Calcium ATPase (PMCA) and GLUT-4 Upregulation in the Transverse Tubule Membrane of Skeletal Muscle from a Rat Model of Chronic Heart Failure
Abstract
1. Introduction
2. Results
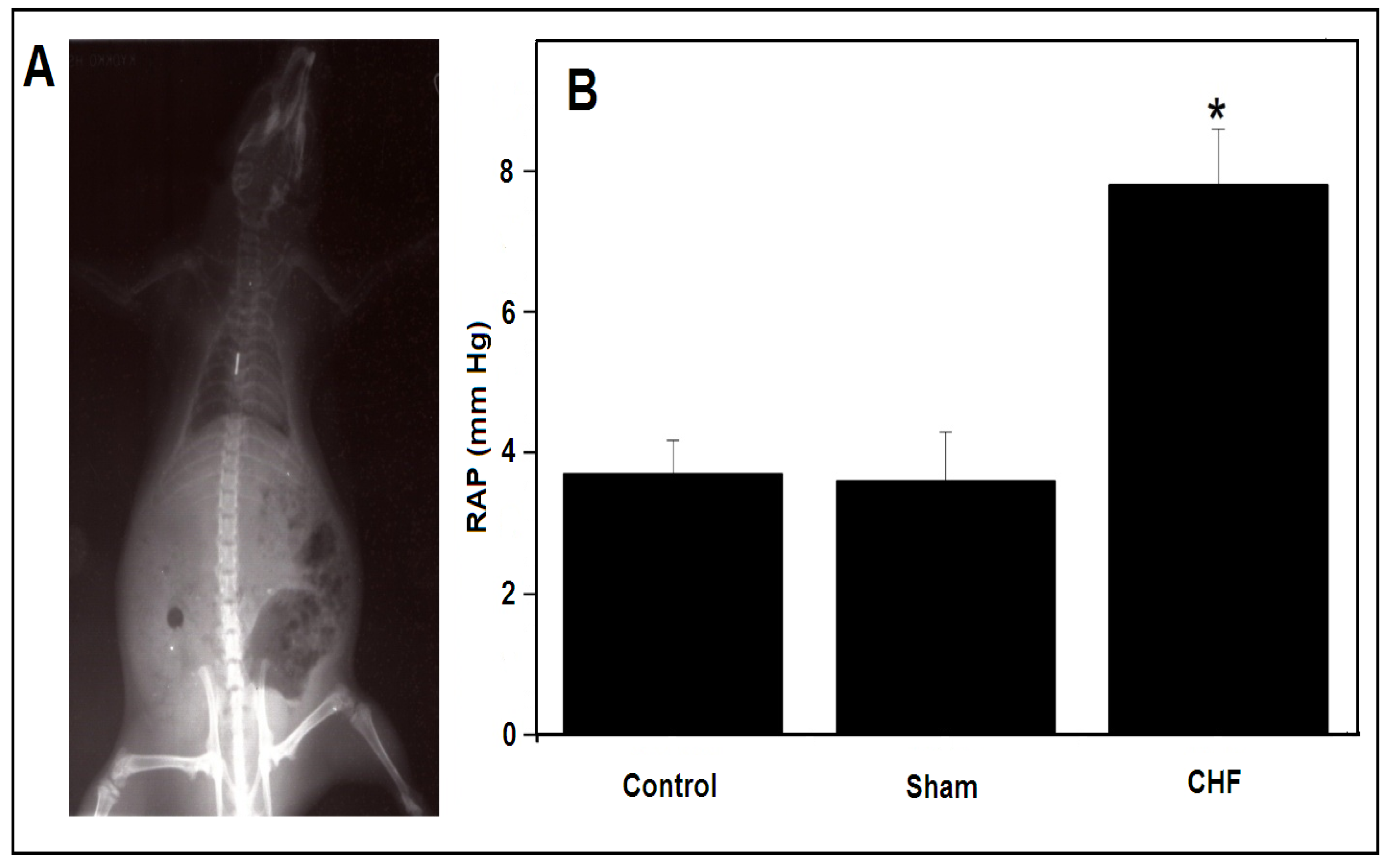
2.1. Myocardial Infarction and Parameters to Determine CHF
2.2. Plasma Concentration of Nitric Oxide
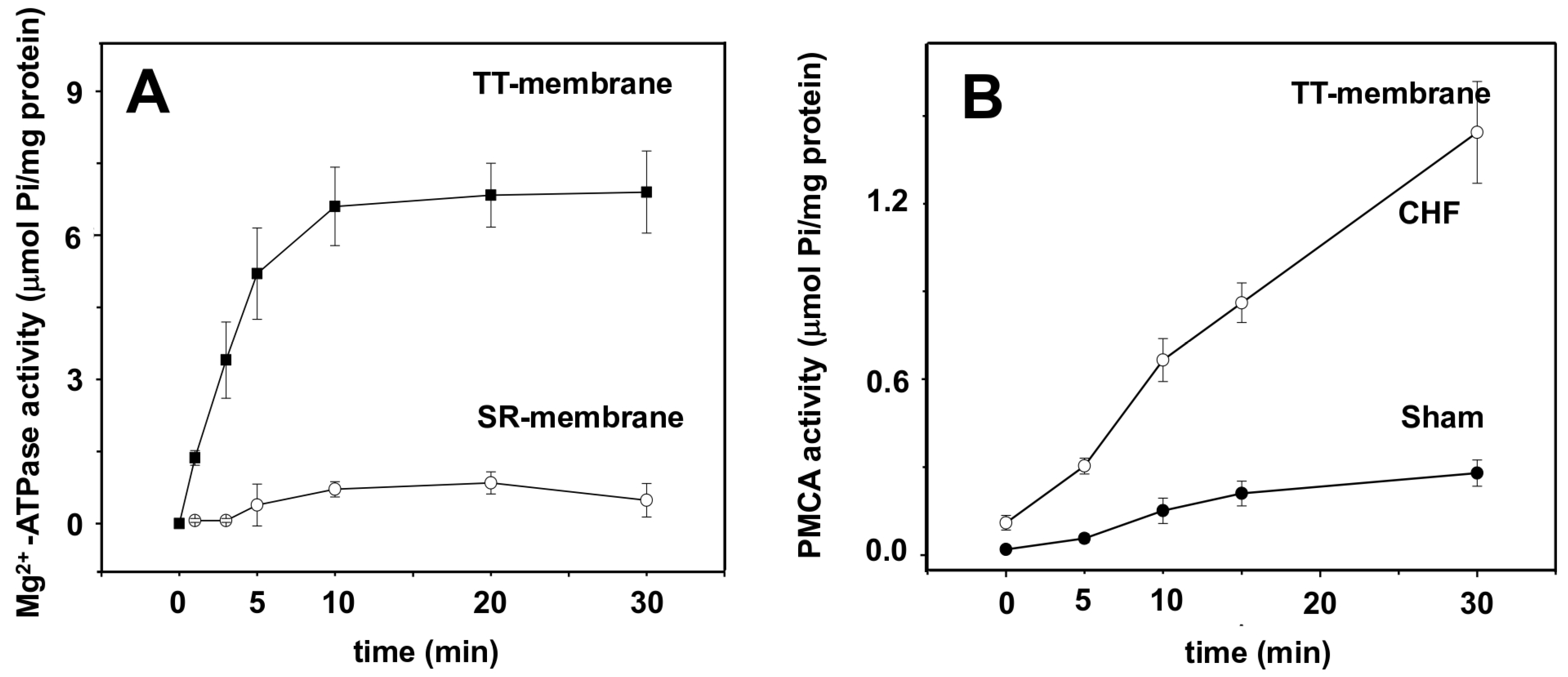
2.3. SERCA1 ATP Hydrolytic Activity and ATP-Dependent Ca2+ Uptake in the LSR
2.4. Transverse Tubule Membrane Mg2+-ATPase and PMCA Hydrolytic Activity
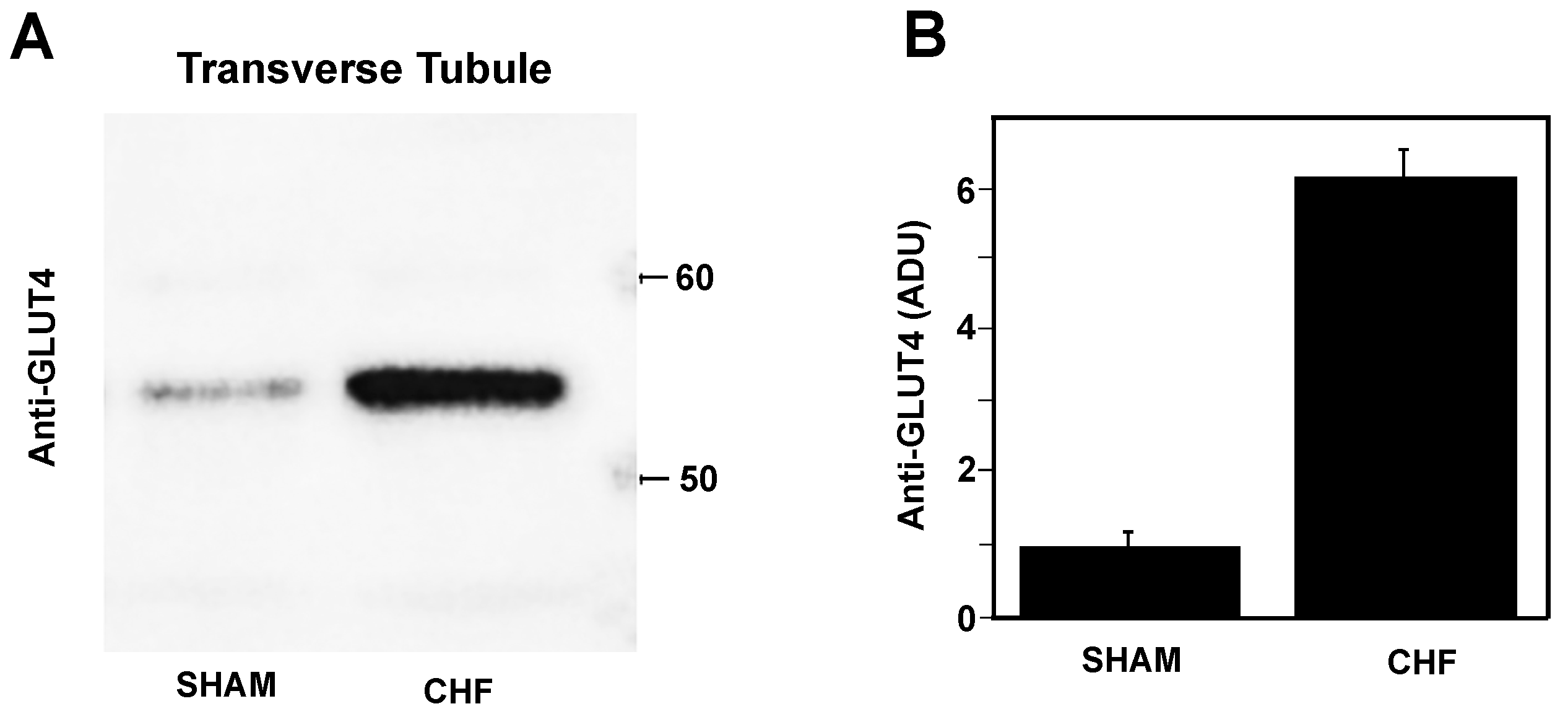
2.5. Glut4 Expression in Transverse Tubule Membranes
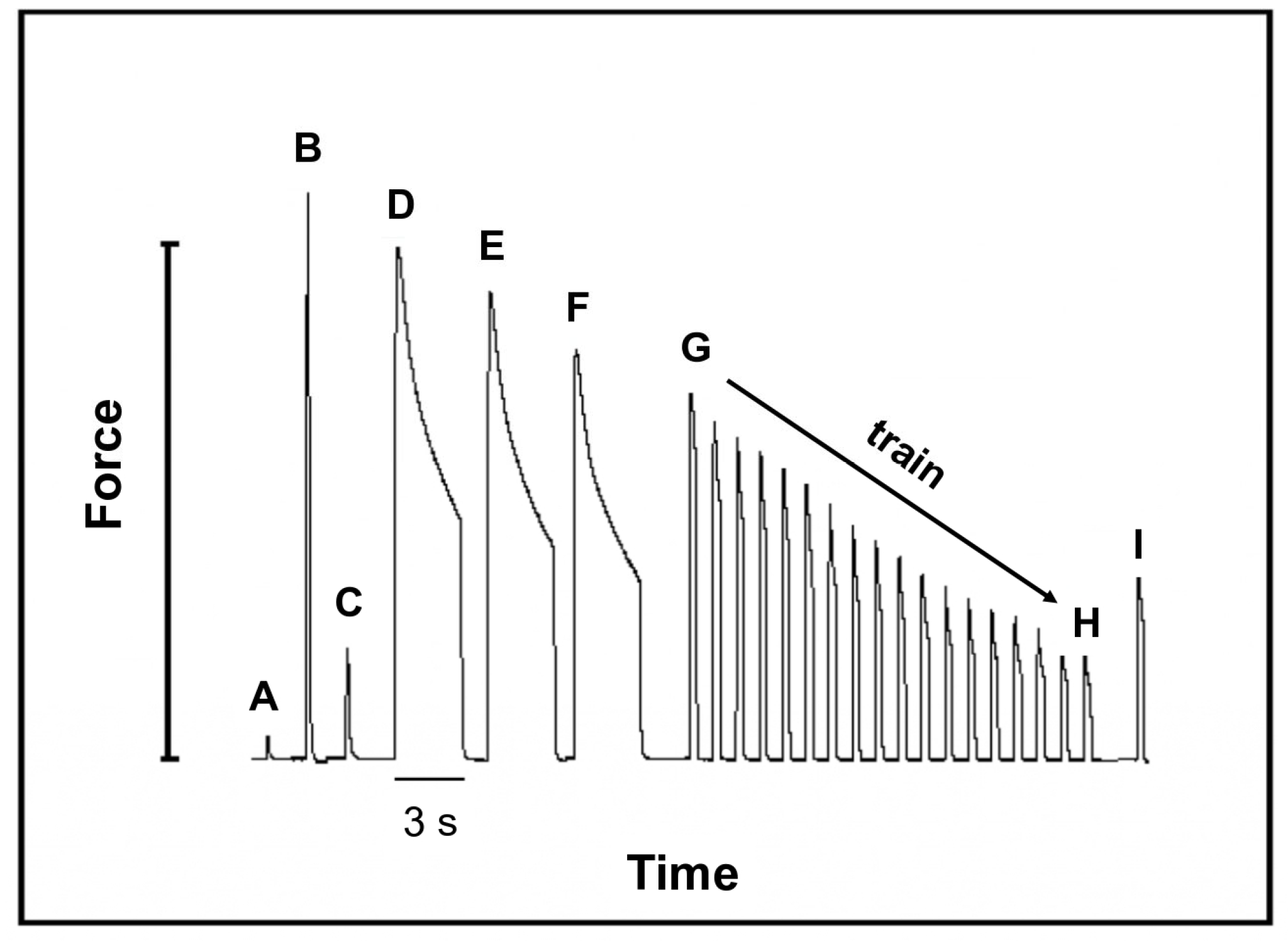
2.6. Mechanical Properties of Fast Skeletal Muscle
3. Discussion
3.1. RAP vs. Left Ventricular End-Diastolic Pressure (LVEDP) to Determine CHF
3.2. [NO] in the Vascular Bloodstream
3.3. SERCA1 Activity Is Not Affected by CHF
3.4. Mg2+-ATPase Activity Marker of TT Membranes Is Not Present in SR
3.5. Ca2+-ATPase (PMCA) Activity in TT Membranes Is Affected by CHF
3.6. TT Membrane GLUT4 Content in SM of CHF Rats
3.7. Mechanical Function in Fast SM Is Preserved after CHF
4. Materials and Methods
4.1. Animals
4.2. Surgical Protocol for Left Anterior Descending Artery Occlusion and Measurement of Right Auricular Pressure (RAP)
4.3. Electrocardiography Trace
4.4. Myocardial Infarct Size Measurement
4.5. Nitrite Determination
4.6. Tissue Sampling
4.7. Experimental Procedure for Muscle Force Recording
4.8. Mechanical Protocol
4.9. Isolation of T-Tubules and SR Membranes
4.10. Immunoblotting
4.11. SERCA1 and PMCA Hydrolytic Activity
4.12. Calcium Uptake
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alessia, L.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure—The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef]
- Perrault, C.L.; Gonzalez, H.; Litwin, S.; Sun, X.; Franzini, C.; Morgan, J. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibres from rats with chronic heart failure. Circ. Res. 1993, 73, 405–412. [Google Scholar] [CrossRef]
- Arnolda, L.; Brosnan, B.; Rajagolapan, B.; Radda, K. Skeletal muscle metabolism in heart failure in rats. Heart Circ. Physiol. 1991, 30, 434–442. [Google Scholar] [CrossRef]
