Intraperitoneal Injection of the Porphyromonas gingivalis Outer Membrane Vesicle (OMV) Stimulated Expressions of Neuroinflammatory Markers and Histopathological Changes in the Brains of Adult Zebrafish
Abstract
1. Introduction
2. Results
2.1. Extraction of P. gingivalis OMVs and Authentication
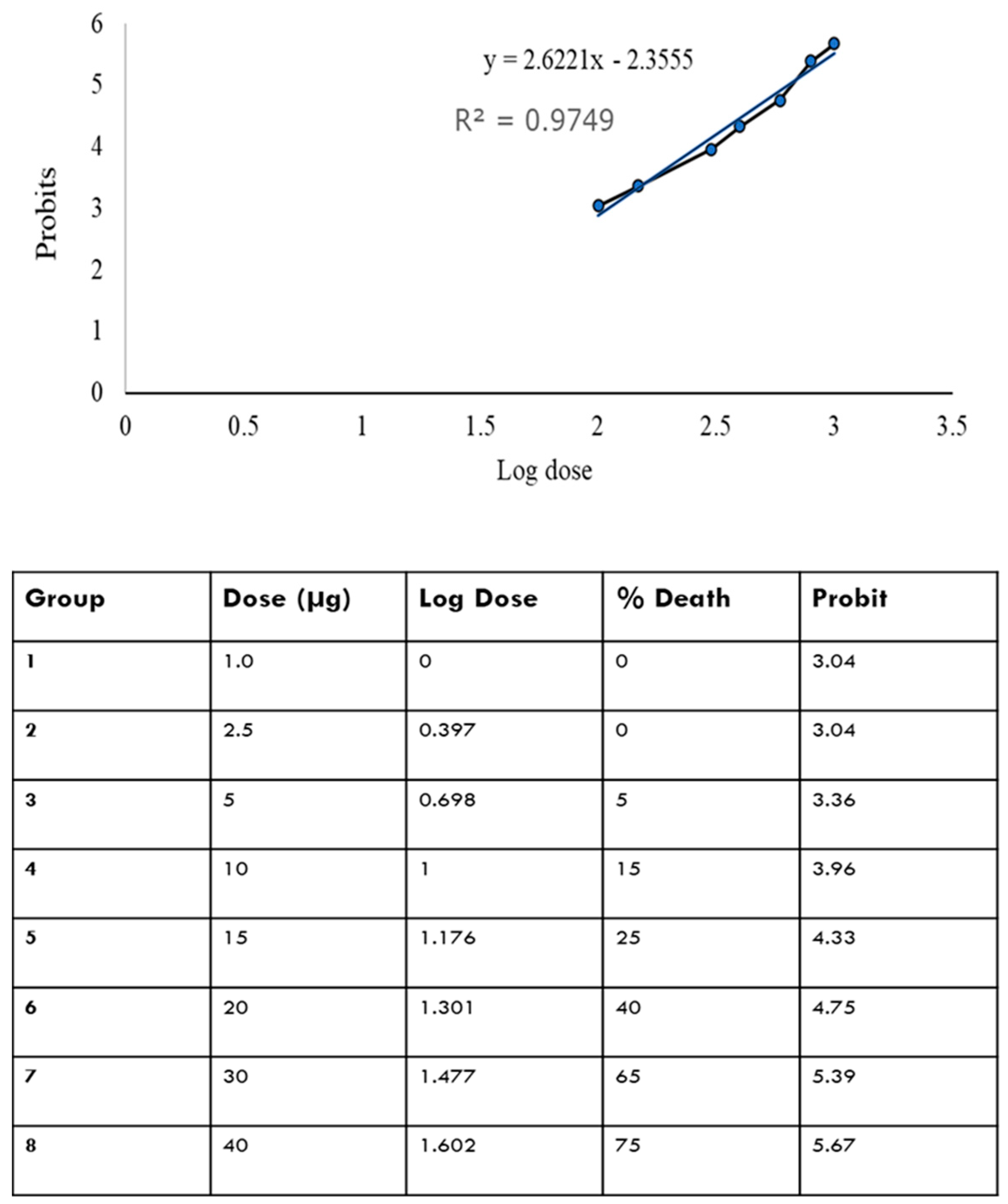
2.2. LD50 of OMVs in Adult Zebrafish
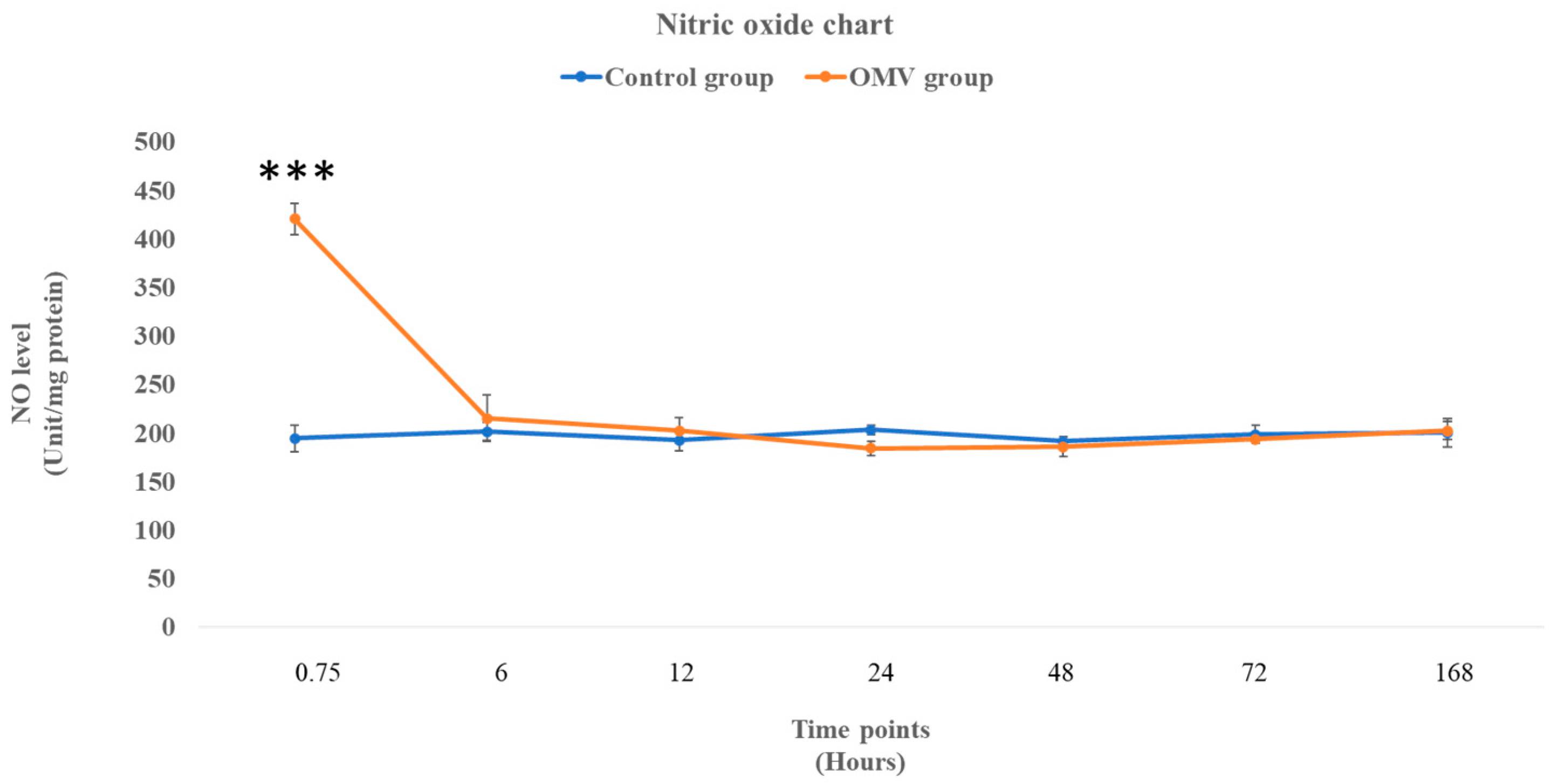
2.3. OMV Triggers Increased NO Production in Zebrafish
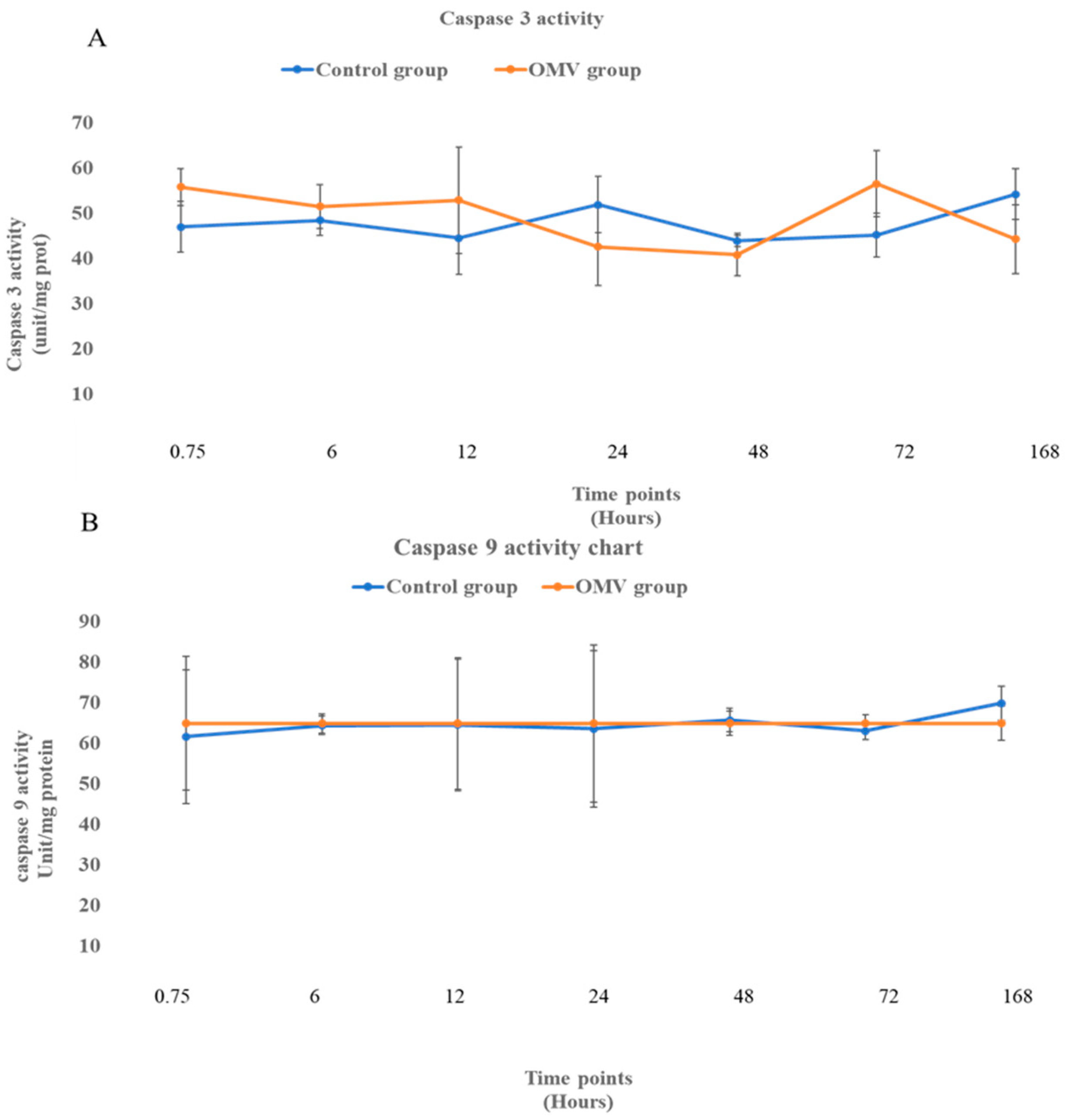
2.4. OMV Does Not Affect Caspase 3 and 9 Activity
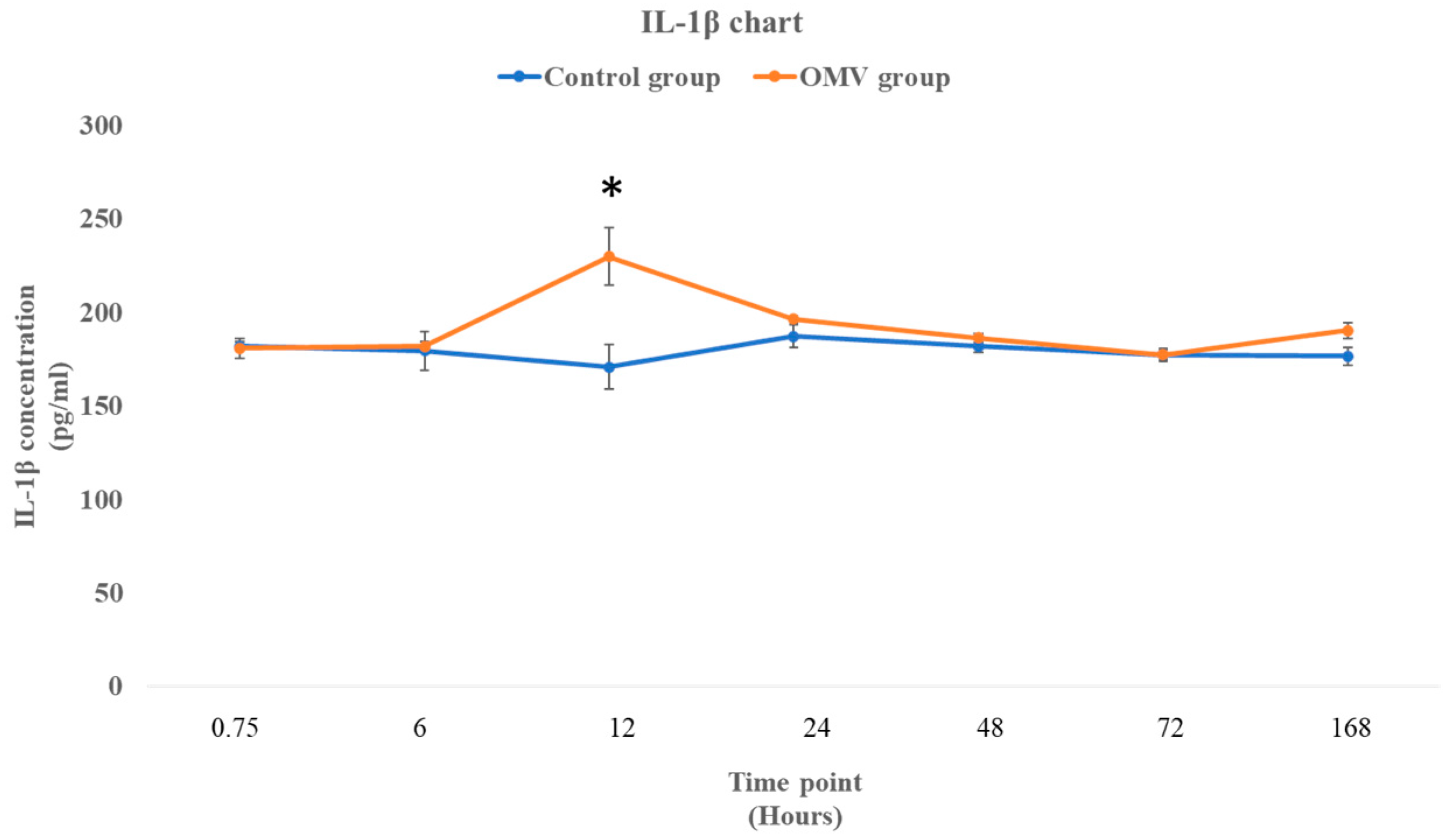
2.5. P. gingivalis OMV Stimulates Production of IL-1β in Zebrafish
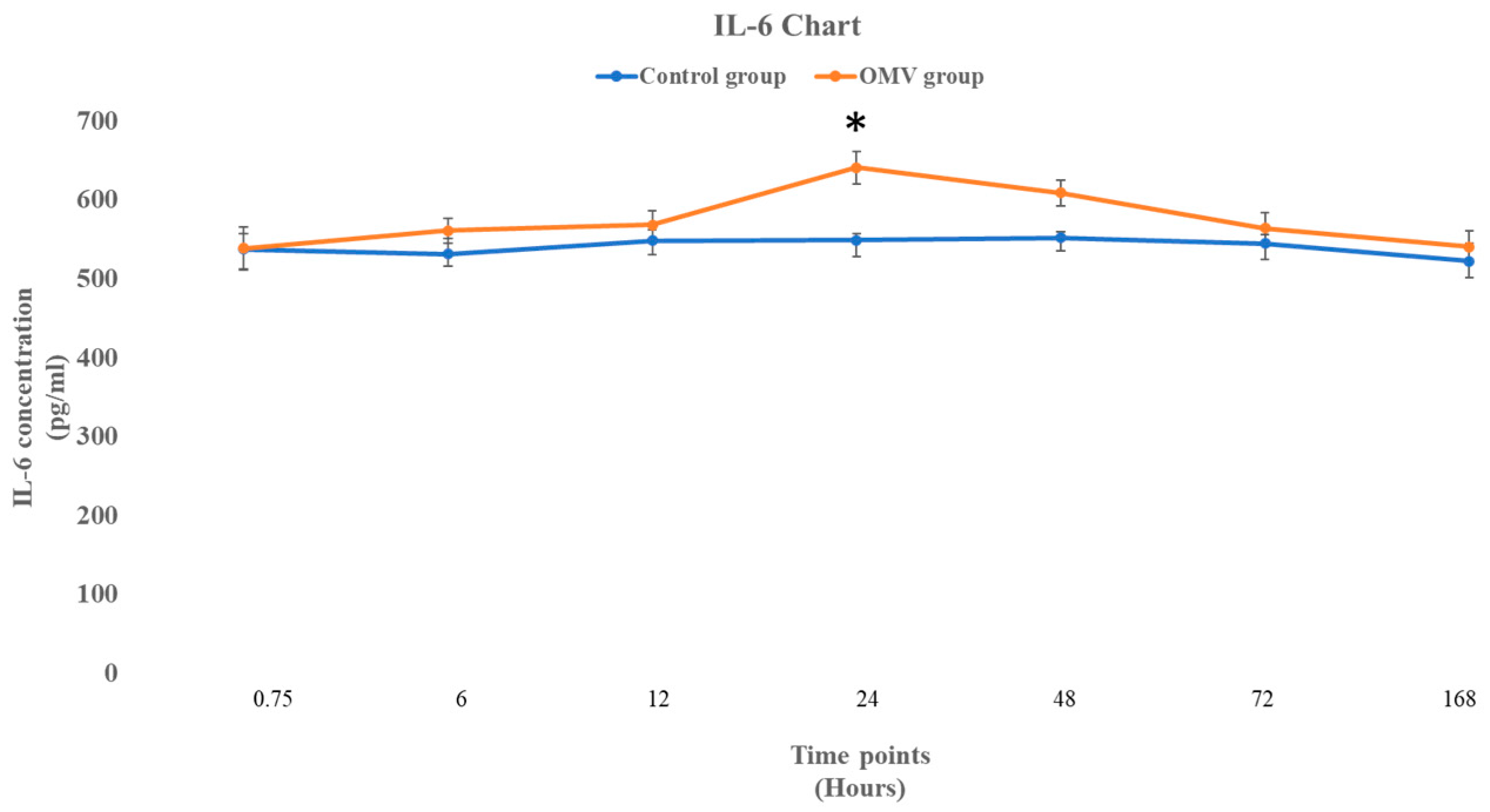
2.6. P. gingivalis OMV Activates Release of IL-6 in Zebrafish
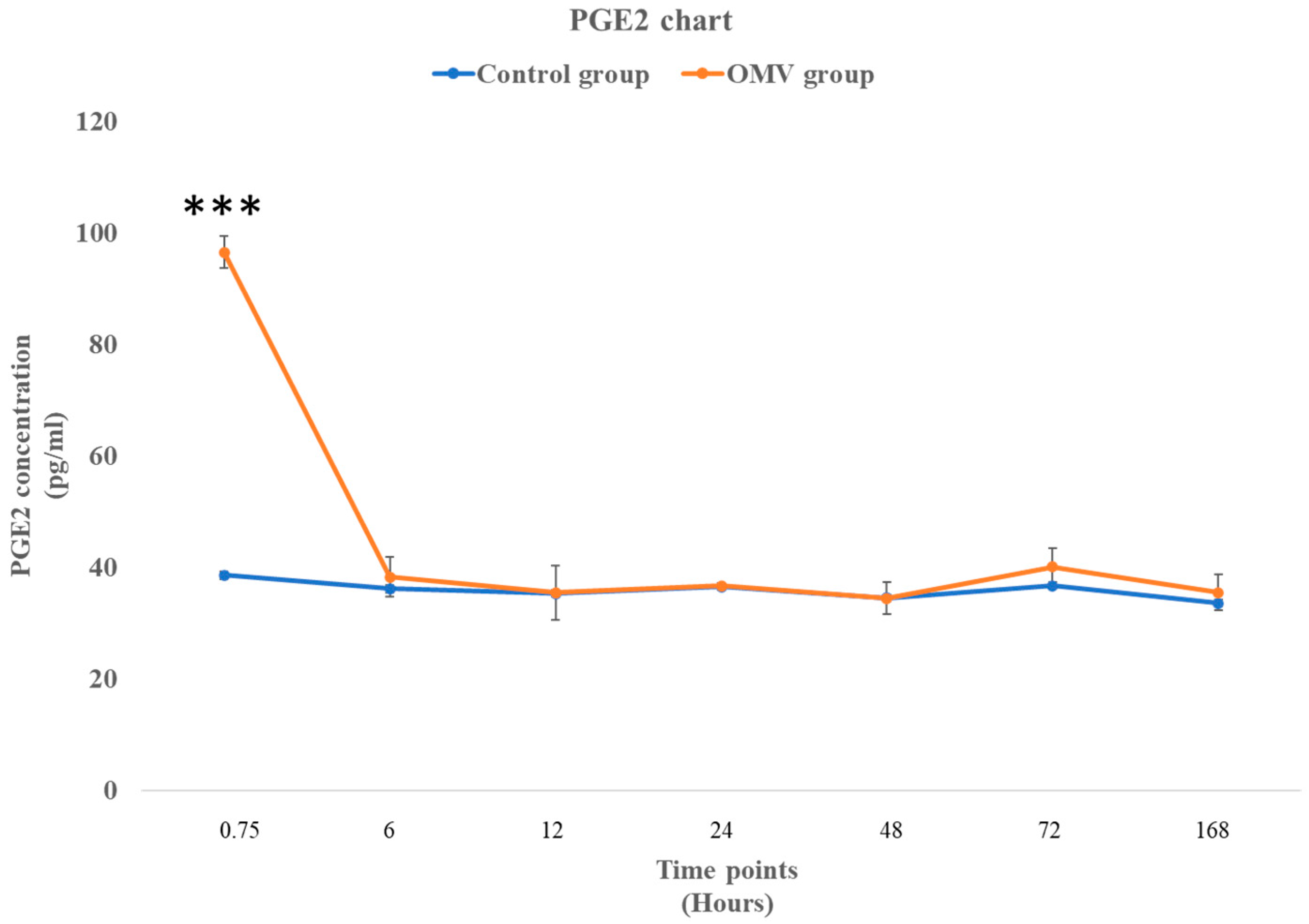
2.7. P. gingivalis OMV Triggers Biosynthesis of PGE2 in Zebrafish
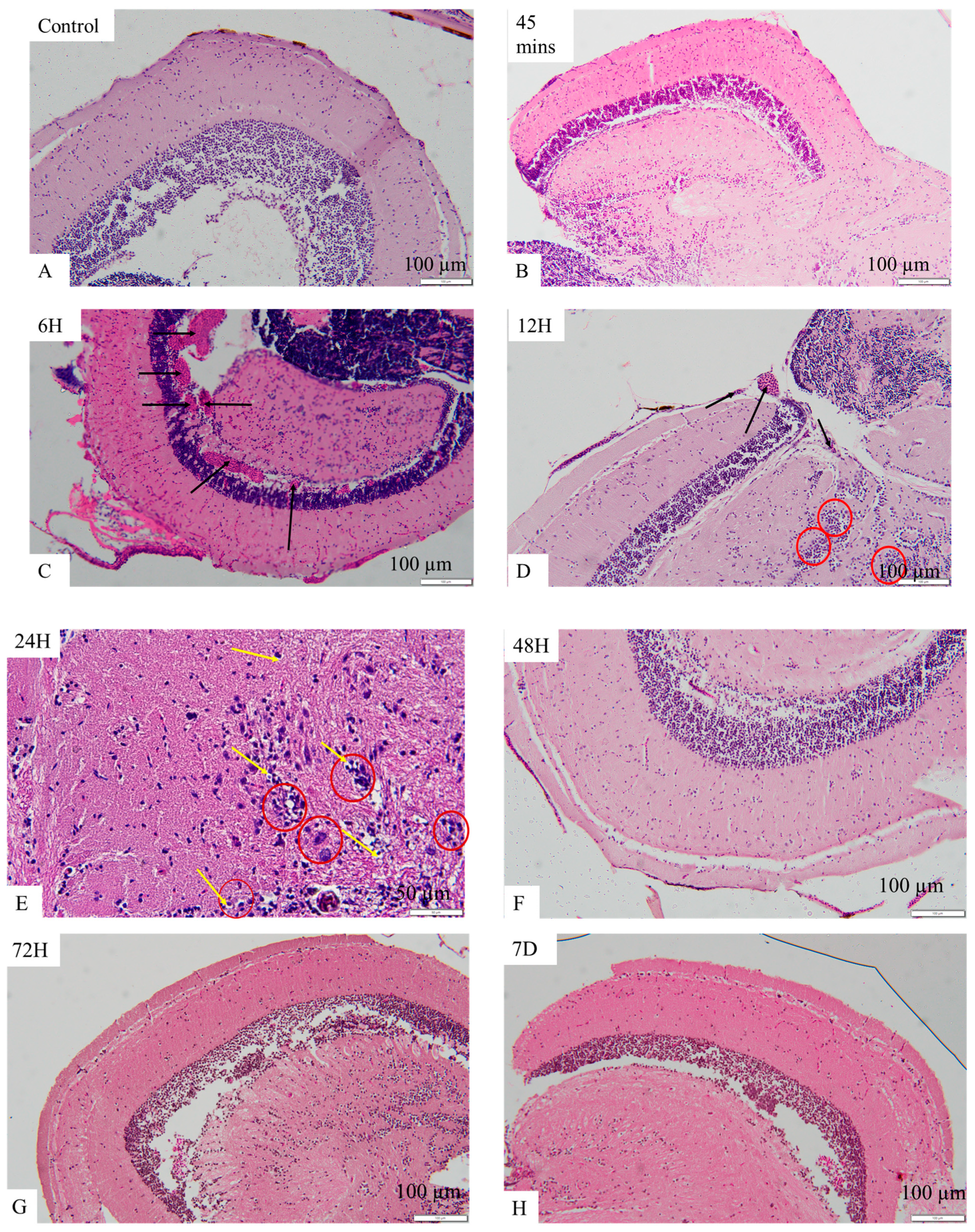
2.8. OMV-Induced Histopathological Changes in the Brains of Zebrafish
3. Discussion
4. Materials and Methods
4.1. P. gingivalis Derived OMV Preparation
4.2. Fish Husbandry and Care
4.3. Intraperitoneal Injection of the P. gingivalis-Derived OMV
4.4. Median Lethal Dose Determination
4.5. Determination of NO Levels, Caspase 3 and 9 Using Colorimetric Assay
4.6. Determination of Concentrations of Interleukin 6 (IL-6), Interleukin 1 Beta (IL-1β), and Prostaglandin E2 (PGE2) Using ELISA
4.7. Evaluation of Zebrafish Brain Histology
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balistreri, C.R.; Monastero, R. Neuroinflammation and Neurodegenerative Diseases: How Much Do We Still Not Know? Brain Sci. 2024, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, M.O.P.; Frazão, D.R.; de Matos, I.G.; Bittencourt, L.O.; Fagundes, N.C.F.; Rösing, C.K.; Maia, L.C.; Lima, R.R. Is There Any Association Between Neurodegenerative Diseases and Periodontitis? A Systematic Review. Front. Aging Neurosci. 2021, 13, 651437. [Google Scholar] [CrossRef] [PubMed]
