/kg, s.c.) for 21 days | SPT, FST, TST | Depression↑ | Ali, S. H. et al. 2015 [103] |
| Swiss albino mice | Male | 20–25 g | 11–12 weeks old | CORT (40 mg/kg, s.c.) for 28 days | SLA, LDT, FST | Depression↑ | Gupta, D. et al. 2015 [104] |
| Fischer 344 rats | Male | 250–320 g | CORT (30 μg, stereotaxic implantation) | EPM | Anxiety↑ | Tran, L. et al. 2015 [105] | |
| Sprague Dawley rats | Male | 3–4 weeks old | CORT (50 μg/mL equivalent to 7.1 mg/kg/day) in drinking water for 21 days | NSF, SPT | Depression↑ | Kvarta, M. D. et al. 2015 [106] | |
| Sprague Dawley rats | Male | 325–350 g | CORT (50 mg/kg, s.c.) for 21 days | OFT, SPT, FST | Depression↑ | Dwivedi, Y. et al. 2015 [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, X.; Wang, H.; Shao, S.; Zhu, J. Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives. Int. J. Mol. Sci. 2024, 25, 11245. https://doi.org/10.3390/ijms252011245
Wang H, Wang X, Wang H, Shao S, Zhu J. Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives. International Journal of Molecular Sciences. 2024; 25(20):11245. https://doi.org/10.3390/ijms252011245
Chicago/Turabian StyleWang, Hao, Xingxing Wang, Huan Wang, Shuijin Shao, and Jing Zhu. 2024. "Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives" International Journal of Molecular Sciences 25, no. 20: 11245. https://doi.org/10.3390/ijms252011245
APA StyleWang, H., Wang, X., Wang, H., Shao, S., & Zhu, J. (2024). Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives. International Journal of Molecular Sciences, 25(20), 11245. https://doi.org/10.3390/ijms252011245






