Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease
Abstract
1. Introduction
2. Hepatic Inflammatory Diseases
3. Liver Inflammation Research Models
4. Canonical and Non-Canonical Mechanism of Nrf2 Activation
5. Farnesoid X Receptor
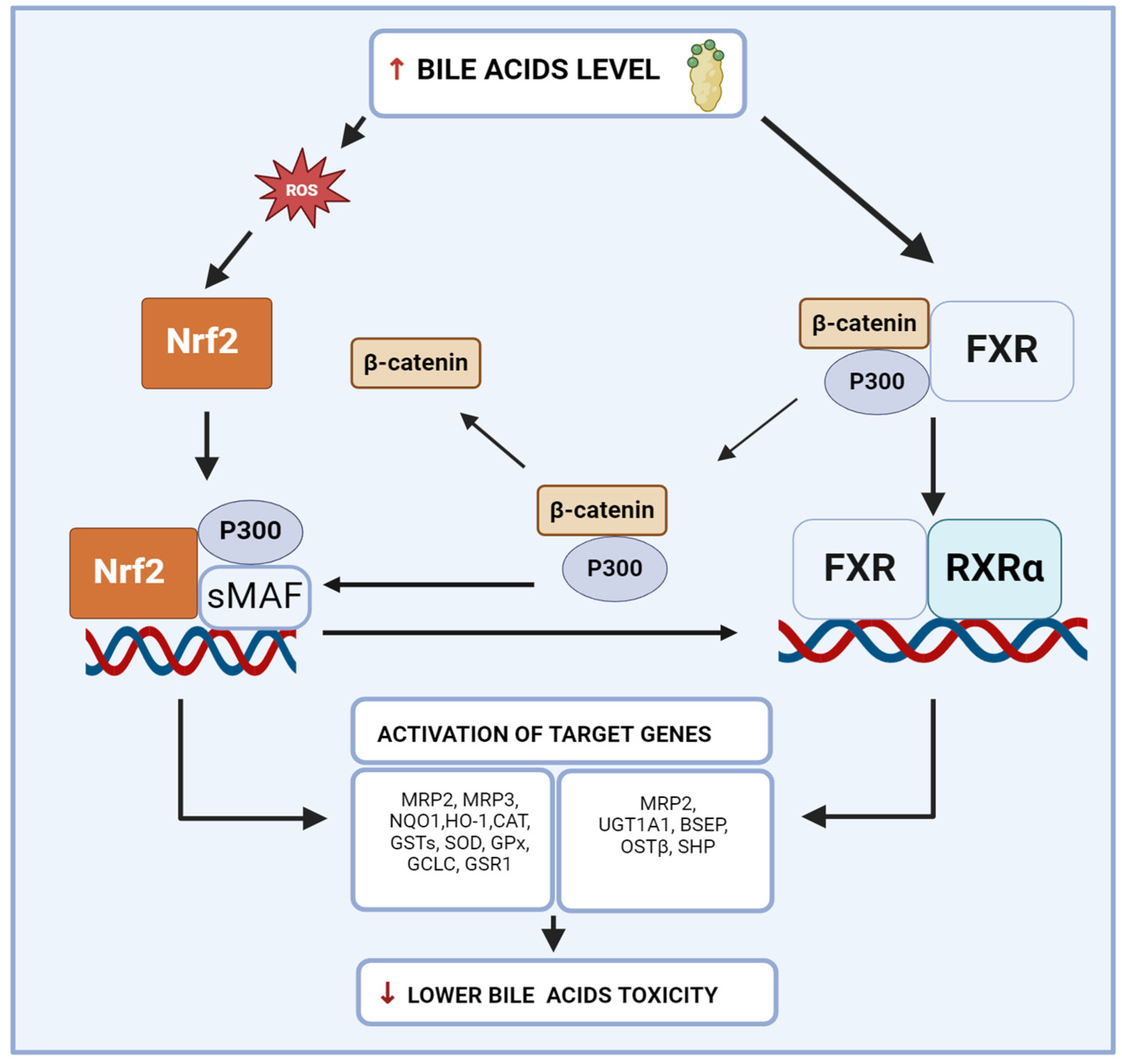
6. Interconnection between Nrf2 and FXR in Liver Inflammation
7. Natural Agonists of FXR and Modulators of Nrf2
7.1. Auraptene
7.2. Cafestol
7.3. Curcumin
7.4. Fargesone A
7.5. Hesperidin
7.6. Lycopene
7.7. Oleanolic Acid
7.8. Resveratrol
7.9. Rutin
7.10. Ursolic Acid
7.11. Withaferin A
8. Limitation of the Use of Natural Agonists of the FXR and Modulators of Nrf2 in Clinical Practice
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2019, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, J.J.; Kullak-Ublick, G.A. The Role of FXR in Disorders of Bile Acid Homeostasis. Physiology 2008, 23, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, F.; Xu, W.; Wu, X.; Lian, N.; Jin, H.; Chen, Q.; Chen, L.; Shao, J.; Wu, L.; et al. Curcumin Attenuates Ethanol-Induced Hepatic Steatosis through Modulating Nrf2/FXR Signaling in Hepatocytes. IUBMB Life 2015, 67, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Choi, G.H.; Choi, H.Y.; Han, S.; Jang, E.S.; Chon, Y.E.; Chang, Y.; Kim, K.-A.; Kim, D.Y.; Yim, H.J.; et al. Core Indicators Related to the Elimination of Hepatitis B and C Virus Infection in South Korea: A Nationwide Study. Clin. Mol. Hepatol. 2023, 29, 779–793. [Google Scholar] [CrossRef]
- Jepsen, P.; Grønbæk, L.; Vilstrup, H. Worldwide Incidence of Autoimmune Liver Disease. Dig. Dis. 2015, 33, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Shubrook, J.H.; Younossi, Z.; Natarajan, Y.; Bugianesi, E.; Rinella, M.E.; Harrison, S.A.; Mantzoros, C.; Pfotenhauer, K.; Klein, S.; et al. Preparing for the NASH Epidemic: A Call to Action. Metabolism 2021, 122, 154822. [Google Scholar] [CrossRef]
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From Hepatitis A to E: A Critical Review of Viral Hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef]
- Muratori, L.; Lohse, A.W.; Lenzi, M. Diagnosis and Management of Autoimmune Hepatitis. BMJ 2023, 380, e070201. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Stalnikowitz, D.K.; Weissbrod, A.B. Liver Fibrosis and Inflammation. A Review. Ann. Hepatol. 2003, 2, 159–163. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver Macrophages in Tissue Homeostasis and Disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. 2024, 100, 100737. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Chauhan, V. Lactate dehydrogenase as an indicator of liver diseases. J. Adv. Med. Dent. Sci. Res. 2015, 3, S20. [Google Scholar]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Fuertes-Agudo, M.; Luque-Tévar, M.; Cucarella, C.; Martín-Sanz, P.; Casado, M. Advances in Understanding the Role of NRF2 in Liver Pathophysiology and Its Relationship with Hepatic-Specific Cyclooxygenase-2 Expression. Antioxidants 2023, 12, 1491. [Google Scholar] [CrossRef]
