Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery
Abstract
1. Introduction
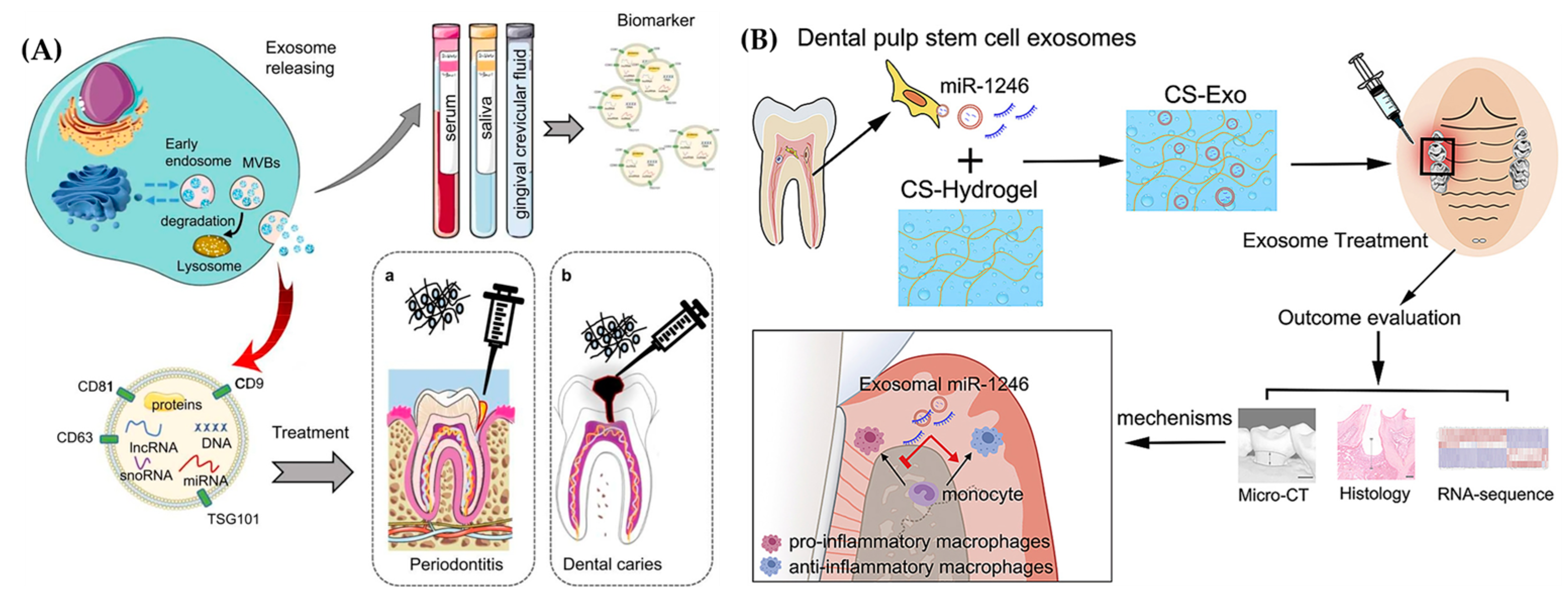
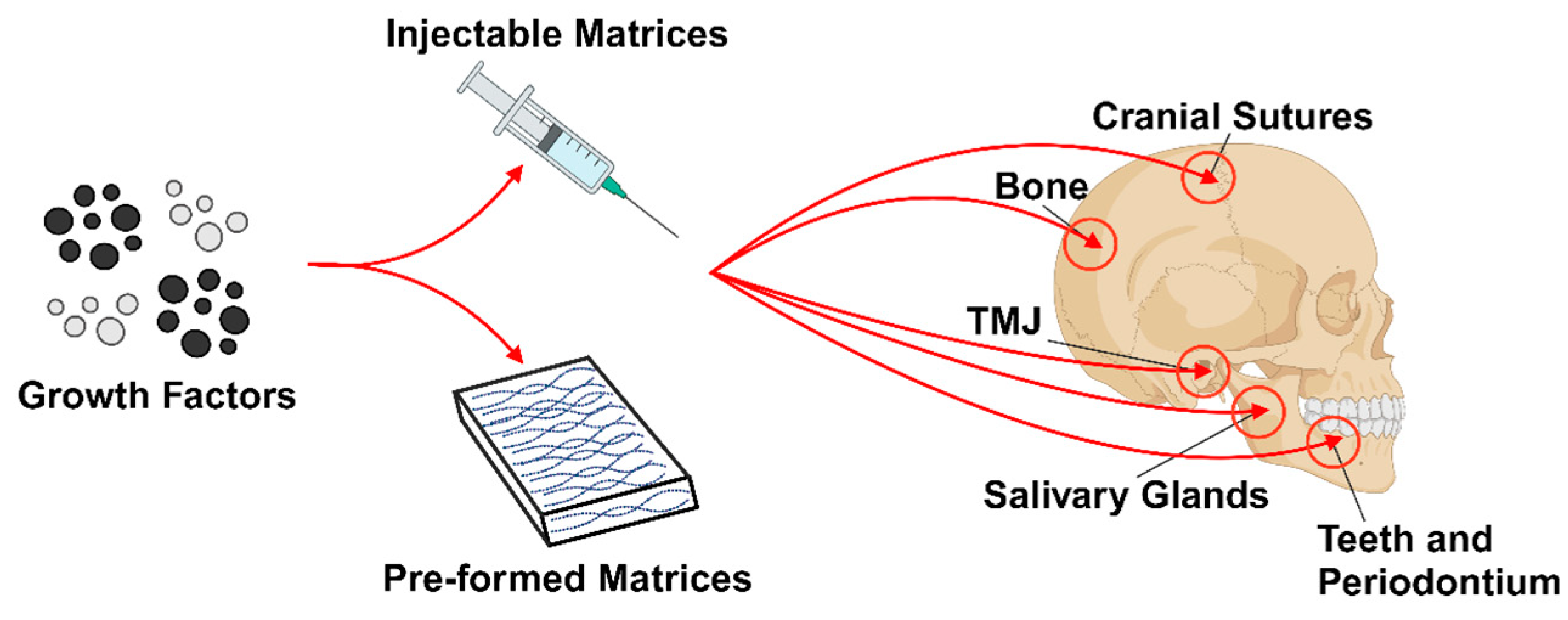
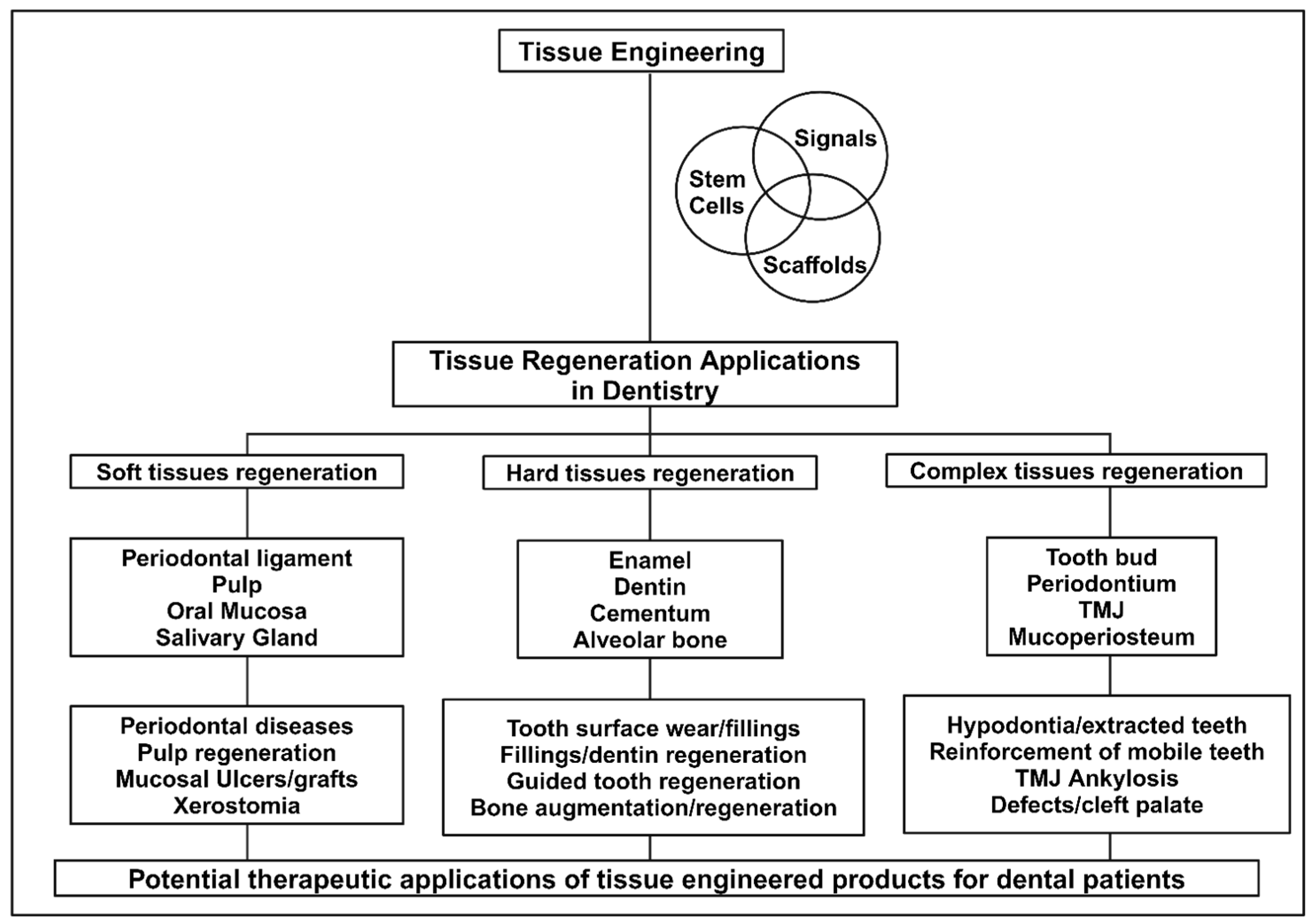
2. Oral Tissue Engineering
2.1. Hydrogels for Oral Tissue Engineering
2.2. Exosomes for Oral Tissue Engineering
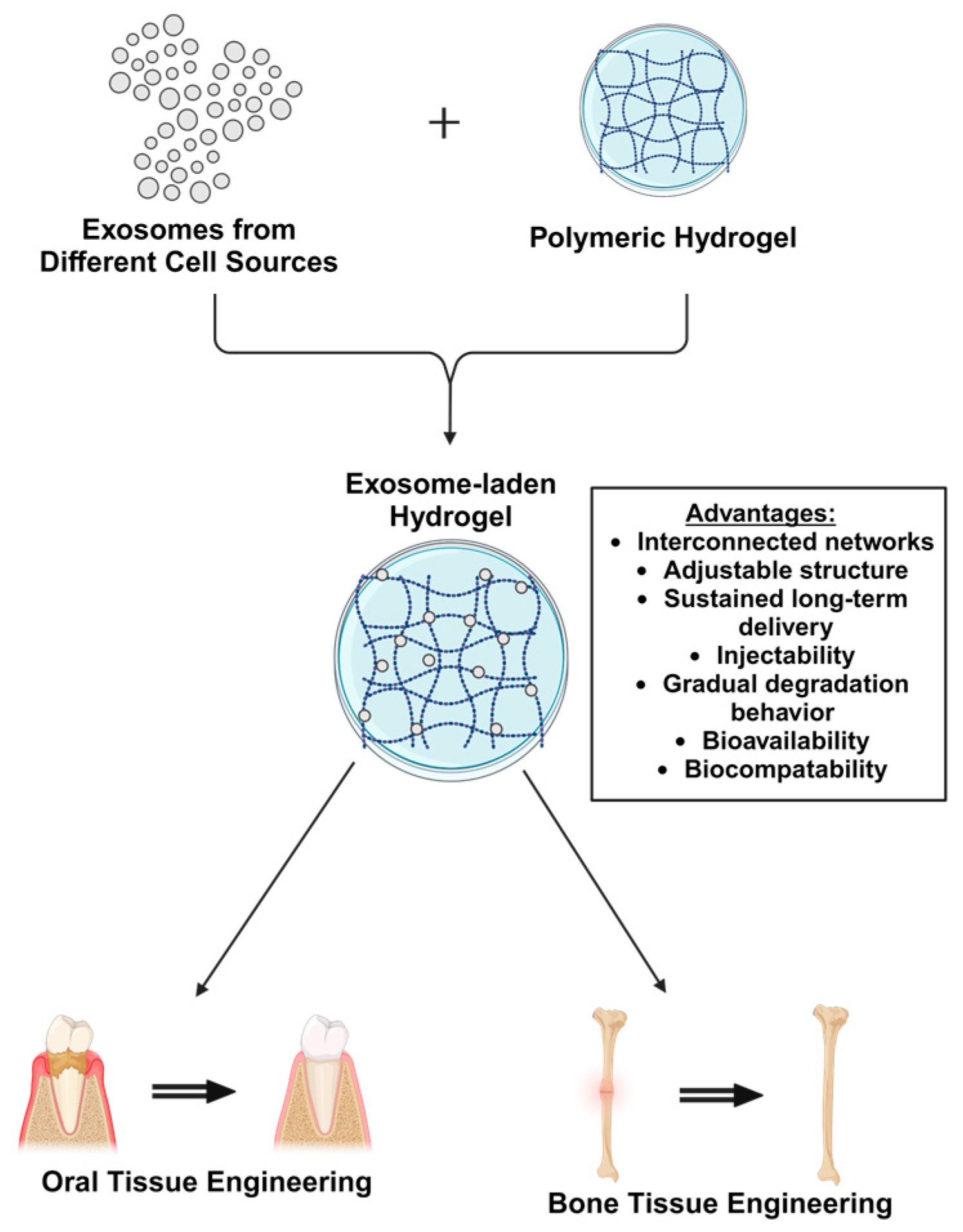
2.3. Exosome Laden Hydrogels for Oral Tissue Engineering
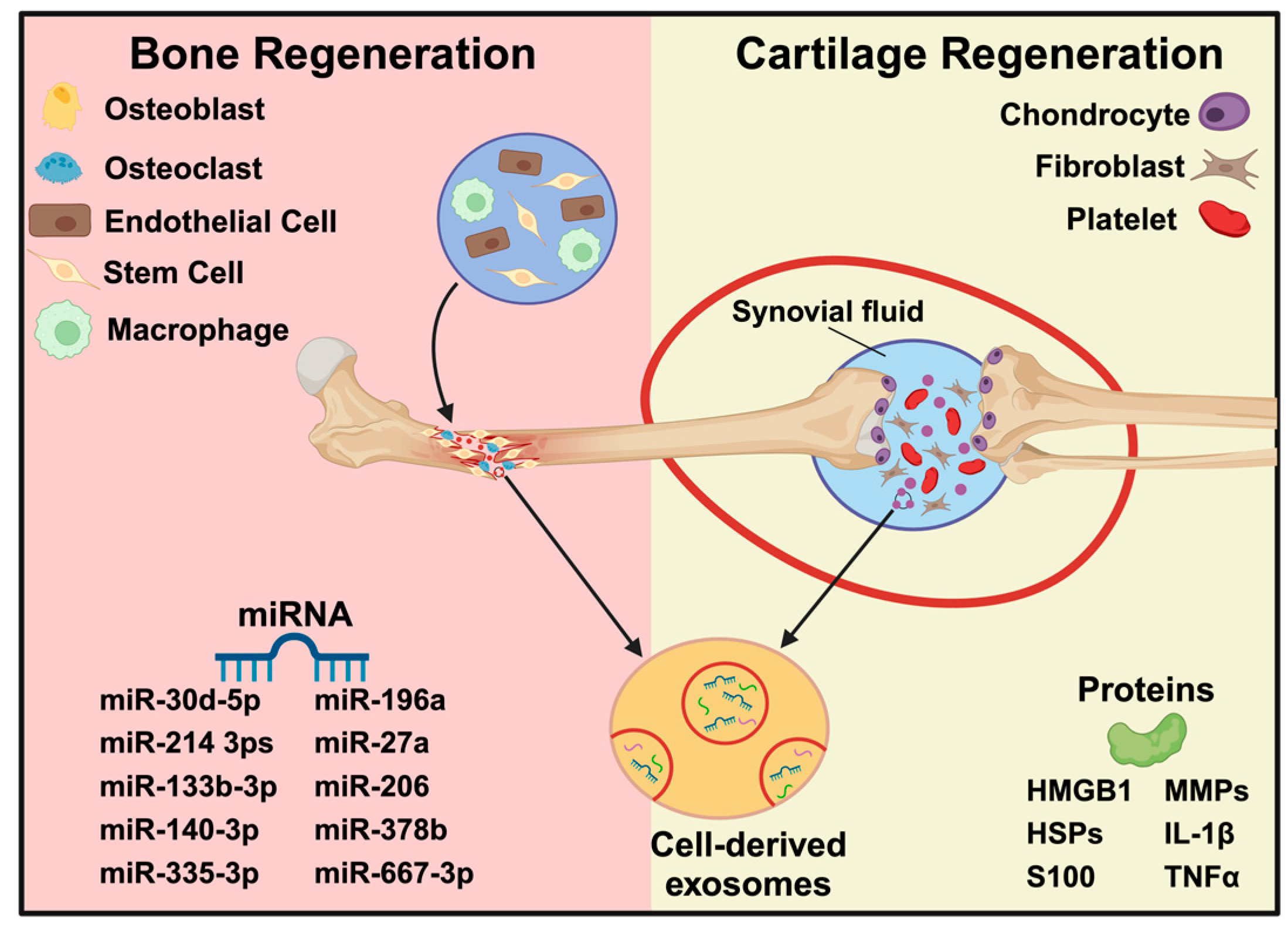
3. Bone Tissue Engineering
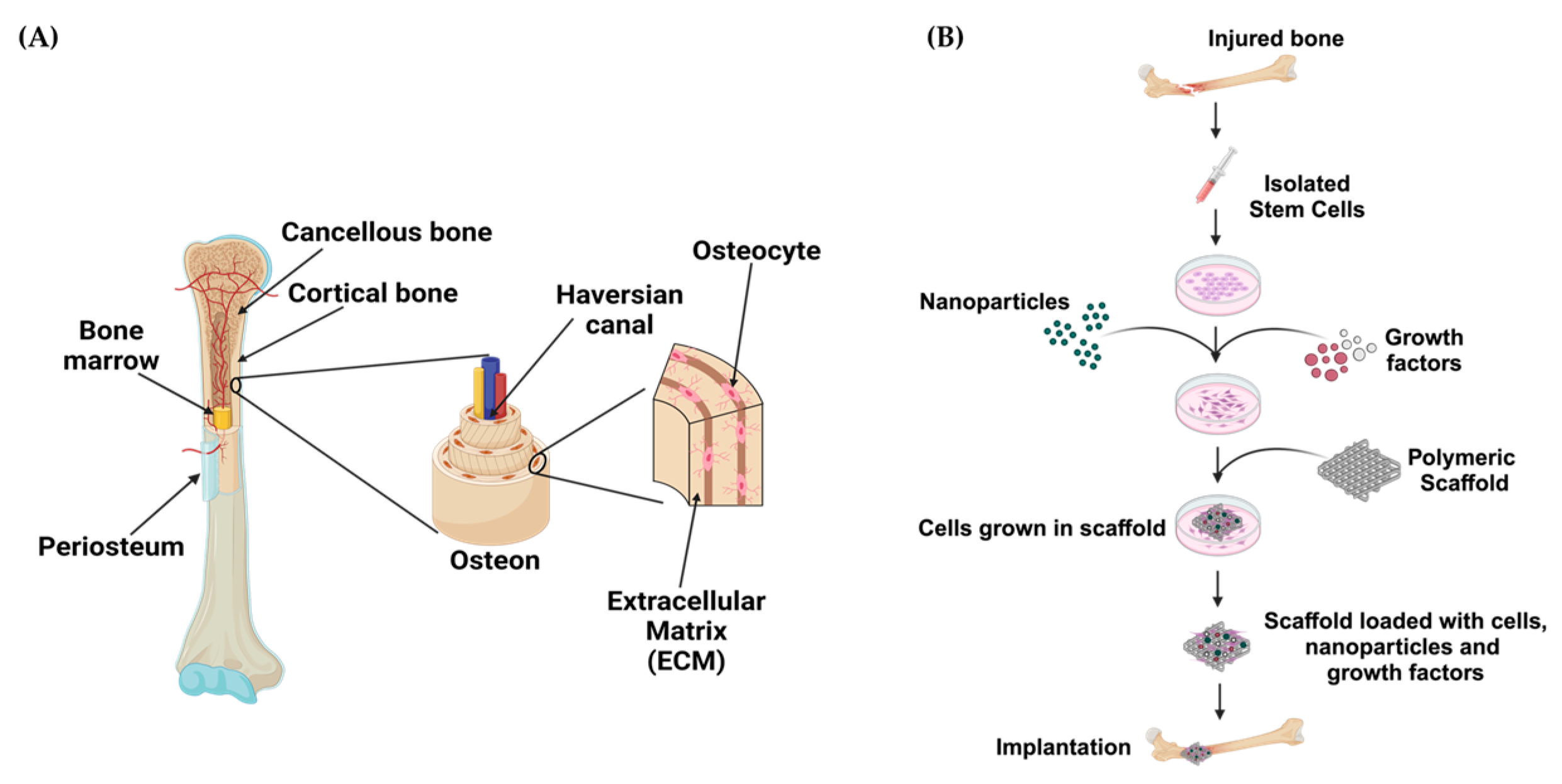
3.1. Hydrogels for Bone Tissue Engineering
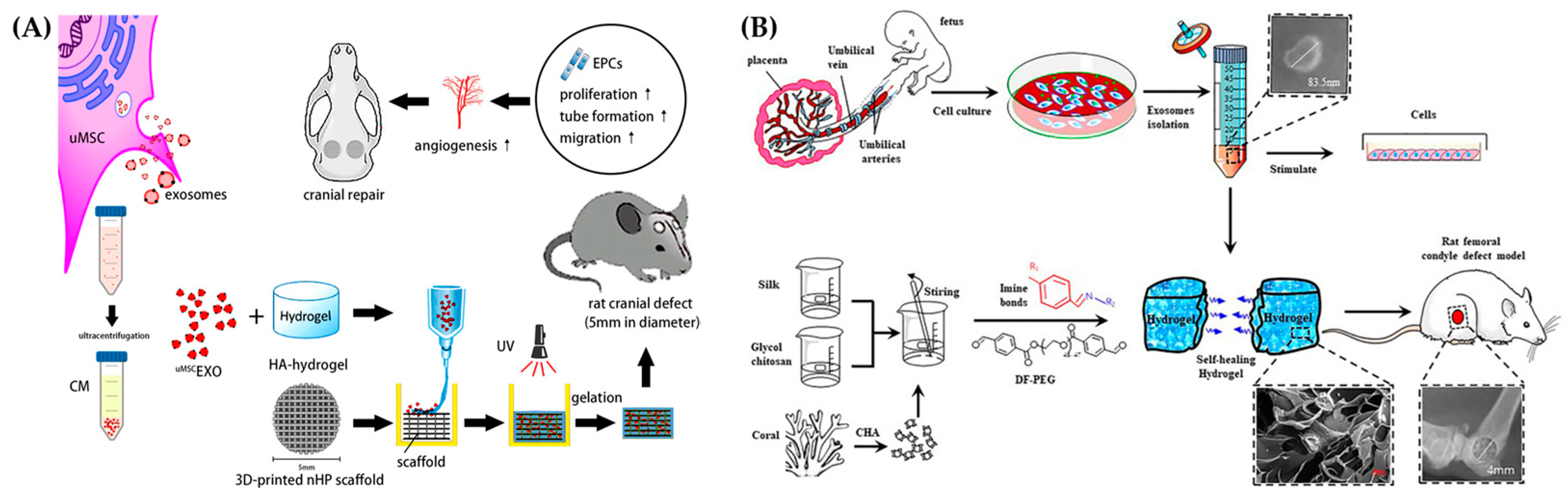
3.2. Exosomes for Bone Tissue Engineering
3.3. Exosome Laden Hydrogels for Bone Tissue Engineering
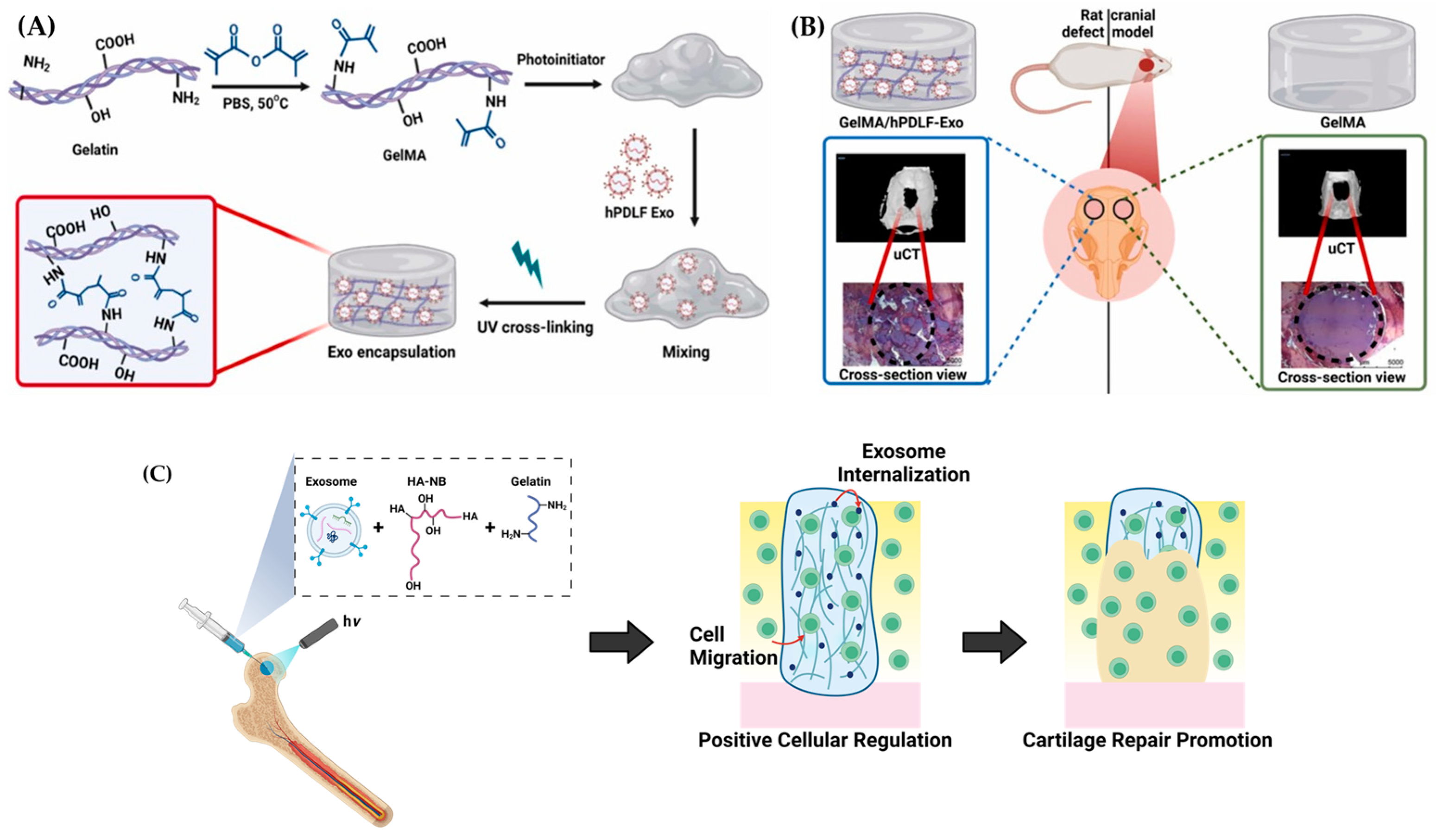
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Xu, S.X.; Wang, R.Y.; Che, Y.A.; Han, C.C.; Feng, W.; Wang, C.W.; Zhao, W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023, 162, 157–178. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Tsolaki, E.; Bertazzo, S. Pathological Mineralization: The Potential of Mineralomics. Materials 2019, 12, 3126. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Cao, Z.; Bian, Y.; Hu, T.; Yang, Y.; Cui, Z.; Wang, T.; Yang, S.; Weng, X.; Liang, R.; Tan, C. Recent advances in two-dimensional nanomaterials for bone tissue engineering. J. Mater. 2023, 9, 930–958. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D printing method for bone tissue engineering scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing-Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, W.P.; Zhang, Z.N.; Sun, Z.J. Tailoring biomaterials for monitoring and evoking tertiary lymphoid structures. Acta Biomater. 2023, 172, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prasathkumar, M.; Dhrisya, C.; Lin, F.-H.; Sadhasivam, S. The Design and Developments of Protein-Polysaccharide Biomaterials for Corneal Tissue Engineering. Adv. Mater. Technol. 2023, 8, 2300171. [Google Scholar] [CrossRef]
- Fu, M.; Yang, C.; Sun, G. Recent advances in immunomodulatory hydrogels biomaterials for bone tissue regeneration. Mol. Immunol. 2023, 163, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Li, Y.; Zhou, L.; Dan, N.; Min, J.; Chen, Y.; Wang, Y. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int. J. Biol. Macromol. 2023, 246, 125672. [Google Scholar] [CrossRef]