- Rehn, T.; Borge, B.; Lunde, P.; Munkvik, M.; Sneve, M.; Grøndahl, F.; Aronsen, J.M.; Sjaastad, I.; Prydz, K.; Kolset, S.O.; et al. Temporary fatigue and altered extracellular matrix in skeletal muscle during progression of heart failure in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 26–33. [Google Scholar] [CrossRef]
- Coirault, C.; Guellich, A.; Barbry, T.; Samuel, J.; Riou, B.; Lecarpentier, Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 1009–1017. [Google Scholar] [CrossRef]
- Williams, J.; Ward, C.; Spangenburg, E.; Nelson, R. Functional aspects of skeletal muscle contractile apparatus and sarcoplasmic reticulum after fatigue. J. Appl. Physiol. 1998, 85, 619–626. [Google Scholar] [CrossRef][Green Version]
- Lunde, P.; Dahlstedt, A.; Bruton, J.; Lännergren, J.; Thorén, P.; Sejersted, O.; Westerblad, H. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circ. Res. 2001, 88, 1299–1305. [Google Scholar] [CrossRef]
- Lunde, P.; Verburg, E.; Vøllestad, N.; Sejersted, O. Skeletal muscle fatigue in normal subjects and heart failure patients. Is there a common mechanism? Acta Physiol. Scand. 1998, 162, 215–228. [Google Scholar] [CrossRef]
- Lunde, P.; Sejersted, O.; Thorud, H.; Tønnessen, T.; Henriksen, U.; Christensen, G.; Westerblad, H.; Bruton, J. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ. Res. 2006, 98, 1514–1519. [Google Scholar] [CrossRef]
- Musch, T.; Terrell, J. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: Rest and exercise. Am. J. Physiol. 1992, 262, 411–419. [Google Scholar] [CrossRef]
- Schiǿtz Thorud, H.; Lunde, P.; Nicolaysen, G.; Nicolaysen, A.; Helge, J.; Nilsson, G.; Sejersted, O. Muscle dysfunction during exercise of a single skeletal muscle in rats with congestive heart failure is not associated with reduced muscle blood supply. Acta Physiol. Scand. 2004, 181, 173–181. [Google Scholar] [CrossRef]
- Brunotte, F.; Thompson, C.H.; Adamopoulos, S.; Coats, A.; Unitt, J.; Lindsay, D.; Kaklamanis, L.; Radda, G.K.; Rajagopalan, B. Rat skeletal muscle metabolism in experimental heart failure: Effects of physical training. Acta Physiol. Scand. 1995, 154, 439–447. [Google Scholar] [CrossRef]
