Real-Time 3D Imaging and Inhibition Analysis of Human Serum Amyloid A Aggregations Using Quantum Dots
Abstract
1. Introduction
2. Results
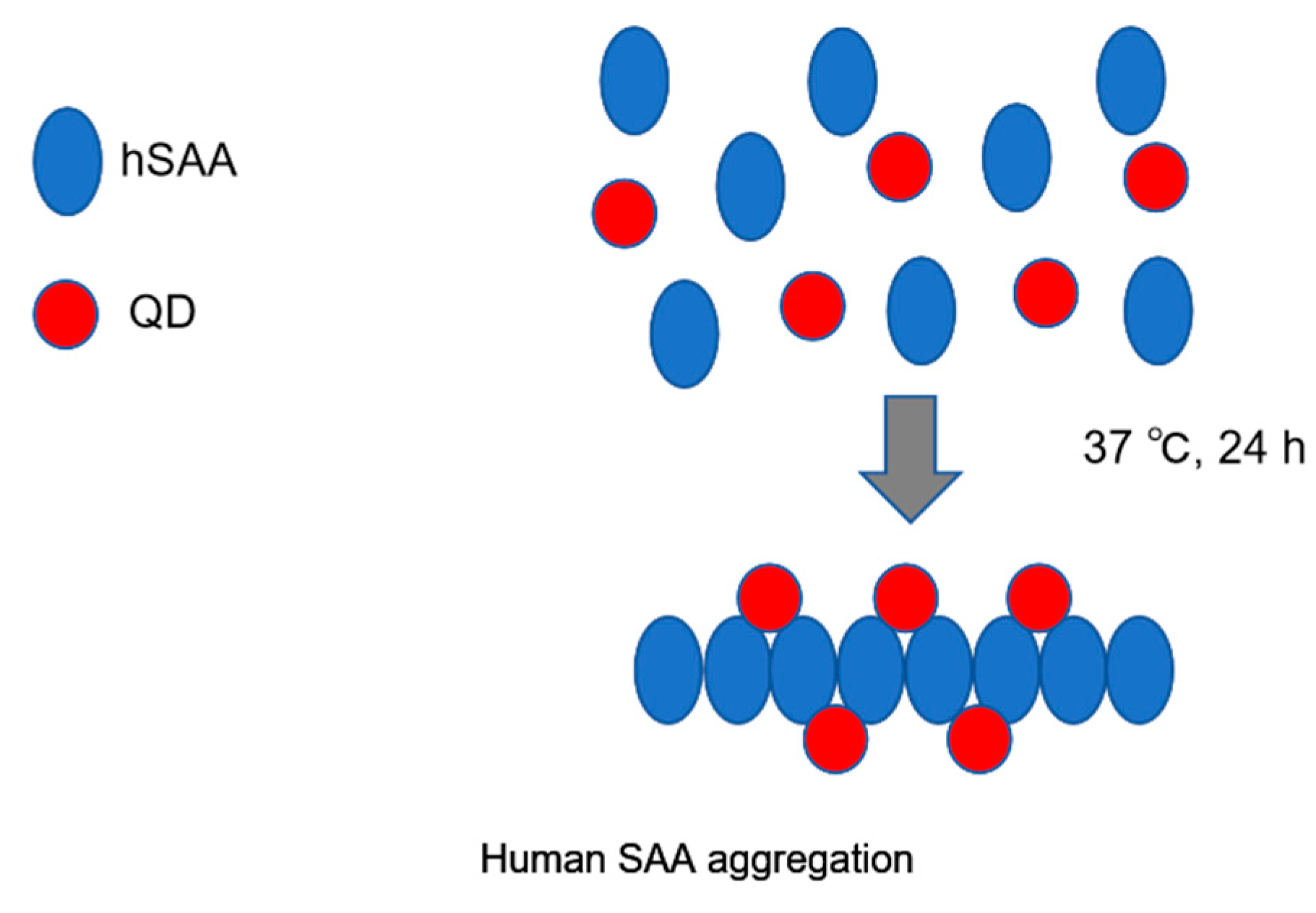
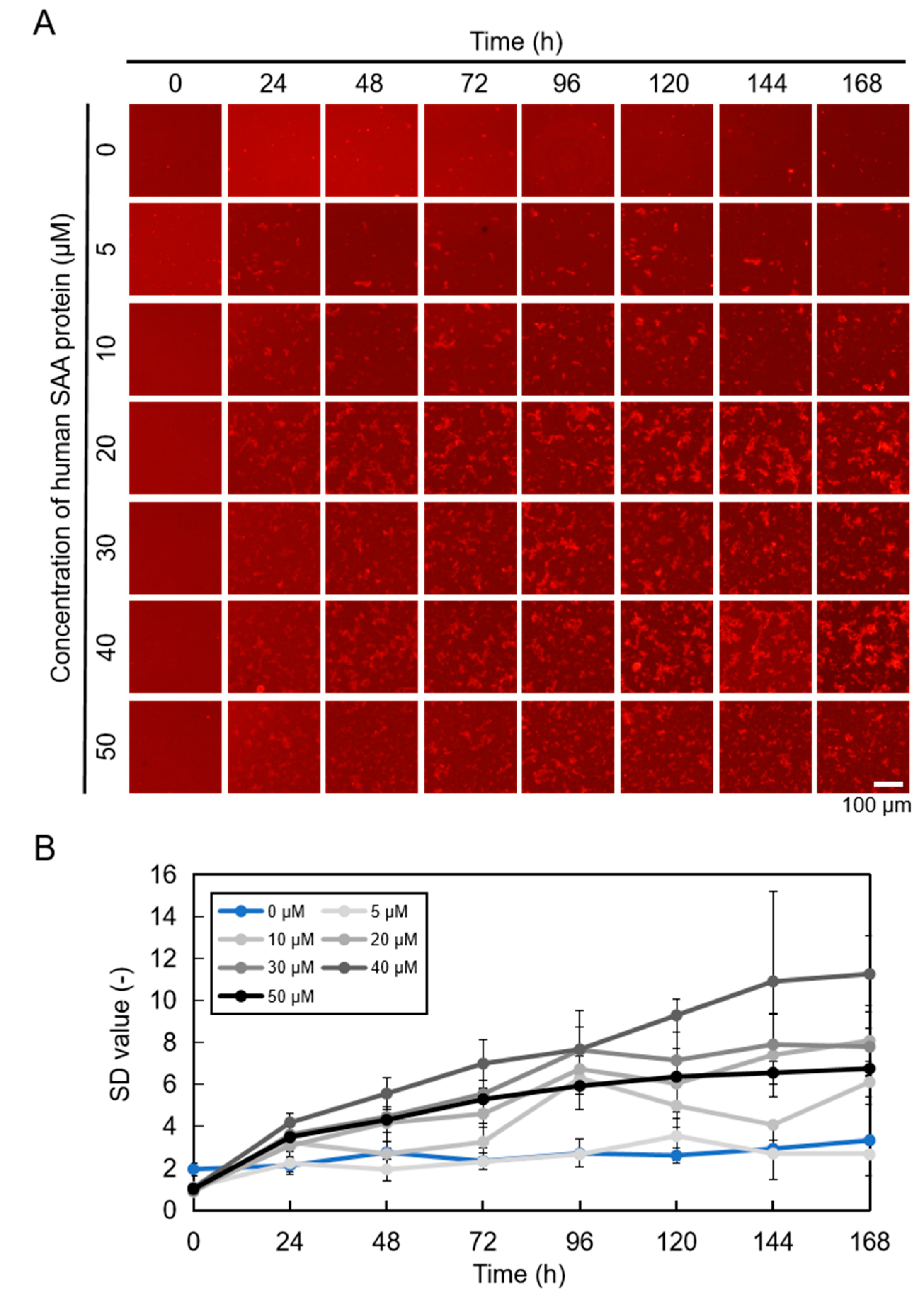
2.1. Imaging hSAA Aggregation Using QDs
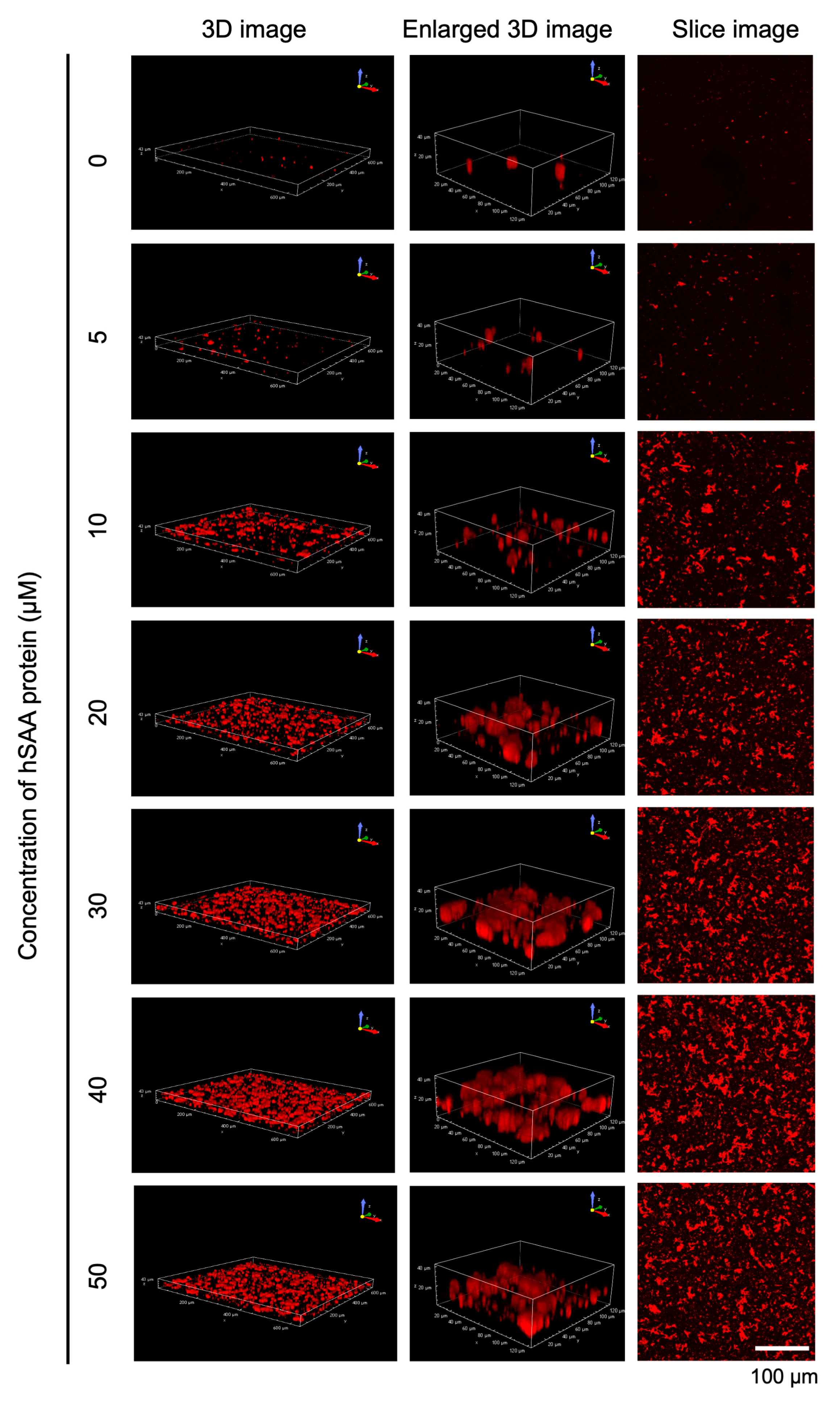
2.2. Three-Dimensional Observation of hSAA Aggregation
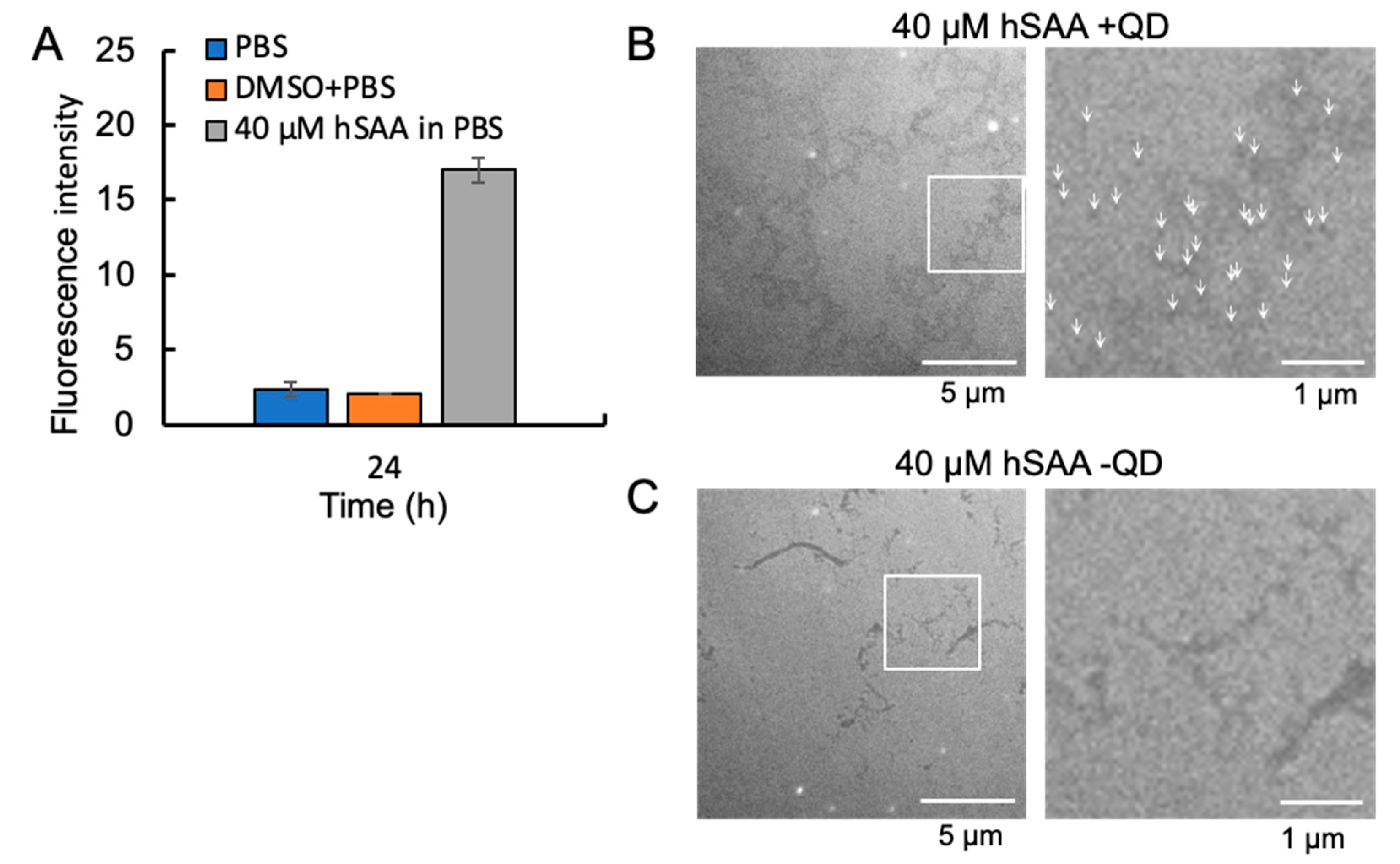
2.3. Thioflavin T (ThT) Fluorescence Measurement Results of hSAA Protein Aggregation
2.4. Visualization of hSAA Aggregation by TEM
2.5. Effect of RA on hSAA Aggregation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Imaging of hSAA Aggregation by QDs
4.3. ThT Assay
4.4. Observation of hSAA Aggregation by TEM
4.5. Measurement of hSAA and Aβ Aggregation Inhibitory Activity of RA Using the MSHTS System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, N.; Kaur, H.; Wajid, S. Renal amyloidosis: An update on diagnosis and pathogenesis. Protoplasma 2020, 257, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Grateau, G.; Jéru, I.; Rouaghe, S.; Cazeneuve, C.; Ravet, N.; Duquesnoy, P.; Cuisset, L.; Dodé, C.; Delpech, M.; Amselem, S. Amyloidosis and auto-inflammatory syndromes. Curr. Drug Targets Inflamm. Allergy 2005, 4, 57–65. [Google Scholar] [CrossRef]
- Dogan, A. Amyloidosis: Insights from proteomics. Annu. Rev. Pathol. 2017, 12, 277–304. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Vieira, M.N.; De Felice, F.G. Soluble protein oligomers as emerging toxins in Alzheimer’s and other amyloid diseases. IUBMB Life 2007, 59, 332–345. [Google Scholar] [CrossRef]