- Ramos, M.J.; Bandiera, L.; Menolascina, F.; Fallowfield, J.A. In Vitro Models for Non-Alcoholic Fatty Liver Disease: Emerging Platforms and Their Applications. iScience 2021, 25, 103549. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Xing, W.; Li, F.; Liang, M.; Li, K.; He, Y.; Wang, J. An Update on Animal Models of Liver Fibrosis. Front. Med. 2023, 10, 1160053. [Google Scholar] [CrossRef]
- Mao, J.; Tan, L.; Tian, C.; Wang, W.; Zhang, H.; Zhu, Z.; Li, Y. Research Progress on Rodent Models and Its Mechanisms of Liver Injury. Life Sci. 2024, 337, 122343. [Google Scholar] [CrossRef]
- Xu, G.; Dai, M.; Zheng, X.; Lin, H.; Liu, A.; Yang, J. Cholestatic Models Induced by Lithocholic Acid and A-naphthylisothiocyanate: Different Etiological Mechanisms for Liver Injury but Shared JNK/STAT3 Signaling. Mol. Med. Rep. 2020, 22, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ji, K.; Shen, X.; Zhang, W.; Wang, R.; Xu, W.; Wei, W. Di(2-Ethylhexyl) Phthalate Promotes Hepatic Fibrosis by Regulation of Oxidative Stress and Inflammation Responses in Rats. Environ. Toxicol. Pharmacol. 2019, 68, 109–119. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Tang, Y.-X.; Qiu, B.-H.; Talukder, M.; Li, X.-N.; Li, J.-L. Di-2-Ethylhexyl Phthalate (DEHP) Induced Lipid Metabolism Disorder in Liver via Activating the LXR/SREBP-1c/PPARα/γ and NF-κB Signaling Pathway. Food Chem. Toxicol. 2022, 165, 113119. [Google Scholar] [CrossRef] [PubMed]
- Nevzorova, Y.A.; Boyer-Diaz, Z.; Cubero, F.J.; Gracia-Sancho, J. Animal Models for Liver Disease—A Practical Approach for Translational Research. J. Hepatol. 2020, 73, 423–440. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.-K.; Kwon, S.W.; Han, D.H.; Lee, Y.-H.; Bae, S.H. SQSTM1/P62 Activates NFE2L2/NRF2 via ULK1-Mediated Autophagic KEAP1 Degradation and Protects Mouse Liver from Lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Shreya, S.; Grosset, C.F.; Jain, B.P. Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Chen, W.-D.; Moore, D.D.; Huang, W. FXR: A Metabolic Regulator and Cell Protector. Cell Res. 2008, 18, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Monte, M.J.; Dominguez, M.; Muntané, J.; Marin, J.J.G. Differential Activation of the Human Farnesoid X Receptor Depends on the Pattern of Expressed Isoforms and the Bile Acid Pool Composition. Biochem. Pharmacol. 2013, 86, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (FXR): Structures and Ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Shaik, F.B.; Prasad, D.V.R.; Narala, V.R. Role of Farnesoid X Receptor in Inflammation and Resolution. Inflamm. Res. 2015, 64, 9–20. [Google Scholar] [CrossRef]
- Xiang, D.; Yang, J.; Liu, L.; Yu, H.; Gong, X.; Liu, D. The Regulation of Tissue-Specific Farnesoid X Receptor on Genes and Diseases Involved in Bile Acid Homeostasis. Biomed. Pharmacother. 2023, 168, 115606. [Google Scholar] [CrossRef] [PubMed]