- Piepoli, M.; Scott, A.; Capucci, A.; Coats, A. Skeletal muscle training in chronic heart failure. Acta Physiol. Scand. 2001, 171, 295–303. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Coats, A.; Brunotte, F.; Arnolda, L.; Meyer, T.; Thompson, C.H.; Dunn, J.F.; Stratton, J.; Kemp, G.J.; Radda, G.K.; et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J. Am. Coll. Cardiol. 1993, 21, 1101–1106. [Google Scholar] [CrossRef]
- Pu, C.; Johnson, M.; Forman, D.; Hausdorff, J.; Roubenoff, R.; Foldvari, M.; Fielding, R.A.; Singh, M.A. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J. Appl. Physiol. 2001, 90, 2341–2350. [Google Scholar] [CrossRef]
- Bernocchi, P.; Cargnoni, A.; Vescovo, G.; Dalla Libera, L.; Parrinello, G.; Boraso, A.; Ceconi, C.; Ferrari, R. Skeletal muscle abnormalities in rats with experimentally induced heart hypertrophy and failure. Basic Res. Cardiol. 2003, 98, 114–123. [Google Scholar] [CrossRef]
- Vescovo, G.; Serafini, F.; Facchin, L.; Tenderini, P.; Carraro, U.; Dalla Libera, L.; Catani, C.; Ambrosio, G.B. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: Differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart 1996, 76, 337–343. [Google Scholar] [CrossRef]
- Toth, M.; Matthews, D.; Ades, P.; Tischler, M.; Van Buren, P.; Previs, M.; LeWinter, M. Skeletal muscle myofibrillar protein metabolism in heart failure: Relationship to immune activation and9functional capacity. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 685–692. [Google Scholar] [CrossRef]
- Mettauer, B.; Zoll, J.; Garnier, A.; Ventura-Clapier, R. Heart failure: A model of cardiac and skeletal muscle energetic failure. Pflugers Arch. 2006, 452, 653–666. [Google Scholar] [CrossRef]
- Rash, J.E.; Fambrough, D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during differentiation of L6 muscle cells. Biochem. Biophys. Res. Commun. 1991, 175, 652–659. [Google Scholar]
- Peachey, L.D. The sarcoplasmic reticulum and the transverse tubules of the frog’s Sartorius. J. Cell Biol. 1995, 25, 209–231. [Google Scholar] [CrossRef]
