Research on the Action and Mechanism of Pharmacological Components of Omphalia lapidescens
Abstract
1. Introduction
2. Pharmacological Components of O. lapidescens
2.1. Protein
2.1.1. Protease
2.1.2. Lectin
2.1.3. Protein pPeOp
2.2. Polysaccharides
2.2.1. Polysaccharide S4001 and S4002
2.2.2. Polysaccharide OL-1, OL-2, and OL-3
2.3. Triterpenes
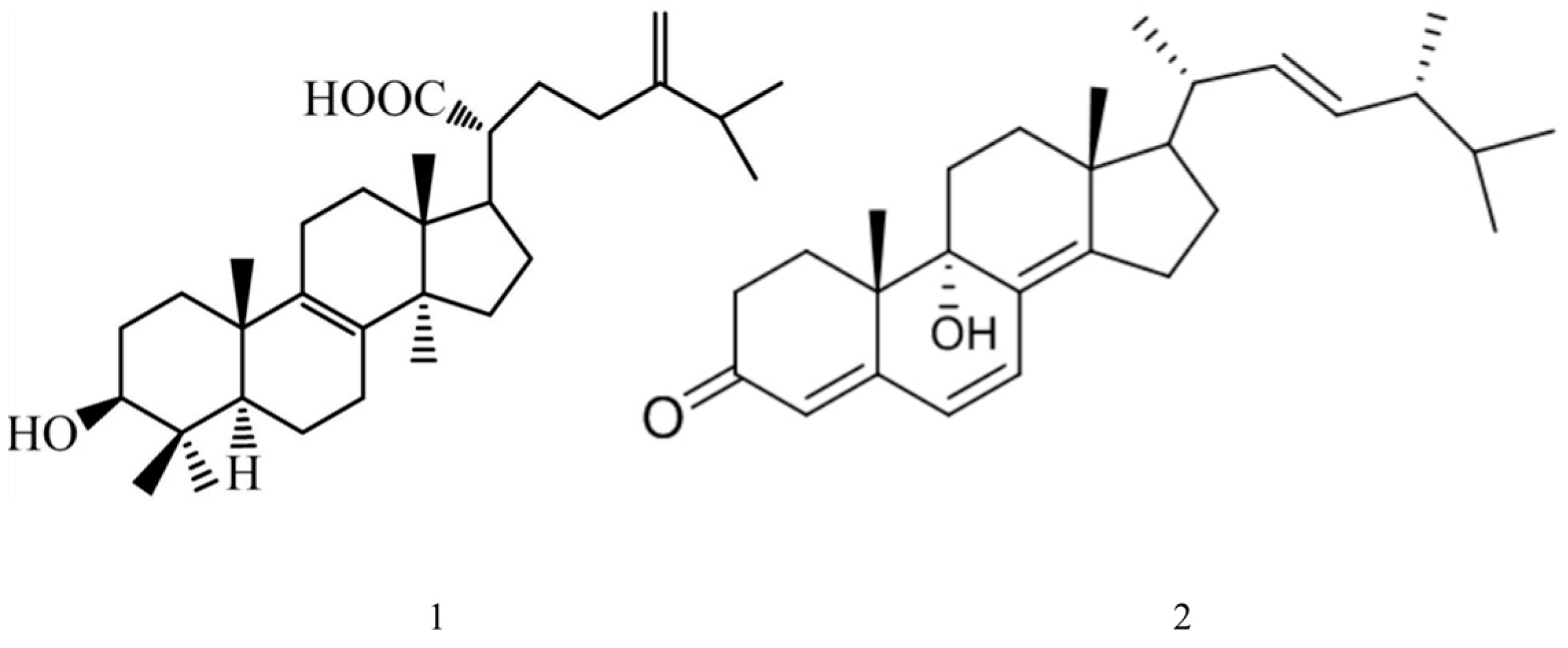
2.3.1. Tetranorlanostane Triterpenoid
2.3.2. Eburicoic Acid and Ganoderma Side D
2.4. Ergosterol
2.5. Other Components
3. Biological Activity
3.1. Antiparasitic
3.2. Antitumor
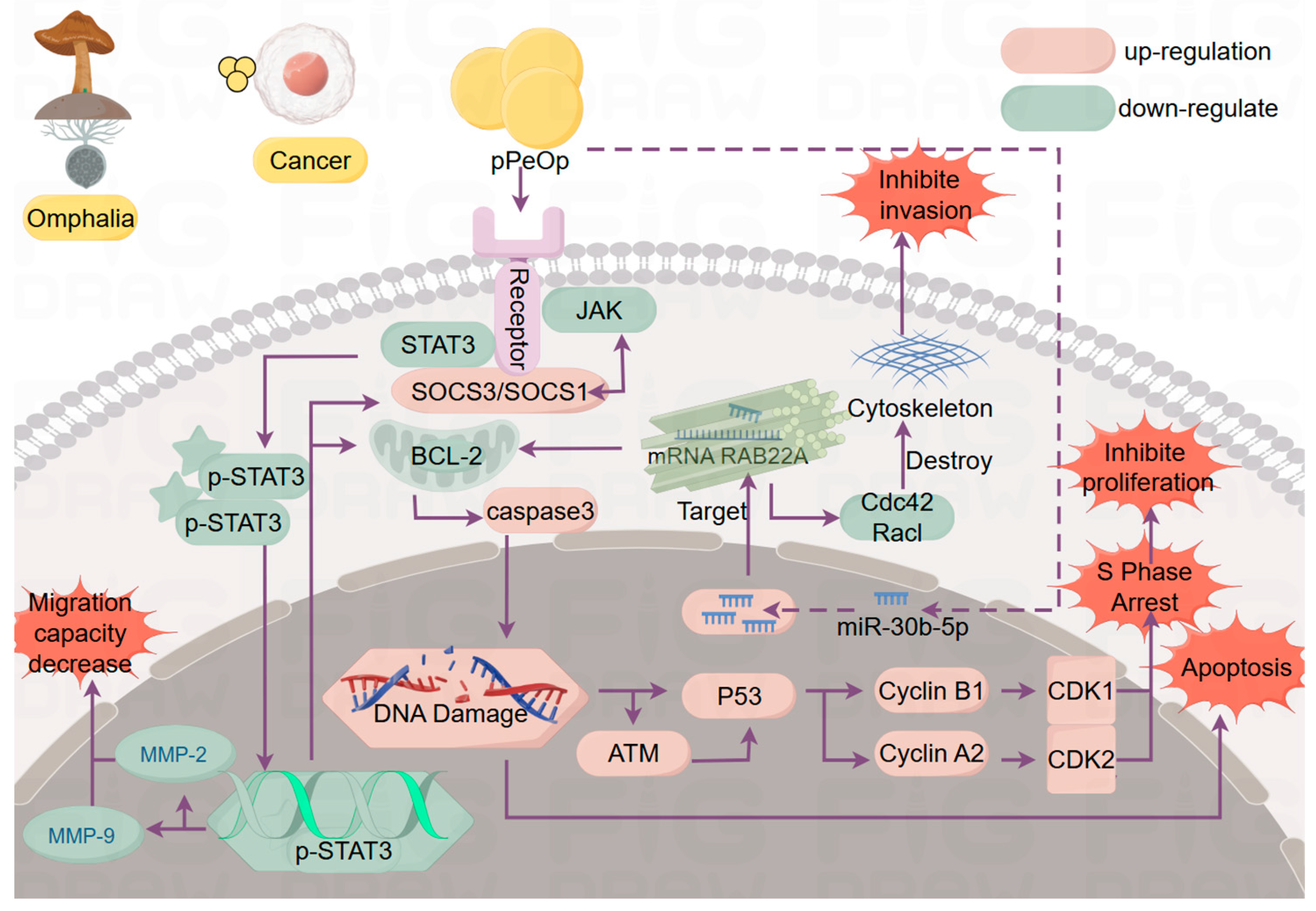
3.2.1. O. lapidescens Protein pPeOp
3.2.2. O. lapidescens Polysaccharides OL-2
3.2.3. Triterpene
3.2.4. O. lapidescens Ergosterol
3.3. Anti-Inflammatory
3.4. Antioxidant
3.5. Others
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Yu, S.; Xu, L.; Jiang, Z.; Xu, S.; Han, J.; Zhu, Y.; Chang, J.; Lin, J.; Xu, F. First report on the nationwide survey of the distribution of parasites in China. Chin. J. Parasitol. Parasit. Dis. 1994, 12, 241–247. [Google Scholar]
- Lin, W.; Zhang, H.; Xia, J.; Dong, X.; Wu, D. The third survey of parasitic diseases in key population in Hubei. China Trop. Med. 2019, 19, 14–18. [Google Scholar]
- Peng, J.; Wu, F.; Yang, Y.; Wang, L.; Du, Z.; Li, B.; Yan, X.; Zi, J.; Cai, X.; Bao, X. Infectious status of human parasites in ecological area of Sichuan basin, Yunnan. China Trop. Med. 2019, 19, 23–26. [Google Scholar]
- Wan, X.; Zhang, W.; Jiang, Z.; Lv, G.; Ou, F.; Wei, H.; Lin, Y.; Tang, W.; Wei, S.; Huang, K.; et al. Investigation on the status of human important parasitic disease in Guangxi in 2015. China Trop. Med. 2019, 19, 19–23. [Google Scholar]
- Yang, Y.; Wu, X.; Tian, H. Investigation report on human Taenia infection in China. China Trop. Med. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Yang, H. Report on the status of the 3rd human important parasitic diseases investigation in Shanxi. China Trop. Med. 2019, 19, 27–30. [Google Scholar]
- Li, Y.; Xie, H.; Zhang, R.; Chen, B.; Lin, C.; Jiang, D.; Xie, X. Three infection investigation of human parasitic diseases in Fujian. China Trop. Med. 2019, 19, 10–13. [Google Scholar]
- Wang, Q.; Xu, J.; Xia, Z.; Han, S.; Zhang, Y.; Qian, M.; Li, S.; Zhou, X. Epidemic status and key tasks for the control and elimination of key parasitic diseases in China. Chin. J. Parasitol. Parasit. Dis. 2024, 42, 1–7. [Google Scholar]
- Wu, Z.; Zhuo, B.; Qiu, H.; Ma, M.; Chen, H.; Zhong, H. Investigation on seroprevalence of Toxoplasma gondii infections among neonates in Fujian Province. Chin. J. Schistosomiasis Control 2021, 33, 71–73. [Google Scholar]
- Xiao, S.H.; Utzinger, J.; Tanner, M.; Keiser, J.; Xue, J. Advances with the Chinese anthelminthic drug tribendimidine in clinical trials and laboratory investigations. Acta Trop. 2013, 126, 115–126. [Google Scholar] [CrossRef]