- Massafra, V.; van Mil, S.W.C. Farnesoid X Receptor: A “Homeostat” for Hepatic Nutrient Metabolism. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR Activation Protects against NAFLD via Bile-Acid-Dependent Reductions in Lipid Absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Rausch, M.; Samodelov, S.L.; Visentin, M.; Kullak-Ublick, G.A. The Farnesoid X Receptor as a Master Regulator of Hepatotoxicity. Int. J. Mol. Sci. 2022, 23, 13967. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lickteig, A.J.; Zhang, Y.; Csanaky, I.L.; Klaassen, C.D. Activation of Nrf2 Decreases Bile Acid Concentrations in Livers of Female Mice. Xenobiotica 2021, 51, 605–615. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Meng, C.; Gu, Q.; Huang, C.; Liu, F.; Xia, C. NRF2 and FXR Dual Signaling Pathways Cooperatively Regulate the Effects of Oleanolic Acid on Cholestatic Liver Injury. Phytomedicine 2023, 108, 154529. [Google Scholar] [CrossRef]
- Kay, H.Y.; Kim, W.D.; Hwang, S.J.; Choi, H.-S.; Gilroy, R.K.; Wan, Y.-J.Y.; Kim, S.G. Nrf2 Inhibits LXRα-Dependent Hepatic Lipogenesis by Competing with FXR for Acetylase Binding. Antioxid. Redox Signal 2011, 15, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Guo, L.; Ma, J.; Yang, Y.; Tang, T.; Zhang, B.; Zhou, W.; Zou, W.; Hou, Z.; Gu, H.; et al. Liquiritin Alleviates Alpha-Naphthylisothiocyanate-Induced Intrahepatic Cholestasis through the Sirt1/FXR/Nrf2 Pathway. J. Appl. Toxicol. 2023, 43, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Henamayee, S.; Parama, D.; Rana, V.; Dutta, U.; Kunnumakkara, A.B. Targeting Farnesoid X Receptor (FXR) for Developing Novel Therapeutics against Cancer. Mol. Biomed. 2021, 2, 21. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic Biomarkers and Mechanisms of Lipotoxicity in Non-Alcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Jaeschke, H.; Klaassen, C.D. Oxidative Stress and the Pathogenesis of Cholestasis. Semin. Liver Dis. 2010, 30, 195–204. [Google Scholar] [CrossRef]
- Masubuchi, N.; Sugihara, M.; Sugita, T.; Amano, K.; Nakano, M.; Matsuura, T. Oxidative Stress Markers, Secondary Bile Acids and Sulfated Bile Acids Classify the Clinical Liver Injury Type: Promising Diagnostic Biomarkers for Cholestasis. Chem. Biol. Interact. 2016, 255, 83–91. [Google Scholar] [CrossRef]
- Chen, W.-D.; Wang, Y.-D.; Meng, Z.; Zhang, L.; Huang, W. Nuclear Bile Acid Receptor FXR in the Hepatic Regeneration. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2011, 1812, 888–892. [Google Scholar] [CrossRef]
- Ho, P.P.; Steinman, L. Obeticholic Acid, a Synthetic Bile Acid Agonist of the Farnesoid X Receptor, Attenuates Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 2016, 113, 1600–1605. [Google Scholar] [CrossRef]
- Gao, X.; Fu, T.; Wang, C.; Ning, C.; Kong, Y.; Liu, Z.; Sun, H.; Ma, X.; Liu, K.; Meng, Q. Computational Discovery and Experimental Verification of Farnesoid X Receptor Agonist Auraptene to Protect against Cholestatic Liver Injury. Biochem. Pharmacol. 2017, 146, 127–138. [Google Scholar] [CrossRef]
- Prince, M.; Li, Y.; Childers, A.; Itoh, K.; Yamamoto, M.; Kleiner, H.E. Comparison of Citrus Coumarins on Carcinogen-Detoxifying Enzymes in Nrf2 Knockout Mice. Toxicol. Lett. 2009, 185, 180–186. [Google Scholar] [CrossRef][Green Version]