- Muñoz, P.; Rosemblatt, M.; Testar, X.; Palacín, M.; Thoidis, G.; Pilch, P.F.; Zorzano, A. The T-tubule is a cell-surface target for insulin-regulated recycling of membrane proteins in skeletal muscle. Biochem. J. 1995, 312, 393–400. [Google Scholar] [CrossRef]
- Burdett, E.; Beeler, T.; Klipp, A. Distribution of glucose transporters and insulin receptors in the plasma membrane and the transverse tubules of skeletal muscle. Arch Biochem. Biophys. 1987, 253, 279–286. [Google Scholar] [CrossRef]
- Ramírez-Oseguera, R.T.; Jiménez-Garduño, A.M.; Álvarez, R.; Heine, K.; Pinzón-Estrada, E.; Torres-Saldaña, I.; Ortega, A. Gestational Undernourishment Modifies the Composition of Skeletal Muscle Transverse Tubule Membranes and the Mechanical Properties of Muscles in Newborn Rats. Cell. Physiol. Biochem. 2013, 32, 1024–1039. [Google Scholar] [CrossRef]
- Toscano, A.E.; Manhñes-de-Castro, R.; Canon, F. Effect of a low-protein diet during pregnancy on skeletal muscle mechanical properties of offspring rats. Nutrition 2008, 24, 270–278. [Google Scholar] [CrossRef]
- Toscano, A.E.; Ferraz, K.M.; Manhñes-de-Castro, R.; Canon, F. Passive stiffness of rat skeletal muscle undernourished during fetal development. Clinics 2010, 65, 1363–1369. [Google Scholar] [CrossRef]
- Hambrecht, R.; Schulze, P.C.; Gielen, S.; Linke, A.; Möbius-Winkler, S.; Yu, J.; Kratzsch, J.Ü.; Baldauf, G.; Busse, M.W.; Schubert, A.; et al. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J. Am. Coll. Cardiol. 2002, 39, 1175–1181. [Google Scholar] [CrossRef]
- Hambrecht, R.; Schulze, P.C.; Gielen, S.; Linke, A.; Möbius-Winkler, S.; Erbs, S.; Kratzsch, J.; Schubert, A.; Adams, V.; Schuler, G. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 4, 401–406. [Google Scholar] [CrossRef]
- Lunde, P.; Verburg, E.; Eriksen, M.; Sejersted, O.M. Contractile properties of in situ perfused skeletal muscles from rats with congestive heart failure. J. Physiol. 2002, 540, 571–580. [Google Scholar] [CrossRef]
- Okada, Y.; Toth, M.J.; Vanburen, P. Skeletal muscle contractile protein function is preserved in human heart failure. J. Appl. Physiol. 2008, 104, 952–957. [Google Scholar] [CrossRef]
- Booth, F.W.; Tseng, B.S.; Flück, M.; Carson, J.A. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol. Scand. 1998, 162, 343–350. [Google Scholar] [CrossRef]