- Lin, X.; Galaqin, N.; Tainaka, R.; Shimamori, K.; Kuragano, M.; Noguchi, T.Q.P.; Tokuraku, K. Real-time 3D imaging and inhibition analysis of various amyloid aggregations using quantum dots. Int. J. Mol. Sci. 2020, 21, 1978. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.; Merlini, G.; Saraiva, M.J.; Westermark, P. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 2014, 21, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, S.A.; Khrabrova, M.S.; Anpilova, A.O.; Dobronravov, V.A.; Rubel, A.A. Noninvasive diagnostics of renal amyloidosis: Current state and perspectives. Int. J. Mol. Sci. 2022, 23, 12662. [Google Scholar] [CrossRef]
- Obici, L.; Merlini, G. AA amyloidosis: Basic knowledge, unmet needs and future treatments. Swiss. Med. Wkly. 2012, 142, w13580. [Google Scholar] [CrossRef]
- Papa, R.; Lachmann, H.J. Secondary AA amyloidosis. Rheum. Dis. Clin. N. Am. 2018, 44, 585–603. [Google Scholar] [CrossRef]
- Bansal, A.; Schmidt, M.; Rennegarbe, M.; Haupt, C.; Liberta, F.; Stecher, S.; Puscalau-Girtu, I.; Biedermann, A.; Fändrich, M. AA amyloid fibrils from diseased tissue are structurally different from in vitro formed SAA fibrils. Nat. Commun. 2021, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ye, R.D. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sack, G.H., Jr. Serum amyloid A (SAA) proteins. Subcell Biochem. 2020, 94, 421–436. [Google Scholar] [CrossRef]