- Sun, J.; Li, G.; Wu, S.; Zou, Y.; Weng, W.; Gai, T.; Chen, X.; Zhang, K.; Zhou, F.; Wang, X.; et al. Engineering preparation and sustained delivery of bone functional exosomes-laden biodegradable hydrogel for in situ bone regeneration. Compos. Part B Eng. 2023, 261, 110803. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, T.; Li, J.; Tao, Y. Advances in development of exosomes for ophthalmic therapeutics. Adv. Drug Deliv. Rev. 2023, 199, 114899. [Google Scholar] [CrossRef]
- Amondarain, M.; Gallego, I.; Puras, G.; Saenz-Del-Burgo, L.; Luzzani, C.; Pedraz, J.L. The role of microfluidics and 3D-bioprinting in the future of exosome therapy. Trends Biotechnol. 2023, 41, 1343–1359. [Google Scholar] [CrossRef]
- Cooper, L.F.; Ravindran, S.; Huang, C.C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2019, 10, 1569. [Google Scholar] [CrossRef]
- Deng, C.; Hu, Y.; Conceicao, M.; Wood, M.J.A.; Zhong, H.; Wang, Y.; Shao, P.; Chen, J.; Qiu, L. Oral delivery of layer-by-layer coated exosomes for colitis therapy. J. Control. Release 2023, 354, 635–650. [Google Scholar] [CrossRef]
- Wang, W.; Liang, X.; Zheng, K.; Ge, G.; Chen, X.; Xu, Y.; Bai, J.; Pan, G.; Geng, D. Horizon of exosome-mediated bone tissue regeneration: The all-rounder role in biomaterial engineering. Mater. Today Bio 2022, 16, 100355. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Malviya, R. Exploring potential of exosomes drug delivery system in the treatment of cancer: Advances and prospective. Med. Drug Discov. 2023, 20, 100163. [Google Scholar] [CrossRef]
- Tzng, E.; Bayardo, N.; Yang, P. Current challenges surrounding exosome treatments. Extracell. Vesicle 2023, 2, 100023. [Google Scholar] [CrossRef]
- Fan, M.-H.; Pi, J.-K.; Zou, C.-Y.; Jiang, Y.-L.; Li, Q.-J.; Zhang, X.-Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.-Q. Hydrogel-exosome system in tissue engineering: A promising therapeutic strategy. Bioact. Mater. 2024, 38, 1–30. [Google Scholar] [CrossRef]
- Rahmati, S.; Khazaei, M.; Nadi, A.; Alizadeh, M.; Rezakhani, L. Exosome-loaded scaffolds for regenerative medicine in hard tissues. Tissue Cell 2023, 82, 102102. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2022, 171, 50–59. [Google Scholar] [CrossRef]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Hamdy, T.M. Dental Biomaterial Scaffolds in Tooth Tissue Engineering: A Review. Curr. Oral Health Rep. 2023, 10, 14–21. [Google Scholar] [CrossRef]