- Bezanilla, F.; Caputo, C.; Gonzalez-Serratos, H. Sodium dependence of the inward spread of activation in isolated twitch muscle fibers of the frog. J. Physiol. 1972, 223, 509–523. [Google Scholar] [CrossRef]
- Ortega, A.; Lepock, J.R. Use of thermal analysis to distinguish magnesium and calcium stimulated ATPase activity in isolated transverse tubules from skeletal muscle. Biochem. Biophys. A 1995, 1233, 7–13. [Google Scholar] [CrossRef][Green Version]
- Becker, V.; González-Serratos, H.; Álvarez, R.; Bäermann, M.; Irles, C.; Ortega, A. Effect of endurance exercise on the Ca2+ pumps from transverse tubule and sarcoplasmic reticulum of rabbit skeletal muscle. J. Appl. Physiol. 2004, 97, 467–474. [Google Scholar] [CrossRef]
- Sjaastad, I.; Sejersted, O.M.; Ilebekk, A.; Bjornerheim, R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J. Appl. Physiol. 2000, 89, 1445–1454. [Google Scholar] [CrossRef]
- Winlaw, D.S.; Keogh, A.M.; Schyvens, C.G.; Spratt, P.M.; Macdonald, P.S.; Smythe, G.A. Increased nitric oxide production in heart failure. Lancet 1994, 344, 373–374. [Google Scholar] [CrossRef]
- Hambrecht, R.; Adams, V.; Gielen, S.; Linke, A.; Möbius-Winkler, S.; Yu, J.; Niebauer, J.; Jiang, H.; Fiehn, E.; Schuler, G. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J. Am. Coll. Cardiol. 1999, 33, 174–179. [Google Scholar] [CrossRef]
- Issa, V.S.; Amaral, A.F.; Cruz, F.D.; Ayub-Ferreira, S.M.; Guimarães, G.V.; Chizzola, P.R.; Souza, G.E.; Bocchi, E.A. Glycemia and prognosis of patients with chronic heart failure—Subanalysis of the Long-term Prospective Randomized Controlled Study Using Repetitive Education at Six-Month Intervals and Monitoring for Adherence in Heart Failure Outpatients (REMADHE) trial. Am. Heart J. 2010, 159, 90–97. [Google Scholar] [CrossRef]
- Rush, J.W.E.; Green, H.J.; MacLean, D.A.; Code, L.M. Oxidative stress and nitric oxide synthase in skeletal muscles of rats with post-infarction, compensated chronic heart failure. Acta Physiol. Scand. 2005, 185, 211–218. [Google Scholar] [CrossRef]
- Didion, S.P.; Mayhan, W.G. Effect of chronic myocardial infarction on in vivo reactivity of skeletal muscle arterioles. Am. J. Physiol. 1997, 272 Pt 2, 2403–2408. [Google Scholar] [CrossRef]
