Hepatitis E Virus Infection in Patients with Systemic and Cutaneous Lupus Erythematosus
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
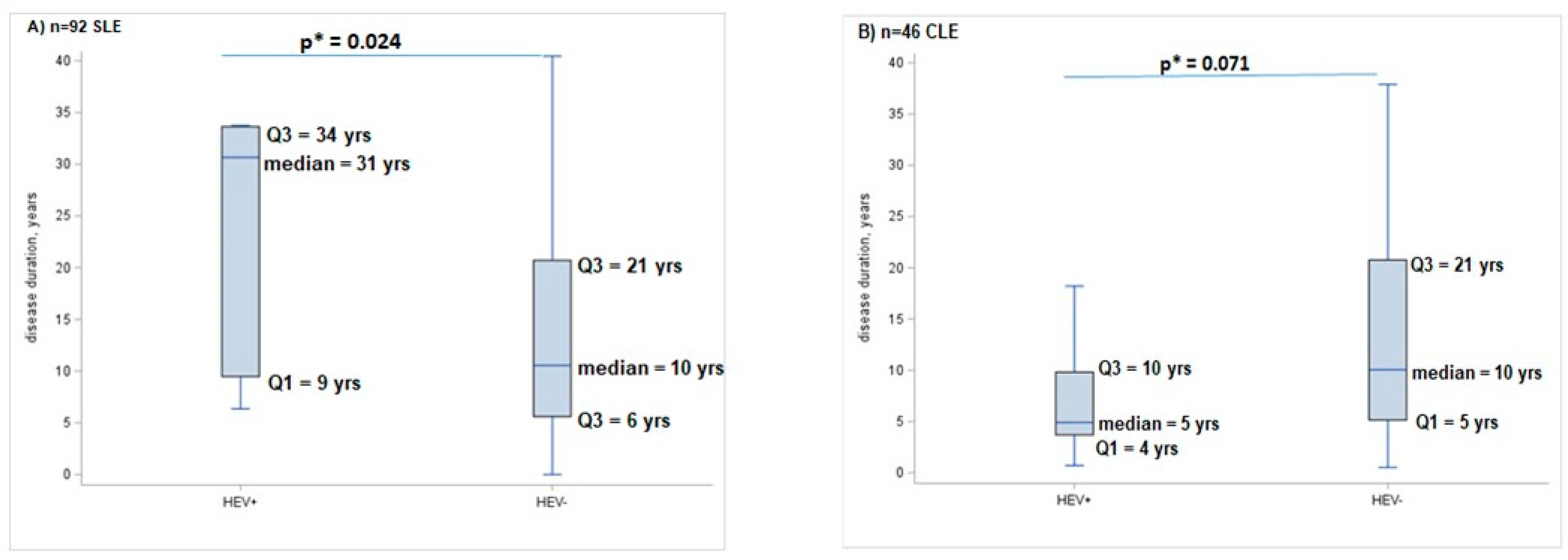
2.2. Hepatitis E Seroprevalence in SLE and CLE Patients
2.3. Factors Associated with HEV Infection among SLE and CLE Patients
3. Discussion
4. Materials and Methods
4.1. Patient Populations
4.2. Serological and Virological Screening HEV
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Perricone, C.; Borgiani, P.; Ciccacci, C.; Rufini, S.; Cipriano, E.; Alessandri, C.; Spinelli, F.R.; Scavalli, A.S.; Novelli, G.; et al. Genetic Factors in Systemic Lupus Erythematosus: Contribution to Disease Phenotype. J. Immunol. Res. 2015, 2015, 745647. [Google Scholar] [CrossRef]
- Nelson, P.; Rylance, P.; Roden, D.; Trela, M.; Tugnet, N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus 2014, 23, 596e605. [Google Scholar] [CrossRef]
- Patel, J.; Borucki, R.; Werth, V.P. An Update on the Pathogenesis of Cutaneous Lupus Erythematosus and Its Role in Clinical Practice. Curr. Rheumatol. Rep. 2020, 22, 69. [Google Scholar] [CrossRef]
- Garelli, C.J.; Refat, M.A.; Nanaware, P.P.; Ramirez-Ortiz, Z.G.; Rashighi, M.; Richmond, J.M. Current Insights in Cutaneous Lupus Erythematosus Immunopathogenesis. Front. Immunol. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis E. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef]
- Donnelly, M.C.; Scobie, L.; Crossan, C.L.; Dalton, H.; Hayes, P.C.; Simpson, K.J. Review article: Hepatitis E—A concise review of virology, epidemiology, clinical presentation and therapy. Aliment. Pharmacol. Ther. 2017, 47, 126–141. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef]
- Izopet, J.; Tremeaux, P.; Marion, O.; Migueres, M.; Capelli, N.; Chapuy-Regaud, S.; Mansuy, J.M.; Abravanel, F.; Kamar, N.; Lhomme, S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019, 120, 20–26. [Google Scholar] [CrossRef]
- Spada, E.; Simeoni, M.; Martina, A.; Pati, I.; Villano, U.; Adriani, D.; D’Angiò, A.; Tritarelli, E.; Taffon, S.; Bellino, S.; et al. Prevalence and risk factors for hepatitis E virus infection in blood donors: A nationwide survey in Italy, 2017 to 2019. Euro Surveill. 2022, 27, 2100516. [Google Scholar] [CrossRef]
- Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis E seroprevalence in Europe: A meta-analysis. Viruses 2016, 8, 211. [Google Scholar] [CrossRef]
- Capai, L.; Falchi, A.; Charrel, R. Meta-analysis of human IgG anti-HEV seroprevalence in industrialized countries and a review of literature. Viruses 2019, 11, 84. [Google Scholar] [CrossRef]
- Spada, E.; Pupella, S.; Pisani, G.; Bruni, R.; Chionne, P.; Madonna, E.; Villano, U.; Simeoni, M.; Fabi, S.; Marano, G.; et al. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018, 16, 413–421. [Google Scholar]
- Praharaj, D.L.; Mallick, B.; Nath, P.; Panigrahi, S.C.; Padhan, P.; Sahu, N. Unusual Presentation of Systemic Lupus Erythematosus in a Young Male: A Case Report. J. Clin. Exp. Hepatol. 2021, 11, 264–269. [Google Scholar] [CrossRef]