- Shadan, S. Drug discovery: Schistosome treatment. Nature 2008, 452, 296. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J.; Asua, V.; Conrad, M.D. Emergence, transmission dynamics and mechanisms of artemisinin partial resistance in malaria parasites in Africa. Nat. Rev. Microbiol. 2024, 22, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; White, N.J.; Padre, L.; Ritter, W.; Fernando, M.T.; Ranoa, C.P.; Laughlin, L.W. Praziquantel pharmacokinetics and side effects in Schistosoma japonicum-infected patients with liver disease. J. Infect. Dis. 1988, 157, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.N.; Yang, Y.Q.; Cheng, B.Z.; Guo, H.F.; Yang, H.Z.; Zhuang, Z.N.; Zhang, C.W.; Lu, X.Y. Toxicity of tribendimidin, a new anti-hookworm drug. Chin. J. Parasitol. Parasit. Dis. 1988, 6, 199–201. [Google Scholar]
- Mei, L.; Liu, C.; Zhao, B.; Yang, H.; Jiang, L.; Wang, W.; Gong, J.; Ni, S. Overview of Pharmacological Research on Pistacia L. J. Anhui Agric. Sci. 2013, 41, 4312–4313+4315. [Google Scholar]
- Xue, F. Chinese Medicine—Chinese Medicine Volume; People’s Medical Publishing House: Beijing, China, 2005. [Google Scholar]
- Lu, Y.; Bao, H. Chinese Fungal Medicine; Central Farmers Publishing House: Gwalior, India, 2020. [Google Scholar]
- Zhang, W.; Wang, C.; Deng, W.; Chang, C.; Zhang, M. A Taxonomic Revision of Rare Medicinal Fungus Leiwan. Acta Edulis Fungi 2024, 31, 31–99. [Google Scholar]
- Du, C.; Li, M. Study on protease properties of Omphalia lapidescens. Chin. Tradit. Herb. Drugs 1987, 18, 18–20. [Google Scholar]
- Zhou, L.; Xu, Q.; Zhang, Y.; Zhou, Z.; Guan, W.; Li, Y. Purification, Characterization and in vitro Anthelmintic Activity of a Neutral Metalloprotease from Laccocephalum mylittae. Chin. J. Chem. Eng. 2010, 18, 122–128. [Google Scholar] [CrossRef]
- Yu, Y.; Gong, J.; Yu, M. Study on purification and physicochemical properties of Omphalia lupidescens Schroet lectin. Mycosystema 2000, 19, 278–282. [Google Scholar]
- Chen, Y.T.; Lu, Q.Y.; Lin, M.A.; Cheng, D.Q.; Ding, Z.S.; Shan, L.T. A PVP-extract fungal protein of Omphalia lapideacens and its antitumor activity on human gastric tumors and normal cells. Oncol. Rep. 2011, 26, 1519–1526. [Google Scholar]
- Goldman, G.H.; Delneste, Y.; Papon, N. Fungal Polysaccharides Promote Protective Immunity. Trends Microbiol. 2021, 29, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Christopoulos, P.F.; Arias, M.A.; Dzovor, D.E.; Oynebraten, I.; Corthay, A.; Inngjerdingen, K.T. Fungal polysaccharides from Inonotus obliquus are agonists for Toll-like receptors and induce macrophage anti-cancer activity. Commun. Biol. 2024, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Zhang, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ji, Y.; Chen, X.; Su, A.; Ma, G.; Chen, G.; Hu, Q.; Zhao, L. Effects of a beta-type glycosidic polysaccharide from Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice. Food Funct. 2020, 11, 4259–4274. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Y.; Wang, S.; Zhang, H.; Gao, C.; Lu, F.; Li, M.; Chen, D.; Lin, Z.; Yang, B. Ganoderma lucidum polysaccharide peptides GL-PPSQ(2) alleviate intestinal ischemia-reperfusion injury via inhibiting cytotoxic neutrophil extracellular traps. Int. J. Biol. Macromol. 2023, 244, 125370. [Google Scholar] [CrossRef]