- Cunha, T.F.; Bacurau, A.V.; Moreira, J.B.; Paixao, N.A.; Campos, J.C. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS ONE 2012, 7, e41701. [Google Scholar] [CrossRef]
- Vázquez, P.; Tirado-Cortés, T.; Alvarez, R.; Ronjat, M.; Amaya, A.; Ortega, A. Reversible oxidation of vicinal-thiols motif in sarcoplasmic reticulum calcium regulatory proteins is involved in muscle fatigue mechanism. Cell Calcium 2016, 60, 245–255. [Google Scholar] [CrossRef]
- Williams, J.H.; Ward, C.W. Changes in skeletal muscle sarcoplasmic reticulum function and force production following myocardial infarction in rats. Exp. Physiol. 1998, 83, 85–94. [Google Scholar] [CrossRef]
- Saborido, A.; Moros, G.; Megias, A. Transverse Tubule Mg2+-ATPase of Skeletal Muscle. Evidence for extracellular orientation of the chicken and rabbit enzymes. J. Biol. Chem. 1991, 266, 23490–23498. [Google Scholar]
- Gonzalez-Serratos, H.; Hilgemann, D.K.; Rozycka, M.; Gauthier, A.; Rasgado-Flores, H. Na+-Ca2+ exchange studies in sarcolemmal skeletal muscle. Ann. N. Y. Acad. Sci. 1996, 79, C556–C560. [Google Scholar] [CrossRef]
- Somlyo, A.V.; McClellan, G.; Gonzalez-Serratos, H.; Somlyo, A.P. Electron probe X-ray microanalysis of post-tetanic Ca2+ and Mg2+ movements across the sarcoplasmic reticulum in situ. J. Biol. Chem. 1985, 260, 6801–6807. [Google Scholar] [CrossRef]
- Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 5 October 2024).
- Johns, T.; Olson, B. Experimental myocardial infarction. A method of coronary occlusion in small animals. Ann. Surg. 1954, 140, 675–682. [Google Scholar] [CrossRef]
- Klein, H.; Puschmann, S.; Schaper, J.; Schaper, W. The mechanism of the tetrazolium reaction in identifying experimental myocardial infarction. Virchows Arch. A Pathol. Anat. Histopathol. 1981, 393, 287–297. [Google Scholar] [CrossRef]
- Tarpey, M.; Wink, D.; Grisham, M. Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, 431–444. [Google Scholar] [CrossRef]
- Vega-Moreno, J.; Tirado-Cortes, A.; Alvarez, R.; Irles, C.; Mas-Oliva, J.; Ortega, A. Cholesterol depletion uncouples β-dystroglycans from discrete sarcolemmal domains, reducing the mechanical activity of skeletal muscle. Cell. Physiol. Biochem. 2012, 29, 905–918. [Google Scholar] [CrossRef]