- Elfert, K.A.; Qasim, H.M.; Faisal, M.M.; Elghazali, A.; Siddiqui, M.Y.A.; Petkar, M.; Sadik, N. Hepatitis E Viral Association with Autoimmune Hepatitis: A Viral Trigger or Cross-Reactivity. Case Rep. Gastroenterol. 2021, 15, 115–122. [Google Scholar] [CrossRef]
- Pischke, S.; Peron, J.M.; von Wulffen, M.; von Felden, J.; Höner Zu Siederdissen, C.; Fournier, S.; Lütgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Par, G.; et al. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses 2019, 11, 186. [Google Scholar] [CrossRef]
- Wu, J.; Guo, N.; Zhu, L.; Zhang, X.; Xiong, C.; Liu, J.; Xu, Y.; Fan, J.; Yu, J.; Pan, Q.; et al. Seroprevalence of AIH-related autoantibodies in patients with acute hepatitis E viral infection: A prospective case-control study in China. Emerg. Microbes Infect. 2020, 9, 332–340. [Google Scholar] [CrossRef]
- Thapa, R.; Biswas, B.; Mallick, D. Henoch-Schönlein purpura triggered by acute hepatitis E virus infection. J. Emerg. Med. 2010, 39, 218–219. [Google Scholar] [CrossRef]
- Dumoulin, F.L.; Liese, H. Acute hepatitis E virus infection and autoimmune thyroiditis: Yet another trigger? BMJ Case Rep. 2012, 2012, bcr1220115441. [Google Scholar] [CrossRef]
- Martínez-Artola, Y.; Poncino, D.; García, M.L.; Munné, M.S.; González, J.; García, D.S. Acute hepatitis E virus infection and association with a subacute thyroiditis. Ann. Hepatol. 2015, 14, 141–142. [Google Scholar] [CrossRef]
- Hui, A.Y.; Chan, H.L.Y.; Chan, F.K.L.; Leung, N.W.Y.; Sung, J.J.Y. Fulminant hepatic failure in a patient with inactive HBsAg carrier state, acute hepatitis E and thyrotoxicosis. Hepatol. Res. 2003, 27, 248–251. [Google Scholar] [CrossRef]
- Kong, S.J.; Min, S.K.; Kim, I.K.; Koo, H.; Park, I.I.; Han, J.P.; Lee, J.Y.; Kim, D.Y.; Lee, S.J.; Baik, G.H.; et al. Two cases of acute hepatitis E in patients with hyperthyroidism. Korean J. Gastroenterol. 2006, 47, 65–71. [Google Scholar]
- Yu, C.; Chang, C.; Zhang, J. Immunologic and genetic considerations of cutaneous lupus erythematosus: A comprehensive review. J. Autoimmun. 2013, 41, 34–45. [Google Scholar] [CrossRef]
- Braunstein, I.; Klein, R.; Okawa, J.; Werth, V.P. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br. J. Dermatol. 2012, 166, 971–975. [Google Scholar] [CrossRef]
- Nabatian, A.S.; Bashir, M.M.; Wysocka, M.; Sharma, M.; Werth, V.P. Tumor necrosis factor α release in peripheral blood mononuclear cells of cutaneous lupus and dermatomyositis patients. Arthritis Res. Ther. 2012, 14, R1. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y. Hepatitis E virus-associated Guillain-Barre syndrome: Revision of the literature. Brain Behav. 2020, 10, e01496. [Google Scholar] [CrossRef]
- Lou, H.; Ling, G.S.; Cao, X. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. J. Autoimmun. 2022, 132, 102861. [Google Scholar] [CrossRef]