- Ricketts, M.-L.; Boekschoten, M.V.; Kreeft, A.J.; Hooiveld, G.J.E.J.; Moen, C.J.A.; Müller, M.; Frants, R.R.; Kasanmoentalib, S.; Post, S.M.; Princen, H.M.G.; et al. The Cholesterol-Raising Factor from Coffee Beans, Cafestol, as an Agonist Ligand for the Farnesoid and Pregnane X Receptors. Mol. Endocrinol. 2007, 21, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Choi, J.H.; Jeong, H.G. Hepatoprotective and Antioxidant Effects of the Coffee Diterpenes Kahweol and Cafestol on Carbon Tetrachloride-Induced Liver Damage in Mice. Food Chem. Toxicol. 2007, 45, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin Regulates Endogenous and Exogenous Metabolism via Nrf2-FXR-LXR Pathway in NAFLD Mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, K.; Dong, H.; Hu, D.; Gao, Y.; Liu, C.; Laphookhieo, S.; Lei, X. Biomimetic Total Synthesis and the Biological Evaluation of Natural Product (−)-Fargesone A as a Novel FXR Agonist. JACS Au 2022, 2, 2830–2838. Available online: https://pubs.acs.org/doi/10.1021/jacsau.2c00600 (accessed on 27 August 2024). [CrossRef]
- Zhang, G.; Sun, X.; Wen, Y.; Shi, A.; Zhang, J.; Wei, Y.; Wu, X. Hesperidin Alleviates Cholestasis via Activation of the Farnesoid X Receptor in Vitro and in Vivo. Eur. J. Pharmacol. 2020, 885, 173498. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin Ameliorates Hepatic Oxidative Stress and Inflammation via the PI3K/AKT-Nrf2-ARE Pathway in Oleic Acid-Induced HepG2 Cells and a Rat Model of High-Fat Diet-Induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Hassanein, E.H.M.; Binmahfouz, L.S.; Bagher, A.M.; Hareeri, R.H.; Algandaby, M.M.; Fadladdin, Y.A.J.; Aleya, L.; Abdel-Daim, M.M. Lycopene Attenuates Chlorpyrifos-Induced Hepatotoxicity in Rats via Activation of Nrf2/HO-1 Axis. Ecotoxicol. Environ. Saf. 2023, 262, 115122. [Google Scholar] [CrossRef]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene Relieves AFB 1 -Induced Liver Injury through Enhancing Hepatic Antioxidation and Detoxification Potential with Nrf2 Activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Hajighasem, A.; Farzanegi, P.; Mazaheri, Z.; Naghizadeh, M.; Salehi, G. Effects of Resveratrol, Exercises and Their Combination on Farnesoid X Receptor, Liver X Receptor and Sirtuin 1 Gene Expression and Apoptosis in the Liver of Elderly Rats with Nonalcoholic Fatty Liver. PeerJ 2018, 6, e5522. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, B.; Li, J.; Yang, L.; Wang, Z. Beneficial Effect of Resveratrol on A-naphthyl Isothiocyanate-induced Cholestasis via Regulation of the FXR Pathway. Mol. Med. Rep. 2018, 17, 1863–1872. [Google Scholar] [CrossRef]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Babaei Khorzoughi, R.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol Alleviates Non-Alcoholic Fatty Liver Disease through Epigenetic Modification of the Nrf2 Signaling Pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.-H.; Lin, S.-Y.; Wang, Y.-Y.; Chen, W.-Y.; Chuang, Y.-H.; Wu, C.-C.; Chen, C.-J. Protective Effects of Rutin on Liver Injury Induced by Biliary Obstruction in Rats. Free Radic. Biol. Med. 2014, 73, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.