- Yoshizaki, K. Pathogenic role of IL-6 combined with TNF-α or IL-1 in the induction of acute phase proteins SAA and CRP in chronic inflammatory diseases. Adv. Exp. Med. Biol. 2011, 691, 141–150. [Google Scholar] [CrossRef]
- Okuda, Y. AA amyloidosis—Benefits and prospects of IL-6 inhibitors. Mod. Rheumatol. 2019, 29, 268–274. [Google Scholar] [CrossRef]
- Wakai, M.; Hayashi, R.; Ueno, Y.; Onishi, K.; Takasago, T.; Uchida, T.; Takigawa, H.; Yuge, R.; Urabe, Y.; Oka, S.; et al. Promoting mechanism of serum amyloid a family expression in mouse intestinal epithelial cells. PLoS ONE 2022, 17, e0264836. [Google Scholar] [CrossRef]
- Murata, E.; Kozaki, S.; Murakami, T.; Shimizu, K.; Okada, A.; Ishiguro, N.; Inoshima, Y. Differential expression of serum amyloid A1 and A3 in bovine epithelia. J. Vet. Med. Sci. 2020, 82, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; May, K.S.; Zhang, X.S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: Evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef]
- Real de Asúa, D.; Costa, R.; Galván, J.M.; Filigheddu, M.T.; Trujillo, D.; Cadiñanos, J. Systemic AA amyloidosis: Epidemiology, diagnosis, and management. Clin. Epidemiol. 2014, 6, 369–377. [Google Scholar] [CrossRef]
- Tanaka, M.; Takarada, T.; Nadanaka, S.; Kojima, R.; Hosoi, K.; Machiba, Y.; Kitagawa, H.; Yamada, T. Influences of amino-terminal modifications on amyloid fibril formation of human serum amyloid A. Arch. Biochem. Biophys. 2023, 742, 109615. [Google Scholar] [CrossRef]