- Kuhn, A.; Herrmann, M.; Kleber, S.; Beckmann-Welle, M.; Fehsel, K.; Martin-Villalba, A.; Lehmann, P.; Ruzicka, T.; Krammer, P.H.; Kolb-Bachofen, V. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheumatol. 2006, 54, 939–950. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, R.; Soomro, M.H.; Hu, F.; Du, F.; She, R. Hepatitis E virus induces hepatocyte apoptosis via mitochondrial pathway in Mongolian gerbils. Front. Microbiol. 2018, 9, 460. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Sanchez-Guerrero, J.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996, 39, 363–369. [Google Scholar] [CrossRef]
| Variables | OR * | (95% CI) | p-Value |
|---|---|---|---|
| part (a) CLE vs. SLE | |||
| HEV+ vs. HEV− | 3.82 | (1.37–10.65) | 0.010 |
| age (51–74) vs. (18–50) | 1.93 | (0.94–3.96) | 0.072 |
| males vs. females | 4.14 | (1.57–10.89) | 0.004 |
| part (b) CLE vs. SLE | |||
| HEV+ vs. HEV− | 2.88 | (0.96–8.68) | 0.060 |
| age (51–74) vs. (18–50) | 1.98 | (0.88–4.45) | 0.098 |
| males vs. females | 4.84 | (1.72–13.63) | 0.003 |
| (a) Risk Factors in CLE | OR | 95% CI | p-Value |
| contact with animals vs. no | 9.70 * | (0.10–961.45) | 0.332 |
| eating homemade sausages/uncooked meat vs. no | 2.07 | (0.22–19.35) | 0.524 |
| gardening/vegetable gardening vs. no | 0.97 | (0.24–3.95) | 0.963 |
| eating raw seafood vs. no | 8.25 * | (0.38–179.19) | 0.179 |
| (b) Risk Factors in SLE | OR | 95% CI | p-Value |
| contact with animals vs. no | 2.23 * | (0.02–30.98) | 0.679 |
| eating homemade sausages/uncooked meat vs. no | 0.45 | (0.09–2.29) | 0.425 |
| gardening/vegetable gardening vs. no | 2.21 | (0.46–10.51) | 0.319 |
| eating raw seafood vs. no | 0.45 | (0.09–2.15) | 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, F.; Dorrucci, M.; Pirone, C.; Mataj, E.; Garufi, C.; Farchi, F.; Bruni, R.; Villano, U.; Madonna, E.; Iaiani, G.; et al. Hepatitis E Virus Infection in Patients with Systemic and Cutaneous Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 11162. https://doi.org/10.3390/ijms252011162
Ceccarelli F, Dorrucci M, Pirone C, Mataj E, Garufi C, Farchi F, Bruni R, Villano U, Madonna E, Iaiani G, et al. Hepatitis E Virus Infection in Patients with Systemic and Cutaneous Lupus Erythematosus. International Journal of Molecular Sciences. 2024; 25(20):11162. https://doi.org/10.3390/ijms252011162
Chicago/Turabian StyleCeccarelli, Fulvia, Maria Dorrucci, Carmelo Pirone, Elida Mataj, Cristina Garufi, Francesca Farchi, Roberto Bruni, Umbertina Villano, Elisabetta Madonna, Giancarlo Iaiani, and et al. 2024. "Hepatitis E Virus Infection in Patients with Systemic and Cutaneous Lupus Erythematosus" International Journal of Molecular Sciences 25, no. 20: 11162. https://doi.org/10.3390/ijms252011162
APA StyleCeccarelli, F., Dorrucci, M., Pirone, C., Mataj, E., Garufi, C., Farchi, F., Bruni, R., Villano, U., Madonna, E., Iaiani, G., Ciccozzi, M., Ciccaglione, A. R., Conti, F., & Lo Presti, A. (2024). Hepatitis E Virus Infection in Patients with Systemic and Cutaneous Lupus Erythematosus. International Journal of Molecular Sciences, 25(20), 11162. https://doi.org/10.3390/ijms252011162