-F.; Wu, Y.; Wang, Y.; et al. Rutin Ameliorated Lipid Metabolism Dysfunction of Diabetic NAFLD via AMPK/SREBP1 Pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, W.; Li, N.; Guo, S.; Gao, L.; Ge, N. Effect of Ursolic Acid Extracted from Hippophae rhamnoides L. on FXR Signaling Pathway in Liver of Rats with Alcoholic Liver Injury. Sci. Technol. Food Ind. 2023, 44, 363–370. [Google Scholar]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Protective Effects of Ursolic Acid in an Experimental Model of Liver Fibrosis through Nrf2/ARE Pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, W.; Wang, X.; Qin, L.; Zhong, M.; Liu, Y.; Xiong, Y.; Yi, X.; Wang, X.; Zhang, H. Ursolic Acid Attenuates Cholestasis through NRF2-Mediated Regulation of UGT2B7 and BSEP/MRP2. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2257–2267. [Google Scholar] [CrossRef]
- Shiragannavar, V.D.; Sannappa Gowda, N.G.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The Ameliorating Effect of Withaferin A on High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Acting as an LXR/FXR Dual Receptor Activator. Front. Pharmacol. 2023, 14, 1135952. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A Induces Nrf2-Dependent Protection against Liver Injury: Role of Keap1-Independent Mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Mofed, D.; Ahmed, W.; Zekri, A.-R.; Said, O.; Rahouma, M.; Faraag, A.H.I. The Antiviral Efficacy of Withania somnifera (Ashwagandha) against Hepatitis C Virus Activity: In Vitro and in Silico Study. Adv. Microbiol. 2020, 10, 463–477. [Google Scholar] [CrossRef]
- Bibak, B.; Shakeri, F.; Barreto, G.E.; Keshavarzi, Z.; Sathyapalan, T.; Sahebkar, A. A Review of the Pharmacological and Therapeutic Effects of Auraptene. Biofactors 2019, 45, 867–879. Available online: https://iubmb.onlinelibrary.wiley.com/doi/full/10.1002/biof.1550 (accessed on 27 August 2024). [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-W.; Huang, C.-Y.; Yang, S.-Y.; Peng, Y.-H.; Yu, C.-P.; Chao, P.-D.L.; Hou, Y.-C. Oral Intake of Curcumin Markedly Activated CYP 3A4: In Vivo and Ex-Vivo Studies. Sci. Rep. 2014, 4, 6587. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. The Ameliorative Effect of Turmeric (Curcuma longa Linn) Extract and Its Major Constituent, Curcumin, and Its Analogs on Ethanol Toxicity. Phytother. Res. 2024, 38, 2165–2181. [Google Scholar] [CrossRef]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin-Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Navarro-González, I.; González-Barrio, R.; Martín-Pozuelo, G.; Doménech, G.; Seva, J.; García-Alonso, J.; Periago-Castón, M.J. Tomato Juice Supplementation Influences the Gene Expression Related to Steatosis in Rats. Nutrients 2018, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K. Therapeutic Potential of Oleanolic Acid in Liver Diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 4537–4554. [Google Scholar] [CrossRef]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The Role of Resveratrol in Liver Disease: A Comprehensive Review from In Vitro to Clinical Trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- El-hawary, S.S.; Ali, Z.Y.; Younis, I.Y. Hepatoprotective Potential of Standardized Ficus Species in Intrahepatic Cholestasis Rat Model: Involvement of Nuclear Factor-κB, and Farnesoid X Receptor Signaling Pathways. J. Ethnopharmacol. 2019, 231, 262–274. [Google Scholar] [CrossRef]