- Sirisereephap, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Domon, H.; Isono, T.; Terao, Y.; Maeda, T.; Tabeta, K. Osteoimmunology in Periodontitis: Local Proteins and Compounds to Alleviate Periodontitis. Int. J. Mol. Sci. 2022, 23, 5540. [Google Scholar] [CrossRef] [PubMed]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef]
- Li, X.; Kiprowska, M.; Kansara, T.; Kansara, P.; Li, P. Neuroinflammation: A Distal Consequence of Periodontitis. J. Dent. Res. 2022, 101, 1441–1449. [Google Scholar] [CrossRef]
- Pastorello, Y.; Carare, R.O.; Banescu, C.; Potempa, L.; Di Napoli, M.; Slevin, M. Monomeric C-reactive protein: A novel biomarker predicting neurodegenerative disease and vascular dysfunction. Brain Pathol. 2023, 33, e13164. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Gjaltema, J.; Hagens, E.R.C.; Hashemi, Z.; Knüppe, T.B.; Kootstra, T.J.; Visser, A.; Bloem, B.R. Periodontitis: A Plausible Modifiable Risk Factor for Neurodegenerative Diseases? A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4504. [Google Scholar] [CrossRef]
- Gare, J.; Kanoute, A.; Meda, N.; Viennot, S.; Bourgeois, D.; Carrouel, F. Periodontal conditions and pathogens associated with pre-eclampsia: A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 7194. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, B.; Yang, W.; Jia, Y.; Guo, B.; Yang, W.; Zhao, Q.; Jia, W.; Wu, Y. Rho kinase mediates Porphyromonas gingivalis outer membrane vesicle-induced suppression of endothelial nitric oxide synthase through ERK1/2 and p38 MAPK. Arch. Oral Biol. 2015, 60, 488–495. [Google Scholar] [CrossRef]