- Cao, L.; Su, H.; Si, M.; Xu, J.; Chang, X.; Lv, J.; Zhai, Y. Tissue Engineering in Stomatology: A Review of Potential Approaches for Oral Disease Treatments. Front. Bioeng. Biotechnol. 2021, 9, 662418. [Google Scholar] [CrossRef]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Dard, M.; Sewing, A.; Meyer, J.; Verrier, S.; Roessler, S.; Scharnweber, D. Tools for tissue engineering of mineralized oral structures. Clin. Oral Investig. 2000, 4, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Sanchez-Schmitz, G.; Ramos-Payan, R. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Front. Mater. 2021, 8, 708945. [Google Scholar] [CrossRef]
- Jhaveri-Desai, H.; Khetarpal, S. Tissue Engineering in Regenerative Dental Therapy. J. Healthc. Eng. 2011, 2, 598263. [Google Scholar] [CrossRef]
- Vurat, M.T.; Seker, S.; Lalegul-Ulker, O.; Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M. Development of a multicellular 3D-bioprinted microtissue model of human periodontal ligament-alveolar bone biointerface: Towards a pre-clinical model of periodontal diseases and personalized periodontal tissue engineering. Genes Dis. 2022, 9, 1008–1023. [Google Scholar] [CrossRef]
- Sangkert, S.; Kamolmatyakul, S.; Gelinsky, M.; Meesane, J. 3D printed scaffolds of alginate/polyvinylalcohol with silk fibroin based on mimicked extracellular matrix for bone tissue engineering in maxillofacial surgery. Mater. Today Commun. 2021, 26, 102140. [Google Scholar] [CrossRef]
- Mohaghegh, S.; Sadat Haeri Boroojeni, H.; Nokhbatolfoghahaei, H.; Khojasteh, A. Application of biodegradable Patient-specific scaffolds for maxillofacial bone regeneration: A scoping review of clinical studies. Br. J. Oral Maxillofac. Surg. 2023, 61, 587–597. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A.C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X.; et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem. Eng. J. 2021, 426, 130634. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D Printing of Microenvironment-Specific Bioinspired and Exosome-Reinforced Hydrogel Scaffolds for Efficient Cartilage and Subchondral Bone Regeneration. Adv. Sci. 2023, 10, 2303650. [Google Scholar] [CrossRef]
- Zarei, M.; Shabani Dargah, M.; Hasanzadeh Azar, M.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly(lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef]
- Almansoori, A.A.; Kim, B.; Lee, J.H.; Tran, S.D. Tissue Engineering of Oral Mucosa and Salivary Gland: Disease Modeling and Clinical Applications. Micromachines 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Deng, S.; Lai, J.; Li, J.; Chen, W.; Varma, S.N.; Zhang, J.; Lei, C.; Liu, C.; Huang, L. Hydrogels for Oral Tissue Engineering: Challenges and Opportunities. Molecules 2023, 28, 3946. [Google Scholar] [CrossRef] [PubMed]
