Epigenetic Modifications Are Involved in Transgenerational Inheritance of Cadmium Reproductive Toxicity in Mouse Oocytes
Abstract
1. Introduction
2. Results
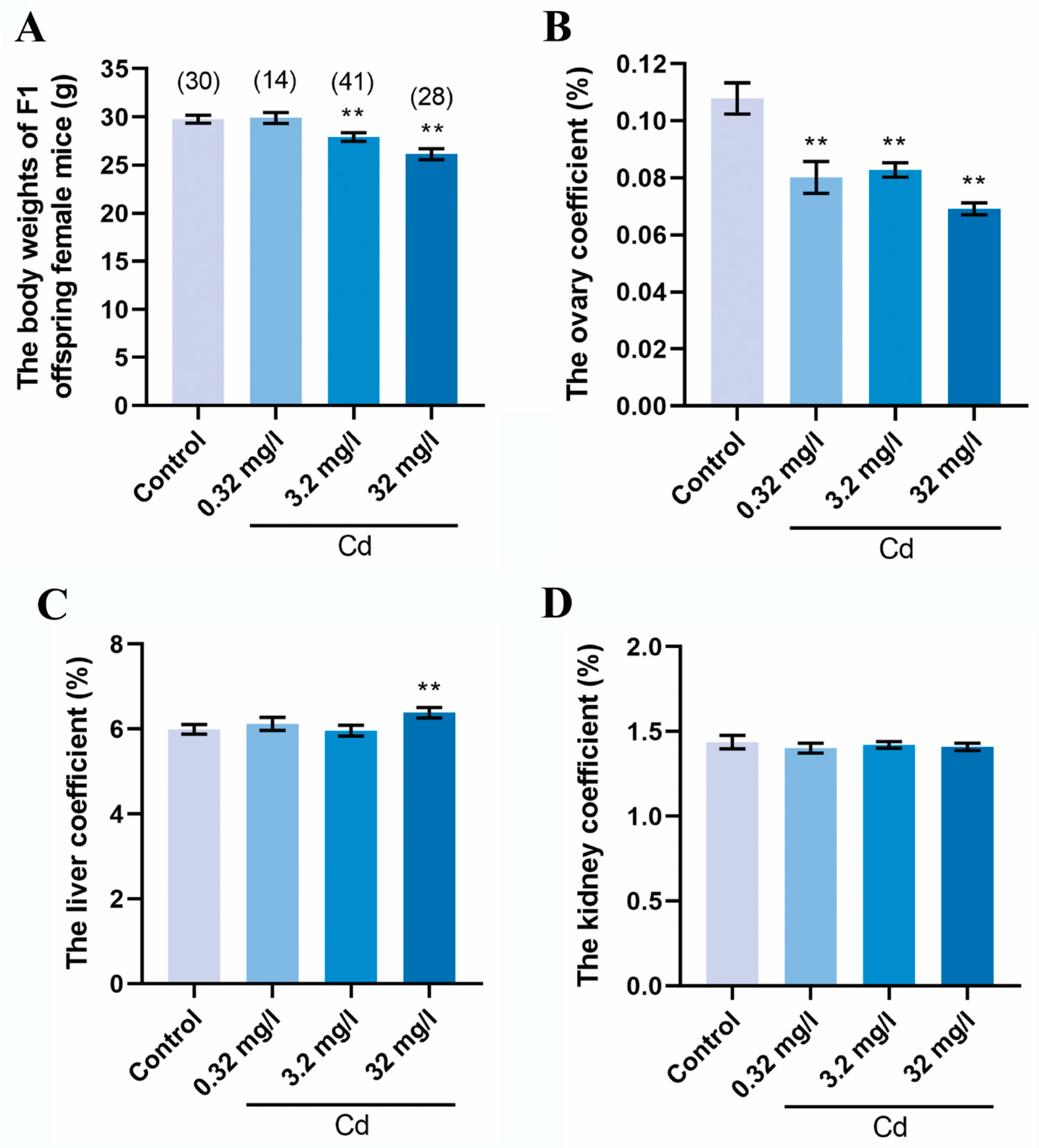
2.1. Cadmium Exposure during Pregnancy