- Nakao, R.; Hasegawa, H.; Ochiai, K.; Takashiba, S.; Ainai, A.; Ohnishi, M.; Watanabe, H.; Senpuku, H. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS ONE 2011, 6, e26163. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.d.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Chen, Q.; Mao, H.; Zhang, Y.; Ren, H.; Xu, M.; Chen, H.; Yang, D. Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front. Cell. Infect. Microbiol. 2022, 12, 925435. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Neuroprotective effects of Neurotrophin-3 in MPTP-induced zebrafish Parkinson’s disease model. Front. Pharmacol. 2023, 14, 1307447. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, P.; Chen, J.; Zhang, L.; Shang, N.; Chen, J.; Fan, R.; Wang, Y.; Huang, T.; Niu, Q.; et al. Necrostatin-1 Relieves Learning and Memory Deficits in a Zebrafish Model of Alzheimer’s Disease Induced by Aluminum. Neurotox. Res. 2022, 40, 198–214. [Google Scholar] [CrossRef]
- Koehler, D.; Shah, Z.A.; Hensley, K.; Williams, F.E. Lanthionine ketimine-5-ethyl ester provides neuroprotection in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem. Int. 2018, 115, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sahu, K.; Kapil, L.; Singh, C.; Singh, A. Quercetin ameliorates lipopolysaccharide-induced neuroinflammation and oxidative stress in adult zebrafish. Mol. Biol. Rep. 2022, 49, 3247–3258. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, K.; Kapil, L.; Singh, C.; Singh, A. Administration of Quercetin Ameliorates Lipopolysaccharide Induced Neuroinammation and Oxidative Stress in Adult Zebrash. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Martínez-Vázquez, J.M.; Riesco, M.F.; Robles, V. Probiotics reduce anxiety-related behavior in zebrafish. Heliyon 2020, 6, e03973. [Google Scholar] [CrossRef]