- Li, F.; Liu, T.; Liu, X.; Han, C.; Li, L.; Zhang, Q.; Sui, X. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int. J. Biol. Macromol. 2024, 260 Pt 2, 129682. [Google Scholar] [CrossRef]
- Na, L.; Yi, Y.; Liang, H.; Yan, Z.; Shouyan, C.; Qiusheng, T. Comparative Study on the Active Ingredients and Antioxidant Activity of Polysaccharides between Artificially Cultivated and Wild Omphalia. Lishizhen Med. Mater. Medica Res. 2020, 31, 2499–2502. [Google Scholar]
- Wang, W.J.; Zhu, X.Y. The Antiinflammatory and Immunostimulating Activities Of S-4001—A Polysaccharide Isolated from Lei Wan (Polyporus mylitiae). Acta Pharm. Sin. 1989, 24, 151–154. [Google Scholar]
- Saito, K.; Nishijima, M.; Ohno, N.; Yadomae, T.; Miyazaki, T. Structure and antitumor activity of the less-branched derivatives of an alkali-soluble glucan isolated from Omphalia lapidescens. (Studies on fungal polysaccharide. XXXVIII). Chem. Pharm. Bull. 1992, 40, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nishijima, M.; Miyazaki, T. Structure of a heteroglycan isolated from the fungus Omphalia lapidescens. Carbohydr. Res. 1992, 224, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, J.F.; Wang, Y.; Guo, L.; Zhou, Q.M.; Peng, C.; Xiong, L. Cytotoxicity of lanostane-type triterpenoids and ergosteroids isolated from Omphalia lapidescens on MDA-MB-231 and HGC-27 cells. Biomed. Pharmacother. 2019, 118, 109273. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Eburicoic Acid, a Triterpenoid Compound from Antrodia camphorata, Displays Antidiabetic and Antihyperlipidemic Effects in Palmitate-Treated C2C12 Myotubes and in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef]
- Du, Y.; Tian, L.; Wang, Y.; Li, Z.; Xu, Z. Chemodiversity, pharmacological activity, and biosynthesis of specialized metabolites from medicinal model fungi Ganoderma lucidum. Chin. Med. 2024, 19, 51. [Google Scholar] [CrossRef]
- Gao, W.; Chai, C.; He, Y.; Li, F.; Hao, X.; Cao, F.; Gu, L.; Liu, J.; Hu, Z.; Zhang, Y. Periconiastone A, an Antibacterial Ergosterol with a Pentacyclo [8.7.0.01,5.02,14.010,15] heptadecane System from Periconia sp. TJ403-rc01. Org. Lett. 2019, 21, 8469–8472. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.; Rinker, D.L.; Tsao, R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef]
- Caz, V.; Gil-Ramirez, A.; Santamaria, M.; Tabernero, M.; Soler-Rivas, C.; Martin-Hernandez, R.; Marin, F.R.; Reglero, G.; Largo, C. Plasma Cholesterol-Lowering Activity of Lard Functionalized with Mushroom Extracts is Independent of Niemann-Pick C1-like 1 Protein and ABC Sterol Transporter Gene Expression in Hypercholesterolemic Mice. J. Agric. Food Chem. 2016, 64, 1686–1694. [Google Scholar] [CrossRef]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef]
- Yazawa, Y.; Ikarashi, N.; Hoshino, M.; Kikkawa, H.; Sakuma, F.; Sugiyama, K. Inhibitory effect of ergosterol on bladder carcinogenesis is due to androgen signaling inhibition by brassicasterol, a metabolite of ergosterol. J. Nat. Med. 2020, 74, 680–688. [Google Scholar] [CrossRef]