- Solares-Perez, A.; Alvarez, R.; Crosbie, R.H.; Vega-Moreno, J.; Medina-Monares, J.; Estrada, F.J.; Ortega, A.; Coral-Vazquez, R. Altered calcium pump and secondary deficiency of γ-sarcoglycan and microspan in sarcoplasmic reticulum membranes isolated from δ-sarcoglycan knockout mice. Cell Calcium 2010, 48, 28–36. [Google Scholar] [CrossRef]
- Ramirez-Soto, I.; Ortega-Aguilar, A. Skeletal Muscle is a Source of α-Synuclein with a Sarcolemmal Non-Lipid Raft Distribution. Cell. Physiol. Biochem. 2022, 56, 382–400. [Google Scholar] [CrossRef]
- Lanzetta, P.A.; Alvarez, L.J.; Reinach, P.S.; Candia, O.A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979, 100, 95–97. [Google Scholar] [CrossRef]
- Rosemblatt, M.; Hidalgo, C.; Vergara, C.; Ikemoto, N. Immunological and bio- chemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J. Biol. Chem. 1981, 256, 8140–8148. [Google Scholar] [CrossRef]


| Sham | CHF | |
|---|---|---|
| Initial body wt (g) | 277.5 ± 9.2 | 262.6 ± 15.8 |
| Final body wt (FBW) (g) | 442 ± 45.8 | 458.1 ± 45 |
| RAP (mm Hg) | 3.6 ± 0.7 | 7.8 ± 0.8 * |
| Liver wt (mg) | 16686 ± 3043 | 15910 ± 2902 |
| Heart wt (mg) | 1375 ± 221 | 1376 ± 150 |
| LV wt (mg) | 595.6 ± 105 | 596.3 ± 82 |
| RV wt (mg) | 436.3 ± 66 | 381.8 ± 71 |
| Septum wt (mg) | 313.8 ± 70 | 296.8 ± 44 |
| Liver/FBW (mg/g) | 37.8 ± 6.1 | 34.62 ± 6.47 |
| Heart/FBW (mg/g) | 3.1 ± 0.31 | 3.05 ± 0.63 |
| LV/FBW (mg/g) | 1.31 ± 0.21 | 1.32 ± 0.3 |
| RV/FBW (mg/g) | 0.96 ± 0.13 | 0.84 ± 0.18 |
| Septum/FBW (mg/g) | 0.69 ± 0.14 | 0.65 ± 0.14 |
| Pleural effusion (μL) | 0 | 125 ± 35.4 * |
| Ascites (μL) | 0 | 171.7 ± 20.3 * |
| Extensor Digitorum Longus | Sham | CHF |
|---|---|---|
| [A] Twitch, optimum muscle length (1 V) (N) | 0.110 ± 0.032 | 0.135 ± 0.042 |
| [B] Tetanus, maximum force (N) | 1.358 ± 0.301 | 1.363 ± 0.338 |
| [C] Twitch (100 V) (N) | 0.101 ± 0.022 | 0.134 ± 0.035 * |
| [D] First tetanus, maximum force (N) | 1.261 ± 0.302 | 1.320 ± 0.403 |
| [D] First tetanus, minimum force (N) | 0.902 ± 0.373 | 1.036 ± 0.433 |