- Kaur, K.; Narang, R.K.; Singh, S. AlCl3 induced learning and memory deficit in zebrafish. Neurotoxicology 2022, 92, 67–76. [Google Scholar] [CrossRef]
- Zanandrea, R.; Abreu, M.S.; Piato, A.; Barcellos, L.J.; Giacomini, A.C. Lithium prevents scopolamine-induced memory impairment in zebrafish. Neurosci. Lett. 2018, 664, 34–37. [Google Scholar] [CrossRef]
- Singsai, K.; Ladpala, N.; Dangja, N.; Boonchuen, T.; Jaikhamfu, N.; Fakthong, P. Effect of Streblus asper Leaf Extract on Scopolamine-Induced Memory Deficits in Zebrafish: The Model of Alzheimer’s Disease. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6666726. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.S. Gut–brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Mottaz, H.; Schönenberger, R.; Fischer, S.; Eggen, R.I.; Schirmer, K.; Groh, K.J. Dose-dependent effects of morphine on lipopolysaccharide (LPS)-induced inflammation, and involvement of multixenobiotic resistance (MXR) transporters in LPS efflux in teleost fish. Environ. Pollut. 2017, 221, 105–115. [Google Scholar] [CrossRef]
- Zhang, Q.; Kopp, M.; Babiak, I.; Fernandes, J.M. Low incubation temperature during early development negatively affects survival and related innate immune processes in zebrafish larvae exposed to lipopolysaccharide. Sci. Rep. 2018, 8, 4142. [Google Scholar] [CrossRef] [PubMed]
- Widziolek, M.; Prajsnar, T.K.; Tazzyman, S.; Stafford, G.P.; Potempa, J.; Murdoch, C. Zebrafish as a new model to study effects of periodontal pathogens on cardiovascular diseases. Sci. Rep. 2016, 6, 36023. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Potempa, J.; Wilkinson, R.N.; Chen, Y.; Murdoch, C.; Widziolek, M. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 2021, 288, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Murdoch, C. Porphyromonas gingivalis Outer Membrane Vesicles Increase Vascular Permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef]