- Liu, J.F.; Xia, J.J.; Nie, K.L.; Wang, F.; Deng, L. Outline of the biosynthesis and regulation of ergosterol in yeast. World J. Microbiol. Biotechnol. 2019, 35, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, X.; Yang, Y.; Qin, S.; Li, Q.; Zhao, L.; Ding, Z. A new natural nucleotide and other antibacterial metabolites from an endophytic Nocardia sp. Nat. Prod. Res. 2015, 29, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hong, K.; Chen, G.D.; Wang, C.X.; Tang, J.S.; Yu, Y.; Jiang, M.M.; Li, M.M.; Wang, N.L.; Yao, X.S. New oxidized sterols from Aspergillus awamori and the endo-boat conformation adopted by the cyclohexene oxide system. Magn. Reson. Chem. 2010, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Motoyashiki, N.; Yamada, T.; Shibatani, K.; Ninomiya, K.; Morikawa, T.; Tanaka, R. Ergostane-Type Sterols from King Trumpet Mushroom (Pleurotus eryngii) and Their Inhibitory Effects on Aromatase. Int. J. Mol. Sci. 2017, 18, 2479. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C.B.; Li, C.W.; Hua, W.; Wu, C.J.; Zhu, T.J.; Gu, Q.Q. Activation of dormant secondary metabolite production by introducing neomycin resistance into the deep-sea fungus, Aspergillus versicolor ZBY-3. Mar. Drugs 2014, 12, 4326–4352. [Google Scholar] [CrossRef]
- Shaker, S.; Sun, T.T.; Wang, L.Y.; Ma, W.Z.; Wu, D.L.; Guo, Y.W.; Dong, J.; Chen, Y.X.; Zhu, L.P.; Yang, D.P.; et al. Reactive oxygen species altering the metabolite profile of the marine-derived fungus Dichotomomyces cejpii F31-1. Nat. Prod. Res. 2021, 35, 41–48. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yu, Q.M.; Qiao, Z.H.; Li, J.; Tang, H.X.; Chen, G.Y.; Fu, Y.H. Chemical constituents from Morinda citrifolia and their inhibitory activities on proliferation of synoviocytes in vitro. China J. Chin. Mater. Medica 2021, 46, 2519–2526. [Google Scholar]
- Yin, T.P.; Xing, Y.; Cai, L.; Yu, J.; Luo, P.; Ding, Z.T. A new polyketide glycoside from the rhizospheric Clonostachys rogersoniana associated with Panax notoginseng. J. Asian Nat. Prod. Res. 2017, 19, 1258–1263. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Y.; Lu, D.; Du, B.; Xiong, L.; Shi, J.; Yang, L.; Liu, W.; Yuan, X.; Zhang, G.; et al. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3beta,5alpha,8alpha-triol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19). Toxicol. Appl. Pharmacol. 2014, 279, 23–32. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhang, L.; Mao, J.R.; Cheng, X.H.; Lin, R.C.; Zhang, Y.; Sun, W.J. Ergosta-4,6,8(14),22-tetraen-3-one isolated from Polyporus umbellatus prevents early renal injury in aristolochic acid-induced nephropathy rats. J. Pharm. Pharmacol. 2011, 63, 1581–1586. [Google Scholar] [CrossRef]