- Ugurlu, S.; Hacioglu, A.; Adibnia, Y.; Hamuryudan, V.; Ozdogan, H. Tocilizumab in the treatment of twelve cases with aa amyloidosis secondary to familial Mediterranean fever. Orphanet. J. Rare Dis. 2017, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Tanaka, H.; Akama, H.; Ogara, T.; Uwai, K.; Tokuraku, K. A microliter-scale high-throughput screening system with quantum-dot nanoprobes for amyloid-β aggregation inhibitors. PLoS ONE 2013, 8, e72992. [Google Scholar] [CrossRef]
- Tokuraku, K.; Marquardt, M.; Ikezu, T. Real-time imaging and quantification of amyloid-beta peptide aggregates by novel quantum-dot nanoprobes. PLoS ONE 2009, 4, e8492. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Tainaka, R.; Ando, Y.; Hashi, Y.; Deepak, H.V.; Suga, Y.; Murai, Y.; Anetai, M.; Monde, K.; Ohta, K.; et al. An automated microliter-scale high-throughput screening system (MSHTS) for real-time monitoring of protein aggregation using quantum-dot nanoprobes. Sci. Rep. 2019, 9, 2587. [Google Scholar] [CrossRef]
- Kuragano, M.; Yoshinari, W.; Lin, X.; Shimamori, K.; Uwai, K.; Tokuraku, K. Evaluation of amyloid β42 aggregation inhibitory activity of commercial dressings by a microliter-scale high-throughput screening system using quantum-dot nanoprobes. Foods 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef]
- Lin, X.; Watanabe, K.; Kuragano, M.; Kurotaki, Y.; Nakanishi, U.; Tokuraku, K. Dietary intake of rosmarinic acid increases serum inhibitory activity in amyloid A aggregation and suppresses deposition in the organs of mice. Int. J. Mol. Sci. 2020, 21, 6031. [Google Scholar] [CrossRef]