- Shanmugam, S.; Sivaraj, D.; dos Santos Lima, B.; dos Passos Menezes, P.; de Carvalho, Y.M.B.G.; de Souza Araújo, A.A.; Narain, N.; Serafini, M.R.; Quintans Júnior, L.J.; Scotti, L.; et al. Polyphenols Rich Passiflora leschenaultii Leaves Modulating Farnesoid X Receptor and Pregnane X Receptor against Paracetamol-Induced Hepatotoxicity in Rats. Biomed. Pharmacother. 2017, 88, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Kadasah, S.F.; Radwan, M.O. Overview of Ursolic Acid Potential for the Treatment of Metabolic Disorders, Autoimmune Diseases, and Cancers via Nuclear Receptor Pathways. Biomedicines 2023, 11, 2845. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yan, M.; Wang, P.; Hamada, K.; Yan, N.; Hao, H.; Gonzalez, F.J.; Yan, T. Withaferin A in the Treatment of Liver Diseases: Progress and Pharmacokinetic Insights. Drug Metab. Dispos. 2022, 50, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Lycopene: A Biologically Important Carotenoid for Humans? Arch. Biochem. Biophys. 1996, 336, 1–9. [Google Scholar] [CrossRef]
- Sobreiro, M.A.; Della Torre, A.; de Araújo, M.E.M.B.; Canella, P.R.B.C.; de Carvalho, J.E.; Carvalho, P.D.O.; Ruiz, A.L.T.G. Enzymatic Hydrolysis of Rutin: Evaluation of Kinetic Parameters and Anti-Proliferative, Mutagenic and Anti-Mutagenic Effects. Life 2023, 13, 549. [Google Scholar] [CrossRef]
- Riangjanapatee, P.; Müller, R.H.; Keck, C.M.; Okonogi, S. Development of Lycopene-Loaded Nanostructured Lipid Carriers: Effect of Rice Oil and Cholesterol. Pharmazie 2013, 68, 723–731. [Google Scholar]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]

| Natural Compounds /Origin | Chemical Name | Model/Efficient Dose | Activation of FXR or Nrf2 Pathway | References |
|---|---|---|---|---|
| Auraptene Citrus aurantium | 7-{[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy}-2H-1-benzopyran-2-one (purity > 98%) | Model: cholestatic liver injury In vitro: mouse primary cultured hepatocytes (5, 10 and 20 μM) In vivo: C57BL/6 mice (7.5, 15, 30 mg/kg) | ↓ ALT, AST, ALP, TBIL, and TBA serum levels ↑ BSEP, MRP2 mRNA, and protein levels ↓ NTCP mRNA and protein level ↓ CYP7A1 and CYP8B1 mRNA levels ↑ SULT2A1 and SHP mRNA levels ↑ FXR protein level | [49] |
| In vitro: HepG2 cells (25, 50, 75 and 100 µM) In vivo: ICR mice (150 mg/kg) | ↑ GST activities | [50] | ||
| Cafestol Coffea arabica | 3,18-(epoxymetheno)-19-nor-5β, 8α, 9β, 10α, 13β, 16β-kaur-3-ene-16α, 17-diol (purity > 98%) | In vivo: HepG2 cells (56 μM) and CV-1 cells (1, 10, 20 μM) In vivo: C57BL6 mice (400 mg/kg) | ↓ CYP7A1, CYP8B1, and NTCP mRNA levels ↑ SHP and BSEP mRNA levels | [51] |
| Model: carbon tetrachloride-induced liver damage In vivo: ICR mice (10–100 mg/kg) | ↓ ALT and AST in serum levels ↑ GST activities | [52] | ||
| Curcumin Curcuma longa | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione (purity > 98%) | Model: MAFLD In vitro: primary hepatocytes from the liver of C57BL/6 mice (10 µM) In vivo: C57BL/6 mice (50, 100 mg/kg) | ↓ TG, TC, and ALT serum levels ↑ CYP3A and CYP7A protein level ↓ SREBP-1c protein and mRNA level, FAS mRNA level ↑ FXR, SHP, and Nrf2 protein levels | [53] |