- Fangkrathok, N.; Sripanidkulchai, B.; Umehara, K.; Noguchi, H. Bioactive ergostanoids and a new polyhydroxyoctane from Lentinus polychrous mycelia and their inhibitory effects on E2-enhanced cell proliferation of T47D cells. Nat. Prod. Res. 2013, 27, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Xiong, J.; Zhai, W.Z.; Cao, L.; Zhang, S.P.; Tang, Y.; Wang, J.; Su, J.J.; Yang, G.X.; Zhao, Y.; et al. Sterols from the polypore macrofungus Fomes fomentarius. Phytochem. 2013, 92, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nahla, E.; Arya, P.; Maneesha, P.; Chitra, K.C. Exposure to the plasticizer dibutyl phthalate causes oxidative stress and neurotoxicity in brain tissue. Environ. Sci. Pollut. Res. Int. 2024, 31, 21399–21414. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Mou, R.; Bao, H.; He, P.; Chang, A. Using transmission electron microscopy to observe the effects of Omphalia albendazole,and praziquantel on the cysticercus of Taenia asiatica. J. Pathog. Biol. 2016, 11, 34–40. [Google Scholar]
- Song, G.; Li, J.; Chen, Y.; Xu, J. Effects of Omphalia lapidescens and Praziquantel on the Infectivity and Ultrastructure of Spirometra erinacei Plerocercoids. Chin. J. Parasitol. Parasit. Dis. 2015, 33, 40–44. [Google Scholar]
- Shen, J. Experimental Study on Six Kinds of Traditional Chinese Medicines against Giardia; Yunnan University of Chinese Medicine: Kunming, China, 2021. [Google Scholar]
- Zhao, G.; You, J.; Xu, C.; Guo, M.; Feng, M. Fermentation and extraction of Leiwan (Omphalia lapidescens Schroet) proteinase and its activation against cysticercus cellulosae in vitro. Chin. Pharm. J. 1997, 32, 43–45. [Google Scholar]
- Zhao, G.; Xu, Z.; Feng, M.; You, J.; Guo, M.; Li, H. Histological changes of Cysticercus cellulosae under the action of proteinase of Omphalia lapidescens in vitro. Chin. J. Parasitol. Parasit. Dis. 1998, 16, 113–116. [Google Scholar]
- Yi, C.; Fu, M.; Cao, X.; Tong, S.; Zheng, Q.; Firempong, C.K.; Jiang, X.; Xu, X.; Yu, J. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J. Agric. Food Chem. 2013, 61, 5961–5971. [Google Scholar] [CrossRef]
- Yi, C.; Zhong, H.; Tong, S.; Cao, X.; Firempong, C.K.; Liu, H.; Fu, M.; Yang, Y.; Feng, Y.; Zhang, H.; et al. Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. Int. J. Nanomed. 2012, 7, 5067–5078. [Google Scholar]
- Yi, C.; Sun, C.; Tong, S.; Cao, X.; Feng, Y.; Firempong, C.K.; Jiang, X.; Xu, X.; Yu, J. Cytotoxic effect of novel Flammulina velutipes sterols and its oral bioavailability via mixed micellar nanoformulation. Int. J. Pharm. 2013, 448, 44–50. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Park, Y.J. Ergosterol Peroxide from the Medicinal Mushroom Ganoderma lucidum Inhibits Differentiation and Lipid Accumulation of 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, T.; Zhang, Y.; Su, J.; Jiao, C.; Xie, Y. Anti-tumor and Anti-angiogenic Ergosterols from Ganoderma lucidum. Front. Chem. 2017, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ma, H. Extraction of Omphalia lapidoscens protease and its inhibitory effect on sarcoma 180 in mice. J. Ningxia Med. Univ. 1979, 50–52. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RE1hRqpi5T24IlAlcMWesyoAcsDWFCBm-7UTirhqtzCxMfpnTaCNtk41LAw6mAt3SaZyF2qF4_bs8KwWRRta0pN678eCj60NnwPFJhtJOuBQDmMozh6o8HONQuqaX0ZP0nmQDEdVY6IE3ISUSAGEsT4mQJH0iKQ4&uniplatform=NZKPT (accessed on 20 August 2024).