- Guo, J.; Lin, K.; Wang, S.; He, X.; Huang, Z.; Zheng, M. Effects and mechanisms of Porphyromonas gingivalis outer membrane vesicles induced cardiovascular injury. BMC Oral Health 2024, 24, 112. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Chen, Z.; Jalabi, W.; Shpargel, K.B.; Farabaugh, K.T.; Dutta, R.; Yin, X.; Kidd, G.J.; Bergmann, C.C.; Stohlman, S.A.; Trapp, B.D. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J. Neurosci. 2012, 32, 11706–11715. [Google Scholar] [CrossRef] [PubMed]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Olivera, G.C.; Ren, X.; Vodnala, S.K.; Lu, J.; Coppo, L.; Leepiyasakulchai, C.; Holmgren, A.; Kristensson, K.; Rottenberg, M.E. Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier. PLoS Pathog. 2016, 12, e1005442. [Google Scholar] [CrossRef]
- Mao, X.; Xia, B.; Zheng, M.; Zhou, Z. Assessment of the anti-inflammatory, analgesic and sedative effects of oleuropein from Olea europaea L. Cell. Mol. Biol. 2019, 65, 52–55. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural basis of IL-1 family cytokine signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Martin, P.; Goldstein, J.D.; Mermoud, L.; Diaz-Barreiro, A.; Palmer, G. IL-1 Family Antagonists in Mouse and Human Skin Inflammation. Front. Immunol. 2021, 12, 652846. [Google Scholar] [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin, C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [Google Scholar] [CrossRef]