- Simons, J.P.; Al-Shawi, R.; Ellmerich, S.; Speck, I.; Aslam, S.; Hutchinson, W.L.; Mangione, P.P.; Disterer, P.; Gilbertson, J.A.; Hunt, T.; et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc. Natl. Acad. Sci. USA 2013, 110, 16115–16120. [Google Scholar] [CrossRef]
- Afjadi, M.N.; Dabirmanesh, B.; Uversky, V.N. Therapeutic approaches in proteinopathies. Prog. Mol. Biol. Transl. Sci. 2024, 206, 341–388. [Google Scholar] [CrossRef]
- Ankarcrona, M.; Winblad, B.; Monteiro, C.; Fearns, C.; Powers, E.T.; Johansson, J.; Westermark, G.T.; Presto, J.; Ericzon, B.G.; Kelly, J.W. Current and future treatment of amyloid diseases. J. Intern. Med. 2016, 280, 177–202. [Google Scholar] [CrossRef]
- Chung, H.; Brazil, M.I.; Soe, T.T.; Maxfield, F.R. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid beta-peptide by microglial cells. J. Biol. Chem. 1999, 274, 32301–32308. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kiyota, T.; Walsh, S.M.; Liu, J.; Kipnis, J.; Ikezu, T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J. Immunol. 2008, 181, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Stilwell, J.L.; Gerion, D.; Ding, L.; Elboudwarej, O.; Cooke, P.A.; Gray, J.W.; Alivisatos, A.P.; Chen, F.F. Cellular effect of high doses of silica-coated quantum dot profiled with high throughput gene expression analysis and high content cellomics measurements. Nano Lett. 2006, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, J.; Wang, S.; Wang, Y.; Liu, D.; Wang, J. Quantum dots in cell imaging and their safety issues. J. Mater. Chem. B 2021, 9, 5765–5779. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, P.; Yang, L.; Quimby, J.L.; Sun, Y.P. Carbon “quantum” dots for bioapplications. Exp. Biol. Med. 2022, 247, 300–309. [Google Scholar] [CrossRef]
- Taguchi, R.; Hatayama, K.; Takahashi, T.; Hayashi, T.; Sato, Y.; Sato, D.; Ohta, K.; Nakano, H.; Seki, C.; Endo, Y.; et al. Structure–activity relations of rosmarinic acid derivatives for the amyloid β aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 2017, 138, 1066–1075. [Google Scholar] [CrossRef]
- Desai, A.M.; Pandey, S.P.; Singh, P.K. Effect of counter-anions on the aggregation of Thioflavin-T. Phys. Chem. Chem. Phys. 2021, 23, 9948–9961. [Google Scholar] [CrossRef] [PubMed]
- Malisauskas, R.; Botyriute, A.; Cannon, J.G.; Smirnovas, V. Flavone derivatives as inhibitors of insulin amyloid-like fibril formation. PLoS ONE 2015, 10, e0121231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poblano, J.; Castillo-Tobías, I.; Berlanga, L.; Tamayo-Ordoñez, M.C.; Del Carmen Rodríguez-Salazar, M.; Silva-Belmares, S.Y.; Aguayo-Morales, H.; Cobos-Puc, L.E. Drugs targeting APOE4 that regulate beta-amyloid aggregation in the brain: Therapeutic potential for Alzheimer’s disease. Basic Clin. Pharmacol. Toxicol. 2024, 135, 237–249. [Google Scholar] [CrossRef]