- Elham, B.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Atila, D.; Kumaravel, V. Advances in antimicrobial hydrogels for dental tissue engineering: Regenerative strategies for endodontics and periodontics. Biomater. Sci. 2023, 11, 6711–6747. [Google Scholar] [CrossRef]
- Hao, M.; Wang, D.; Duan, M.; Kan, S.; Li, S.; Wu, H.; Xiang, J.; Liu, W. Functional drug-delivery hydrogels for oral and maxillofacial wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1241660. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Peng, X.; Zhang, L. Chitosan-based drug delivery systems: Current strategic design and potential application in human hard tissue repair. Eur. Polym. J. 2022, 166, 110979. [Google Scholar] [CrossRef]
- Jo Jang, E.; Patel, R.; Sankpal, N.V.; Bouchard, L.-S.; Patel, M. Alginate, hyaluronic acid, and chitosan-based 3D printing hydrogel for cartilage tissue regeneration. Eur. Polym. J. 2024, 202, 112651. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Xia, Y.; Xu, C.; Meng, K.; Lian, J.; Zhang, X.; Xu, J.; Wang, C.; Zhao, B. Photocross-linked silk fibroin/hyaluronic acid hydrogel loaded with hDPSC for pulp regeneration. Int. J. Biol. Macromol. 2022, 215, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Voytik-Harbin, S.L.; Nor, J.E.; Bottino, M.C. Injectable Highly Tunable Oligomeric Collagen Matrices for Dental Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofac. Res. 2020, 10, 573–577. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Ana, I.D.; Barlian, A.; Hidajah, A.C.; Wijaya, C.H.; Notobroto, H.B.; Kencana Wungu, T.D. Challenges and strategy in treatment with exosomes for cell-free-based tissue engineering in dentistry. Future Sci. OA 2021, 7, FSO751. [Google Scholar] [CrossRef]
- Lv, Z.; Fu, K.; Zhang, Q. Advances of exosomes-based applications in diagnostic biomarkers for dental disease and dental regeneration. Colloids Surf. B Biointerfaces 2023, 229, 113429. [Google Scholar] [CrossRef]
- Ren, J.; Jing, X.; Liu, Y.; Liu, J.; Ning, X.; Zong, M.; Zhang, R.; Cheng, H.; Cui, J.; Li, B.; et al. Exosome-based engineering strategies for the diagnosis and treatment of oral and maxillofacial diseases. J. Nanobiotechnol. 2023, 21, 501. [Google Scholar] [CrossRef]
- Hao, M.; Duan, M.; Yang, Z.; Zhou, H.; Li, S.; Xiang, J.; Wu, H.; Liu, H.; Chang, L.; Wang, D.; et al. Engineered stem cell exosomes for oral and maxillofacial wound healing. Front. Bioeng. Biotechnol. 2022, 10, 1038261. [Google Scholar] [CrossRef]