- Hai, J.; Wan, J.F.; Lin, Q.; Wang, F.; Zhang, L.; Li, H.; Zhang, L.; Chen, Y.Y.; Lu, Y. Cognitive dysfunction induced by chronic cerebral hypoperfusion in a rat model associated with arteriovenous malformations. Brain Res. 2009, 1301, 80–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.; Li, R.; Huang, X.; Liu, Q. Design of outer membrane vesicles as cancer vaccines: A new toolkit for cancer therapy. Cancers 2019, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Adamski, M.G.; Sternak, M.; Mohaissen, T.; Kaczor, D.; Wierońska, J.M.; Malinowska, M.; Czaban, I.; Byk, K.; Lyngsø, K.S.; Przyborowski, K.; et al. Vascular cognitive impairment linked to brain endothelium inflammation in early stages of heart failure in mice. J. Am. Heart Assoc. 2018, 7, e007694. [Google Scholar] [CrossRef] [PubMed]
- Fulop, G.A.; Ahire, C.; Csipo, T.; Tarantini, S.; Kiss, T.; Balasubramanian, P.; Yabluchanskiy, A.; Farkas, E.; Toth, A.; Nyúl-Tóth, Á.; et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019, 41, 575–589. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Yang, W.W.; Guo, B.; Jia, W.Y.; Jia, Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio 2016, 6, 1310–1319. [Google Scholar] [CrossRef]
- Wei, S.; Peng, W.; Mai, Y.; Li, K.; Wei, W.; Hu, L.; Zhu, S.; Zhou, H.; Jie, W.; Wei, Z.; et al. Outer membrane vesicles enhance tau phosphorylation and contribute to cognitive impairment. J. Cell. Physiol. 2020, 235, 4843–4855. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Besio, R.; Xiao, L.; Forlino, A. Outer Membrane Vesicles (OMVs) as Biomedical Tools and Their Relevance as Immune-Modulating Agents against H. pylori Infections: Current Status and Future Prospects. Int. J. Mol. Sci. 2023, 24, 8542. [Google Scholar] [CrossRef]