| [D] ∆ First tetanus (Max F − Min F) − residual force (%) | 69.8 ± 16.3 | 75.5 ± 15 |
| [E] Second tetanus, maximum force (N) | 1.099 ± 0.257 | 1.173 ± 0.387 |
| [E] Second tetanus, minimum force (N) | 0.711 ± 0.261 | 0.920 ± 0.404 |
| [E] ∆ Second tetanus (Max F − Min F) − residual force (%) | 63.4 ± 12.3 | 75.5 ± 12.8 |
| [F] Third tetanus, maximum force (N) | 0.909 ± 0.243 | 1.038 ± 0.370 |
| [F] Third tetanus, minimum force (N) | 0.591 ± 0.320 | 0.768 ± 0.383 |
| [F] ∆ Third tetanus (Max F − Min F) − residual force (%) | 61.8 ± 17.5 | 70.8 ± 14.1 |
| [G] Train, tetanus (Max F: 100%) (N) | 0.793 ± 0.238 | 0.948 ± 0.287 |
| [H] Train, tetanus at 30% respect to Max F (N) | 0.24 ± 0.07 | 0.285 ± 0.091 |
| [H] Time to reach 30% of the initial force in train protocol (development of fatigue) (s) | 66 ± 10.1 | 68.2 ± 7.6 |
| [I] Recovery tetanus − 5 min (%) | 54 ± 8.4 | 52.9 ± 10.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitler, S.; Ramirez-Soto, I.; Jiménez-Graduño, A.; Ortega, A. Calcium ATPase (PMCA) and GLUT-4 Upregulation in the Transverse Tubule Membrane of Skeletal Muscle from a Rat Model of Chronic Heart Failure. Int. J. Mol. Sci. 2024, 25, 11180. https://doi.org/10.3390/ijms252011180
Gitler S, Ramirez-Soto I, Jiménez-Graduño A, Ortega A. Calcium ATPase (PMCA) and GLUT-4 Upregulation in the Transverse Tubule Membrane of Skeletal Muscle from a Rat Model of Chronic Heart Failure. International Journal of Molecular Sciences. 2024; 25(20):11180. https://doi.org/10.3390/ijms252011180
Chicago/Turabian StyleGitler, Sofia, Ibrahim Ramirez-Soto, Aura Jiménez-Graduño, and Alicia Ortega. 2024. "Calcium ATPase (PMCA) and GLUT-4 Upregulation in the Transverse Tubule Membrane of Skeletal Muscle from a Rat Model of Chronic Heart Failure" International Journal of Molecular Sciences 25, no. 20: 11180. https://doi.org/10.3390/ijms252011180
APA StyleGitler, S., Ramirez-Soto, I., Jiménez-Graduño, A., & Ortega, A. (2024). Calcium ATPase (PMCA) and GLUT-4 Upregulation in the Transverse Tubule Membrane of Skeletal Muscle from a Rat Model of Chronic Heart Failure. International Journal of Molecular Sciences, 25(20), 11180. https://doi.org/10.3390/ijms252011180