- Liang, R. Treatment of 35 cases of extensive stage small cell lung cancer with Lei-wan capsule combined with chemotherapy. J. Tradit. Chin. Med. 2012, 53, 782. [Google Scholar]
- Li, L.; Xiang, B.; Xue, Q.; Liu, J. Clinical observation of Leiwan Capsules combined with gemcitabine in treatment of advanced lung cancer. Drugs Clin. 2018, 33, 390–393. [Google Scholar]
- Yang, Y.L.; Gong, W.Y.; Chen, F.F.; Chen, L.C.; Chen, Y.T. pPeOp from Omphalia lapidescens Schroet induces cell cycle arrest and inhibits the migration of MC-4 gastric tumor cells. Oncol. Lett. 2017, 14, 533–540. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Xu, W.; Lu, Z.; Cheung, M.H.; Lin, M.; Liang, C.; Lou, J.; Chen, Y. Anti-Gastric Cancer Effect of Purified Omphalia lapidescens Protein via Regulating the JAK/STAT3 Signaling Pathway. Nutr. Cancer 2022, 74, 1780–1791. [Google Scholar] [CrossRef]
- Erdogan, F.; Radu, T.B.; Orlova, A.; Qadree, A.K.; de Araujo, E.D.; Israelian, J.; Valent, P.; Mustjoki, S.M.; Herling, M.; Moriggl, R.; et al. JAK-STAT core cancer pathway: An integrative cancer interactome analysis. J. Cell Mol. Med. 2022, 26, 2049–2062. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, Z.; Du, L.; Liang, H.; Chen, Y. Apoptosis mechanism of gastric cancer cell SGC-7901 induced by Omphalia lapidescens protein pPeOp. Chin. Pharmacol. Bull. 2017, 33, 1271–1277. [Google Scholar]
- Lu, Z. The Effect of pPeOp Protein on Gastrointestinal Cancer Was Studied Based on JAK/STAT3 Signaling Pathway; Zhejiang Chinese Medical University: Hangzhou, China, 2018. [Google Scholar]
- Xu, W.; Fu, Z.; Xu, Y.; Cheung, M.H.; Chen, Y.; Lin, M.; Wen, H.; Lv, H.; Liang, C.; Lou, J.; et al. pPe Op inhibits HGC-27 cell proliferation, migration and invasion by upregulating miR-30b-5p and down-regulating the Rac1/Cdc42 pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Kishida, E.; Sone, Y.; Misaki, A. Purification of an antitumor-active, branched (1→3)-beta-D-glucan from Volvariella volvacea, and elucidation of its fine structure. Carbohydr. Res. 1989, 193, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Kim, J.-P.; Na, M.-K.; Jung, H.-J.; Min, B.-S.; Bae, K.-H. Cytotoxicity of Ergosterol Derivatives from the Fruiting Bodies of Hygrophorus russula. Nat. Product. Sci. 2011, 17, 85–89. [Google Scholar]
- Wang, Y.; Dai, O.; Peng, C.; Su, H.-G.; Miao, L.-L.; Liu, L.-S.; Xiong, L. Polyoxygenated ergosteroids from the macrofungus Omphalia lapidescens and the structure-cytotoxicity relationship in a human gastric cancer cell line. Phytochem. Lett. 2018, 25, 99–104. [Google Scholar] [CrossRef]