| Model: ethanol-induced hepatic steatosis In vitro: Human LO2 hepatocyte (10, 20, 40 µM) In vivo: Sprague-Dawley Rats (100, 200, 400 mg/kg) | ↓ AST, ALT, ALP, and LDH serum levels ↓ SREBP-1c and FAS protein and mRNA levels ↑ FXR, Nrf2 mRNA protein, and mRNA levels | [3] | ||
| Fargesone A Magnolia fargesii | (2S,3R,3aR,7S,7aS)-2-(1,3-benzodioxol-5-yl)-3a,4-dimethoxy- 3-methyl-7-prop-2-enyl-2,3,7,7a-tetrahydro-1-benzofuran-6 (purity-no data) | Model: oleic acid-induced lipid accumulation/bile duct ligation in mice In vivo: WRL68 cells (10 µM) In vivo: C57BL/6 mice (3 mg/kg, 30 mg/kg) | ↑ SHP and BSEP mRNA levels ↓ CYP7A1 and CYP8B1 mRNA levels | [54] |
| Hesperidin Citrus aurantium | (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7- [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl] oxan-2-yl]oxy- 2,3-dihydrochromen -4-one (purity > 97%) | Model: ANIT-induced cholestatic In vitro: HepaRG cells (6.25, 12.5, 25 and 50 µM) In vivo: C57BL/6J mice (25, 50, 100 mg/kg) | ↓ ALT, AST, ALP, γ-GT, and TBIL serum levels ↑ FXR, SHP, OATP1A1, BSEP, and MRP2 mRNA levels ↓ NTCP, CYP7A1, and CYP27A1 mRNA levels | [55] |
| Model: MAFLD In vitro: HepG2 cells (2,5; 5 and 10 µM) In vivo: Wistar rats (100, 300 mg/kg) | ↑ SOD, GPx, GCLC, and HO-1 protein levels ↑ Nrf2 and p-Nrf2 protein levels ↑ Nrf2-Keap1 complex dissociation ↑ Nrf2 translocation to the nucleus | [56] | ||
| Lycopene Solanum lycopersicum | (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene (purity > 98%) | Model: chlorpyrifos- induced hepatic toxicity In vivo: Wistar rats (5, 10 mg/kg) | ↓ ALT, AST, ALP, and LDH serum levels ↑ SOD, GSH, and GST activities ↑ Nrf2 protein level ↑ HO-1 mRNA level | [57] |
| Model: aflatoxin B1- induced hepatic toxicity In vivo: kunming mice (5 mg/kg) | ↓ ALT and AST serum activities ↑ SOD, CAT, GSH, and GST activities ↑ Nrf2 protein level ↑ NQO1, SOD1, and CAT mRNA levels | [58] | ||
| Oleanolic acid Olea europaea | (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid (purity > 97%) | Model: ANIT-induced cholestasis In vitro: Human LO2 hepatocyte (20 µM) In vivo: Sprague-Dawley rats (100 mg/kg) | ↓ ALT, AST, ALP, TBIL, γ-GT, and TBA serum levels ↑ SOD and GSH activities ↑ Nrf2, FXR, BSEP, and UGT1A1 mRNA and protein levels ↓ CYP7A1 mRNA levels ↑ Nrf2-Keap1 complex dissociation | [40] |
| Resveratrol Vitis vinifera | 5-[(E)-2-(4-hydroxyphenyl)ethen-1-yl]benzene-1,3-diol (purity > 98%) | Model: MAFLD In vivo: Wistar rats (25 mg/kg) | ↓ AST, ALT, and ALP serum activity ↑ FXR and SIRT1 mRNA levels | [59] |
| Model: ANIT-induced cholestasis In vitro: HEK293 cells, HepG2 cells, primary mouse hepatocytes (3, 10, 30 µM) In vivo: WT C57/BL mice (60 mg/kg) | ↓ ALT, AST, ALP, TBIL, DBIL, and TBA serum levels ↑ BSEP and SHP mRNA levels ↓ OSTß, MRP3, and MRP2 mRNA levels ↓ CYP7A1 and CYP8B1 mRNA levels | [60] | ||
| Model: MAFLD In vitro: HepG2 cells (20 µM) In vivo: C57/BL6 mice (0.4 % resveratrol) | ↓ SREBP-1c and FAS mRNA levels ↓ Keap1 mRNA and protein level ↑ Nrf2 mRNA and protein level ↑ HO-1, NQO1, CAT, and SOD2 mRNA levels ↓ Nrf2 promoter methylation | [61] | ||