- Tobon-Arroyave, S.I.; Celis-Mejia, N.; Cordoba-Hidalgo, M.P.; Isaza-Guzman, D.M. Decreased salivary concentration of CD9 and CD81 exosome-related tetraspanins may be associated with the periodontal clinical status. J. Clin. Periodontol. 2019, 46, 470–480. [Google Scholar] [CrossRef]
- Chen, W.J.; Xie, J.; Lin, X.; Ou, M.H.; Zhou, J.; Wei, X.L.; Chen, W.X. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J. Endod. 2021, 47, 961–969. [Google Scholar] [CrossRef]
- Qiao, X.; Tang, J.; Dou, L.; Yang, S.; Sun, Y.; Mao, H.; Yang, D. Dental Pulp Stem Cell-Derived Exosomes Regulate Anti-Inflammatory and Osteogenesis in Periodontal Ligament Stem Cells and Promote the Repair of Experimental Periodontitis in Rats. Int. J. Nanomed. 2023, 18, 4683–4703. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, X.; Peng, W.; Huang, C.; Du, Y.; Yang, H.; Zhou, J. Fabrication of an exosome-loaded thermosensitive chitin-based hydrogel for dental pulp regeneration. J. Mater. Chem. B 2023, 11, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Z.; Lu, H.; Yang, R.; Wu, J. Crucial Factors Influencing the Involvement of Odontogenic Exosomes in Dental Pulp Regeneration. Stem Cell Rev. Rep. 2023, 19, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Jia, S.; Chen, H.; Duan, Y.; Li, X.; Wang, S.; Wang, T.; Lyu, Y.; Chen, G.; et al. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics 2020, 10, 5914–5931. [Google Scholar] [CrossRef]
- Swanson, W.B.; Gong, T.; Zhang, Z.; Eberle, M.; Niemann, D.; Dong, R.; Rambhia, K.J.; Ma, P.X. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J. Control. Release 2020, 324, 679–694. [Google Scholar] [CrossRef]
- Huang, M.; Huang, Y.; Liu, H.; Tang, Z.; Chen, Y.; Huang, Z.; Xu, S.; Du, J.; Jia, B. Hydrogels for the treatment of oral and maxillofacial diseases: Current research, challenges, and future directions. Biomater. Sci. 2022, 10, 6413–6446. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Delima, A.J.; Oates, T.; Assuma, R.; Schwartz, Z.; Cochran, D.; Amar, S.; Graves, D.T. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J. Clin. Periodontol. 2001, 28, 233–240. [Google Scholar] [CrossRef]