- Wang, Y. Study on Chemical Constituents of Omphalia lapidescens Schroet and Their Cytotoxicities; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2018. [Google Scholar]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Peterson, Q.P.; Goode, D.R.; West, D.C.; Ramsey, K.N.; Lee, J.J.; Hergenrother, P.J. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol. 2009, 388, 144–158. [Google Scholar] [CrossRef]
- Xu, M.; Shen, L.; Wang, K.; Wang, X. Study on Extraction, Isolation and Antioxidant Activity of Polysaccharidefrom Omphalia Lapidescens. J. Chin. Inst. Food Sci. Technol. 2011, 11, 42–46. [Google Scholar]
- Chen, H. Study on Chemical Compositions and Pharmacal Activity of Omphalia lapidescens Schroet. Jilin Agricultural University: Changchun, China, 2012. [Google Scholar]
- Zhang, Y.; Jiang, Y.; Jia, Y.; Pan, X.; Zhao, T.; Wang, K.; Yan, H.; Ma, Z. Separation of anti-TMV active components and modes of action of Omphalia lapidescens. Pestic. Biochem. Physiol. 2024, 198, 105728. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Wei, F.; Xiao, X.; Lin, Y. Pre-column derivatization RP-HPLC determination of 16 amino acids in Omphalia lapidescens Schroet. Chin. J. Pharm. Anal. 2011, 31, 1631–1635. [Google Scholar]
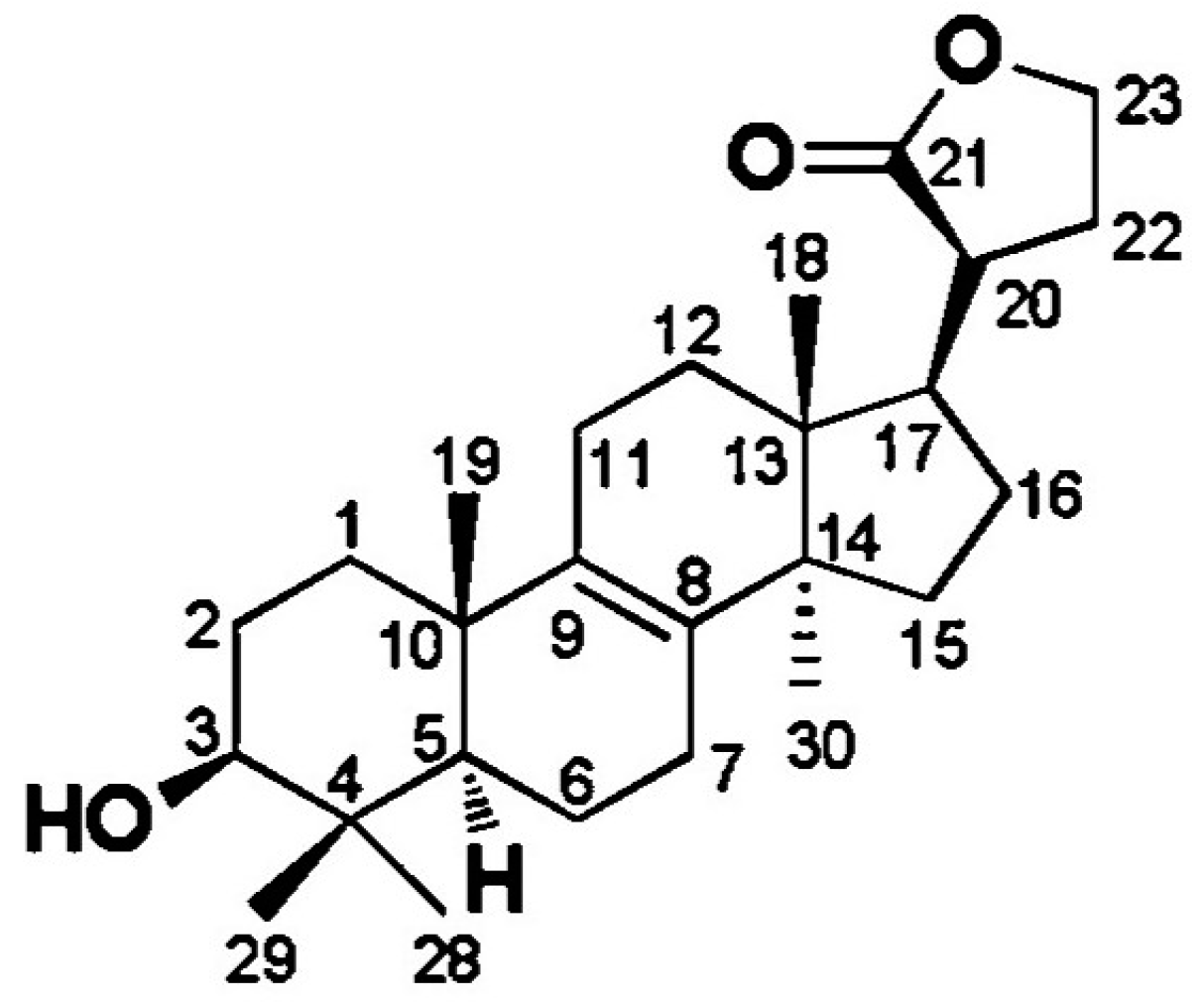
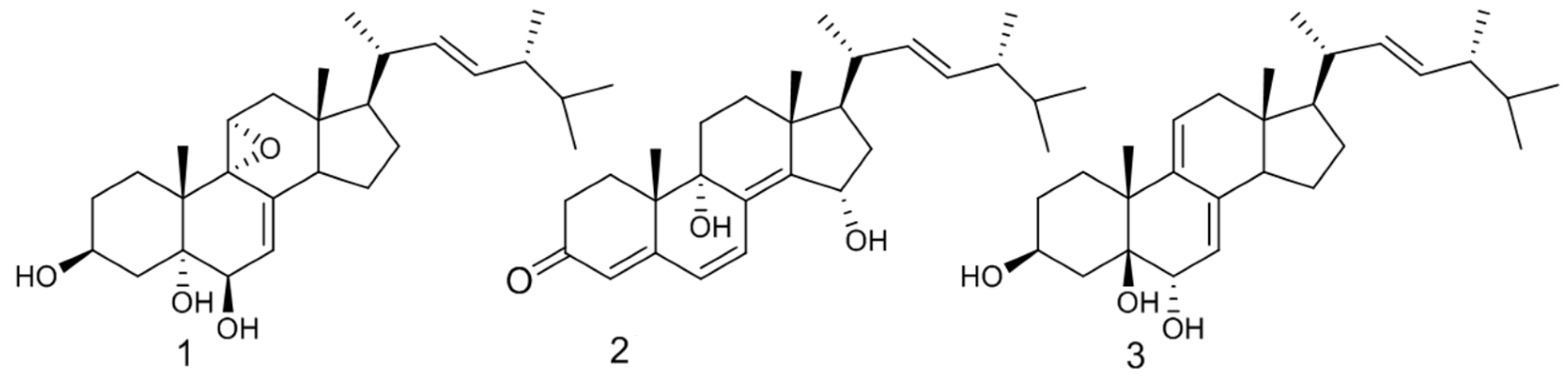
| Classify | Compound Name | Biological Activities |
|---|---|---|
| Epoxides | 5α,6α-epoxyergosta-8,22-diene-3β,7α-diol | Indicated antibacterial activities against Escherichia coli and showed potent inhibitory activity against the proliferation of CACO-2, WiDr, DLD-1, and Colo320 human colorectal adenocarcinoma cells [44]. |
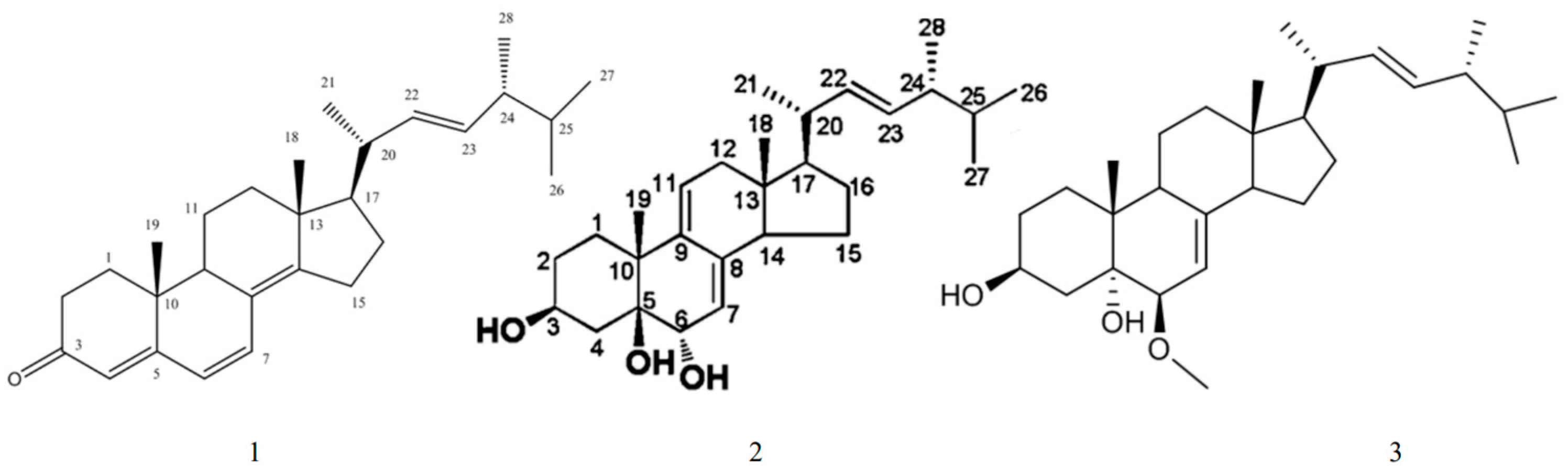
| (22E,24R)-9α,11α-epoxyergosta-7,22-diene-3β,5α,6α-triol | First discovered from O. lapidescens (Figure 3, Compound 1). | |
| 5α,6α-epoxy-3β-hydroxyergosta-22-ene-7-one | No bioactivity was observed. | |
| 5α,6α-epoxy-3β-hydroxy-(22E)-ergosta-8(14),22-dien-7-one | No bioactivity was observed. | |
| 22E-3β-hydroxy-5α,6α,8α,14α-diepoxyergosta-22-en-7-one | Isolated from Aspergillus awamori; mildly toxic to A549 [45]. | |
| 5α,6α-epoxyergosta-8(14),22-diene-3β,7α-diol | Isolated from Pleurotus eryngii, inhibits aromatase [46]. | |
| Hydroxyketones | (22E,24R)-9α,15α-dihydroxyergosta-4,6,8(14),22-tetraene-3-one | First discovered from O. lapidescens (Figure 3, Compound 2). |
| 5α,6α-dihydroxydihydroergosterol | No bioactivity was observed. | |
| 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | Exhibited strong or moderate cytotoxic activities against MCF-7, A549, Hela, and KB cell [47]. | |
| 3β, 5α, 9α-trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | At a concentration of 100 μg/mL, it exhibits an inhibition rate of 51.1% against human chronic myelogenous leukemia (CML) cell K562 and also shows certain inhibitory effects on other human tumor cells such as HL-60, BGC-823, and HeLa cells [47]. | |