| Rutin Fagopyrum esculentum | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl] oxymethyl]oxan-2-yl] oxychromen-4-one (purity > 98%) | Model: BDL-induced liver injury In vivo: Sprague-Dawley rats (25 mg/kg) | ↓ AST, ALT, TBIL, TG, and TC serum levels ↑ Nrf2 and HO-1 protein levels ↑ p-AMPK protein level ↑ liver CAT and SOD1 activities ↑ GSH protein level | [62] |
| Model: diabetic MAFLD In vitro: HeLa cells (150 µM), HepG2 cells (20 µM) In vivo: C57BLKs mice (100 mg/kg, 200 mg/kg) | ↓ ALT, AST, TG, and TC serum levels ↑ Nrf2, HO-1, and NQO1 protein levels ↓ FAS, SREBP1-1c, and SCD1 protein levels ↑ p-AMPK protein level | [63] | ||
| Ursolic acid Malus domestica | (1S,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid (purity > 93%) | Model: ethanol-induced hepatic injury In vivo: rats (150 mg/kg) | ↓ ALT, AST, ALP, and TBA serum levels ↓ SREBP-1c and CYP7A1 mRNA levels ↑ FXR protein level | [64] |
| Model: carbon tetrachloride-induced liver damage In vivo: ICR mice (25 mg/kg, 50 mg/kg) | ↓ AST and ALT in serum activity ↑ SOD, CAT, and GPx activities ↑ Nrf2, NQO1, GST, and HO-1 protein levels | [65] | ||
| Model: ANIT-induced cholestasis In vitro: HepG2 cells (32 µM) In vivo: Sprague-Dawley rats (10 mg/kg, 20 mg/kg, 40 mg/kg) | ↓ ALT, AST, ALP, γ-GT, TBIL, DBIL, and TBA serum levels ↑ Nrf2, BSEP, and MRP2 mRNA levels | [66] | ||
| Withaferin A Withania somnifera | (1S,2R,6S,7R,9R,11S,12S,15R,16S)-6-hydroxy-15-[(1S)-1-[(2R)-5-(hydroxymthyl)-4-methyl-6-oxo-2,3-dihydropyran-2-yl]ethyl]- 2,16-dimethyl-8-oxape tacyclo [9.7.0.02,7.07,9.012,16] octadec-4-en-3-one (purity > 79%) | Model: induced MAFLD In vitro: HepG2 and Huh7 cells (1, 2.5, 5 µM) In vivo: Swill albino mice (1.25 mg/kg) | ↓ AST, ALT, and ALP serum levels ↓ TG and TC serum levels | [67] |
| Model: acetaminophen-induced hepatic toxicity In vitro: mouse embryonic fibroblasts (MEFs) (0–3 µM) In vivo: C57BL/6J mice (7 mg/kg) | ↓ ALT serum level ↑ NQO1, GCLC, GSTP1, UGT1A1, GSTA1, GST1, and HO-1 mRNA levels ↑ Nrf2 protein and mRNA levels | [68] | ||
| Model: lymphocyte cell infection with HCV serum In vitro: human lymphocyte from normal cells (25 mg/mL, 50 mg/mL) | ↑ GSR activity ↑ GST activity | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belka, M.; Gostyńska-Stawna, A.; Stawny, M.; Krajka-Kuźniak, V. Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. Int. J. Mol. Sci. 2024, 25, 11213. https://doi.org/10.3390/ijms252011213
Belka M, Gostyńska-Stawna A, Stawny M, Krajka-Kuźniak V. Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. International Journal of Molecular Sciences. 2024; 25(20):11213. https://doi.org/10.3390/ijms252011213
Chicago/Turabian StyleBelka, Marta, Aleksandra Gostyńska-Stawna, Maciej Stawny, and Violetta Krajka-Kuźniak. 2024. "Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease" International Journal of Molecular Sciences 25, no. 20: 11213. https://doi.org/10.3390/ijms252011213
APA StyleBelka, M., Gostyńska-Stawna, A., Stawny, M., & Krajka-Kuźniak, V. (2024). Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. International Journal of Molecular Sciences, 25(20), 11213. https://doi.org/10.3390/ijms252011213