- Al-Dossary, A.; Isichei, A.; Zhang, S. Pharmaceutical Nanobiotechnology for Targeted Therapy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar]
- Jiang, Y.; Zhou, Z.Z.; Liu, C.; Wang, L.; Li, C. Bacterial outer membrane vesicles as drug delivery carrier for photodynamic anticancer therapy. Front. Chem. 2023, 11, 1284292. [Google Scholar] [CrossRef]
- Mantri, C.K.; Chen, C.H.; Dong, X.; Goodwin, J.S.; Pratap, S.; Paromov, V.; Xie, H. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen 2015, 4, 53–65. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [PubMed]
- Fleetwood, A.J.; Lee, M.K.S.; Singleton, W.; Achuthan, A.; Lee, M.C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Atanasova, K.R.; Bui, P.Q.; Lee, J.; Hung, S.C.; Yilmaz, Ö.; Ojcius, D.M. Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect. 2015, 17, 369–377. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Yao, L.; Jermanus, C.; Barbetta, B.; Choi, C.; Verbeke, P.; Ojcius, D.M.; Yilmaz, Ö. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010, 25, 89–101. [Google Scholar] [CrossRef]
- Hegazy, R.R.; Mansour, D.F.; Salama, A.A.; Abdel-Rahman, R.F.; Hassan, A.M. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacol. Rep. 2019, 71, 879–891. [Google Scholar] [CrossRef]
- Ho, M.H.; Chen, C.H.; Goodwin, J.S.; Wang, B.Y.; Xie, H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS ONE 2015, 10, e0123448. [Google Scholar] [CrossRef]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Parkinson’s disease model in zebrafish using intraperitoneal MPTP injection. Front. Neurosci. 2023, 17, 1236049. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 2010, 42, e2126. [Google Scholar] [CrossRef]