| 5,6β-dihydroxy-5α-ergosta-7,22-dien-3β-ylacetate | No bioactivity was observed. | |
| Endoperoxides | (22E)-5α,8α-epidioxyergosta-6,22-dien-3β-ol | Exhibited moderate cytotoxic activity against human prostate cancer cell line LNCaP-C4-2B [48]. |
| Showed significant anti-rheumatoid arthritis activities, displaying inhibitory effects on the proliferation of MH7 A synovial fibroblast cells [49]. | ||
| Inhibited iNOS activity in LPS-induced macrophages and decreased nitrite levels [50]. | ||
| (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol | Effectively inhibited estrogen biosynthesis and reduced the mRNA and protein expression levels of aromatase in human ovarian granulosa-like KGN cells [51]. | |
| Ergosterol | ergosta-4,6,8(14),22-tetraen-3-one | More common in fungi, serves as novel AchE inhibitor, and prevents early renal injury in aristolochic acid-induced nephropathy rats [52]. |
| (22E,24R)-ergosta-8,22-diene-3β,5α,6β,7α-tetrol | No bioactivity was observed. | |
| (22E,24R)-ergosta-7,22-dien-3β,5α,6β-triol (cerevisterol) | From the mycelia of Lentinus polychrous, a Thai local edible mushroom. Can inhibit estrogen-induced proliferation of breast cancer T47D cells [53]. | |
| (22E)-ergosta-7,22-diene-3β,5β,6α-triol | No bioactivity was observed. | |
| (22E,24R)-ergosta-7,22-diene-3β,5α,6α,9α-tetrol | No bioactivity was observed. | |
| (22E,24R)-3β,5α-dihydroxy-6β-ethoxyergosta-7,22-diene (fomentarol C) | Fomes fomentarius, which has shown slight cytotoxic effects against HCT116 colon cancer cells [54]. | |
| (3β,5α,6β,22E)-6-methoxyergosta-7,22-diene-3,5-diol | No bioactivity was observed. | |
| (22E,24R)-ergosta-7,9(11),22-triene-3β,5β,6α-trio | First discovered from O. lapidescens (Figure 3, Compound 3). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Wang, L.; He, D. Research on the Action and Mechanism of Pharmacological Components of Omphalia lapidescens. Int. J. Mol. Sci. 2024, 25, 11016. https://doi.org/10.3390/ijms252011016
Xu K, Wang L, He D. Research on the Action and Mechanism of Pharmacological Components of Omphalia lapidescens. International Journal of Molecular Sciences. 2024; 25(20):11016. https://doi.org/10.3390/ijms252011016
Chicago/Turabian StyleXu, Keyang, Li Wang, and Dan He. 2024. "Research on the Action and Mechanism of Pharmacological Components of Omphalia lapidescens" International Journal of Molecular Sciences 25, no. 20: 11016. https://doi.org/10.3390/ijms252011016
APA StyleXu, K., Wang, L., & He, D. (2024). Research on the Action and Mechanism of Pharmacological Components of Omphalia lapidescens. International Journal of Molecular Sciences, 25(20), 11016. https://doi.org/10.3390/ijms252011016