- Grauballe, M.B.; Ostergaard, J.A.; Schou, S.; Flyvbjerg, A.; Holmstrup, P. Effects of TNF-alpha blocking on experimental periodontitis and type 2 diabetes in obese diabetic Zucker rats. J. Clin. Periodontol. 2015, 42, 807–816. [Google Scholar] [CrossRef]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guan, Y.; Liu, P.; Gao, L.; Wang, Z.; Huang, S.; Peng, L.; Zhao, Z. Chitosan hydrogel, as a biological macromolecule-based drug delivery system for exosomes and microvesicles in regenerative medicine: A mini review. Cellulose 2022, 29, 1315–1330. [Google Scholar] [CrossRef]
- Shi, W.; Guo, S.; Liu, L.; Liu, Q.; Huo, F.; Ding, Y.; Tian, W. Small Extracellular Vesicles from Lipopolysaccharide-Preconditioned Dental Follicle Cells Promote Periodontal Regeneration in an Inflammatory Microenvironment. ACS Biomater. Sci. Eng. 2020, 6, 5797–5810. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Chao Le Meng, B.; Erin, Y.T.; Mark, S.K.C.; Yuchun, L.; Mahesh, C.; Jerry, K.Y.C. Advances in Bone Tissue Engineering. In Regenerative Medicine and Tissue Engineering; Jose, A.A., Ed.; IntechOpen: Rijeka, Croatia, 2013; Charpter 24. [Google Scholar]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Nie, L.; Shavandi, A. Fabrication of bioactive polyphenolic biomaterials for bone tissue engineering. Mater. Today Sustain. 2023, 24, 100541. [Google Scholar] [CrossRef]
- Kim, H.J.; Kummara, M.R.; Rao, K.S.V.K.; Han, S.S. Bioactive phosphate cross-linked guar gum-based hydrogels with enhanced mineralization ability for application in bone tissue engineering. Ceram. Int. 2023, 49, 39029–39038. [Google Scholar] [CrossRef]
- Hussain, Z.; Mehmood, S.; Liu, X.; Liu, Y.; Wang, G.; Pei, R. Decoding bone-inspired and cell-instructive cues of scaffolds for bone tissue engineering. Eng. Regen. 2024, 5, 21–44. [Google Scholar] [CrossRef]
- Shukla, A.; Dasgupta, N.; Ranjan, S.; Singh, S.; Chidambram, R. Nanotechnology towards prevention of anaemia and osteoporosis: From concept to market. Biotechnol. Biotechnol. Equip. 2017, 31, 863–879. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, J.; Pei, X.; Chen, J.; Wan, Q. Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123246. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Sagadevan, S.; Schirhagl, R.; Rahman, M.Z.; Bin Ismail, M.F.; Lett, J.A.; Fatimah, I.; Mohd Kaus, N.H.; Oh, W.-C. Recent advancements in polymer matrix nanocomposites for bone tissue engineering applications. J. Drug Deliv. Sci. Technol. 2023, 82, 104313. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Liu, X.; Sun, S.; Wang, N.; Kang, R.; Xie, L.; Liu, X. Therapeutic application of hydrogels for bone-related diseases. Front. Bioeng. Biotechnol. 2022, 10, 998988. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- A.Alamir, H.T.; Ismaeel, G.L.; Jalil, A.T.; Hadi, W.a.H.; Jasim, I.K.; Almulla, A.F.; Radhea, Z.A. Advanced injectable hydrogels for bone tissue regeneration. Biophys. Rev. 2023, 15, 223–237. [Google Scholar]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Li, J.; Wang, J.; Yu, Y.; Zhao, Y. Tailoring Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2306554. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.C.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Garske, D.S.; Schmidt-Bleek, K.; Ellinghaus, A.; Dienelt, A.; Gu, L.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters. Tissue Eng. Part A 2020, 26, 852–862. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vuckovic, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-Based Hydrogels and Their Applications for Tissue Engineering and Regenerative Medicine. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar]
- Parmar, P.A.; Skaalure, S.C.; Chow, L.W.; St-Pierre, J.P.; Stoichevska, V.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Temporally degradable collagen-mimetic hydrogels tuned to chondrogenesis of human mesenchymal stem cells. Biomaterials 2016, 99, 56–71. [Google Scholar] [CrossRef]
- Guillen-Carvajal, K.; Valdez-Salas, B.; Beltran-Partida, E.; Salomon-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Zhang, J.; Yuan, Y.; Wang, J. Exosomes: A Novel Therapeutic Agent for Cartilage and Bone Tissue Regeneration. Dose Response 2019, 17, 1559325819892702. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jiang, Y.; Song, J.; Wang, R.; Li, Z.; Yang, L.; Wu, W.; Zhang, L.; Peng, Q. Exosomes as Potential Functional Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2023, 12, e2201989. [Google Scholar] [CrossRef]
- Huang, C.C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. [Google Scholar] [CrossRef]
- Huber, J.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 101–113. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1alpha-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, Z.; Wang, X.; Su, J. Exosome-Laden Hydrogels: A Novel Cell-free Strategy for In-situ Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 866208. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gong, Y.; Liu, X.; He, J.; Zheng, B.; Liu, Y. The Experimental Study of Periodontal Ligament Stem Cells Derived Exosomes with Hydrogel Accelerating Bone Regeneration on Alveolar Bone Defect. Pharmaceutics 2022, 14, 2189. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef]