- Sosnowska, M.; Skibiszewska, S.; Kamińska, E.; Wieczerzak, E.; Jankowska, E. Designing peptidic inhibitors of serum amyloid A aggregation process. Amino Acids 2016, 48, 1069–1078. [Google Scholar] [CrossRef]
- Jana, A.K.; Greenwood, A.B.; Hansmann, U.H.E. Small peptides for inhibiting serum amyloid A aggregation. ACS Med. Chem. Lett. 2021, 12, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Rochet, J.C.; Bieganski, R.M.; Lansbury, P.T., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Huanood, G.; Miura, S.; Kuragano, M.; Tokuraku, K. Real-Time 3D Imaging and Inhibition Analysis of Human Serum Amyloid A Aggregations Using Quantum Dots. Int. J. Mol. Sci. 2024, 25, 11128. https://doi.org/10.3390/ijms252011128
Shi L, Huanood G, Miura S, Kuragano M, Tokuraku K. Real-Time 3D Imaging and Inhibition Analysis of Human Serum Amyloid A Aggregations Using Quantum Dots. International Journal of Molecular Sciences. 2024; 25(20):11128. https://doi.org/10.3390/ijms252011128
Chicago/Turabian StyleShi, Liangquan, Gegentuya Huanood, Shuto Miura, Masahiro Kuragano, and Kiyotaka Tokuraku. 2024. "Real-Time 3D Imaging and Inhibition Analysis of Human Serum Amyloid A Aggregations Using Quantum Dots" International Journal of Molecular Sciences 25, no. 20: 11128. https://doi.org/10.3390/ijms252011128
APA StyleShi, L., Huanood, G., Miura, S., Kuragano, M., & Tokuraku, K. (2024). Real-Time 3D Imaging and Inhibition Analysis of Human Serum Amyloid A Aggregations Using Quantum Dots. International Journal of Molecular Sciences, 25(20), 11128. https://doi.org/10.3390/ijms252011128