- Chandra, M.; Raj, J.; Das Dogra, T.; Rajvanshi, A.C.; Raina, A.N. Determination of median lethal dose of triazophos with DMSO in wistar rats. Asian J. Pharm. Clin. Res. 2014, 7, 64–67. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewoyin, M.; Hamarsha, A.; Akinsola, R.; Teoh, S.L.; Azmai, M.N.A.; Abu Bakar, N.; Nasruddin, N.S. Intraperitoneal Injection of the Porphyromonas gingivalis Outer Membrane Vesicle (OMV) Stimulated Expressions of Neuroinflammatory Markers and Histopathological Changes in the Brains of Adult Zebrafish. Int. J. Mol. Sci. 2024, 25, 11025. https://doi.org/10.3390/ijms252011025
Adewoyin M, Hamarsha A, Akinsola R, Teoh SL, Azmai MNA, Abu Bakar N, Nasruddin NS. Intraperitoneal Injection of the Porphyromonas gingivalis Outer Membrane Vesicle (OMV) Stimulated Expressions of Neuroinflammatory Markers and Histopathological Changes in the Brains of Adult Zebrafish. International Journal of Molecular Sciences. 2024; 25(20):11025. https://doi.org/10.3390/ijms252011025
Chicago/Turabian StyleAdewoyin, Malik, Ahmed Hamarsha, Rasaq Akinsola, Seong Lin Teoh, Mohammad Noor Amal Azmai, Noraini Abu Bakar, and Nurrul Shaqinah Nasruddin. 2024. "Intraperitoneal Injection of the Porphyromonas gingivalis Outer Membrane Vesicle (OMV) Stimulated Expressions of Neuroinflammatory Markers and Histopathological Changes in the Brains of Adult Zebrafish" International Journal of Molecular Sciences 25, no. 20: 11025. https://doi.org/10.3390/ijms252011025
APA StyleAdewoyin, M., Hamarsha, A., Akinsola, R., Teoh, S. L., Azmai, M. N. A., Abu Bakar, N., & Nasruddin, N. S. (2024). Intraperitoneal Injection of the Porphyromonas gingivalis Outer Membrane Vesicle (OMV) Stimulated Expressions of Neuroinflammatory Markers and Histopathological Changes in the Brains of Adult Zebrafish. International Journal of Molecular Sciences, 25(20), 11025. https://doi.org/10.3390/ijms252011025