- Chen, C.; Fu, L.; Luo, Y.; Zeng, W.; Qi, X.; Wei, Y.; Chen, L.; Zhao, X.; Li, D.; Tian, M.; et al. Engineered Exosome-Functionalized Extracellular Matrix-Mimicking Hydrogel for Promoting Bone Repair in Glucocorticoid-Induced Osteonecrosis of the Femoral Head. ACS Appl. Mater. Interfaces 2023, 15, 28891–28906. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Hu, Y.; Chen, R.; Hao, Z.; Wang, Y.; Li, J. Exosome-Hydrogel System in Bone Tissue Engineering: A Promising Therapeutic Strategy. Macromol. Biosci. 2023, 23, e2200496. [Google Scholar] [CrossRef]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21. Front. Bioeng. Biotechnol. 2021, 9, 829136. [Google Scholar] [CrossRef]
- Isik, M.; Vargel, I.; Ozgur, E.; Cam, S.B.; Korkusuz, P.; Emregul, E.; Odabas, S.; Derkus, B. Human periodontal ligament stem cells-derived exosomes-loaded hybrid hydrogel enhances the calvarial defect regeneration in middle-age rats. Mater. Today Commun. 2023, 36, 106869. [Google Scholar] [CrossRef]
- Huang, C.C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021, 126, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Y.; Dong, W.; Jiang, B.; Yu, Y.; Chen, Y. Role of nano-hydrogels coated exosomes in bone tissue repair. Front. Bioeng. Biotechnol. 2023, 11, 1167012. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Zhao, X.; Yan, L.; Jin, X.; Wang, X.; Wang, J.; Yin, Z.; Zhang, Y.; Meng, Z. Thermosensitive Hydrogel Loaded with Primary Chondrocyte-Derived Exosomes Promotes Cartilage Repair by Regulating Macrophage Polarization in Osteoarthritis. Tissue Eng. Regen. Med. 2022, 19, 629–642. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, C.; Murugan, P.; George, A. Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery. Int. J. Mol. Sci. 2024, 25, 11092. https://doi.org/10.3390/ijms252011092
Villani C, Murugan P, George A. Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery. International Journal of Molecular Sciences. 2024; 25(20):11092. https://doi.org/10.3390/ijms252011092
Chicago/Turabian StyleVillani, Cassandra, Prasathkumar Murugan, and Anne George. 2024. "Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery" International Journal of Molecular Sciences 25, no. 20: 11092. https://doi.org/10.3390/ijms252011092
APA StyleVillani, C., Murugan, P., & George, A. (2024). Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery. International Journal of Molecular Sciences, 25(20), 11092. https://doi.org/10.3390/ijms252011092