Reduced the Body Weight and the Ovary Coefficient of F1 Female Mice
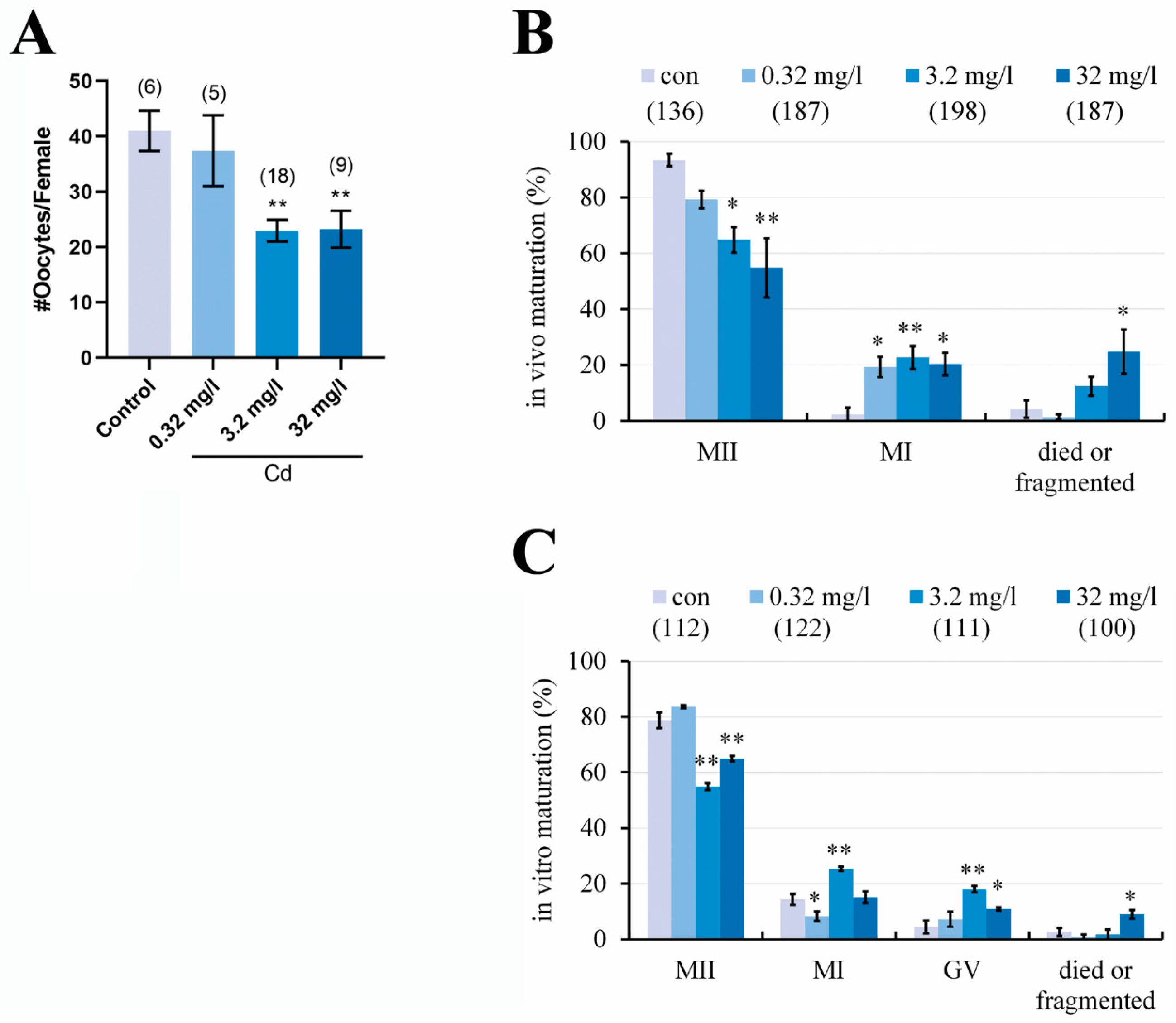
2.2. Cadmium Exposure during Pregnancy Reduced the Number of Ovulated Oocytes and Impaired the Oocyte Maturation in F1 Mice
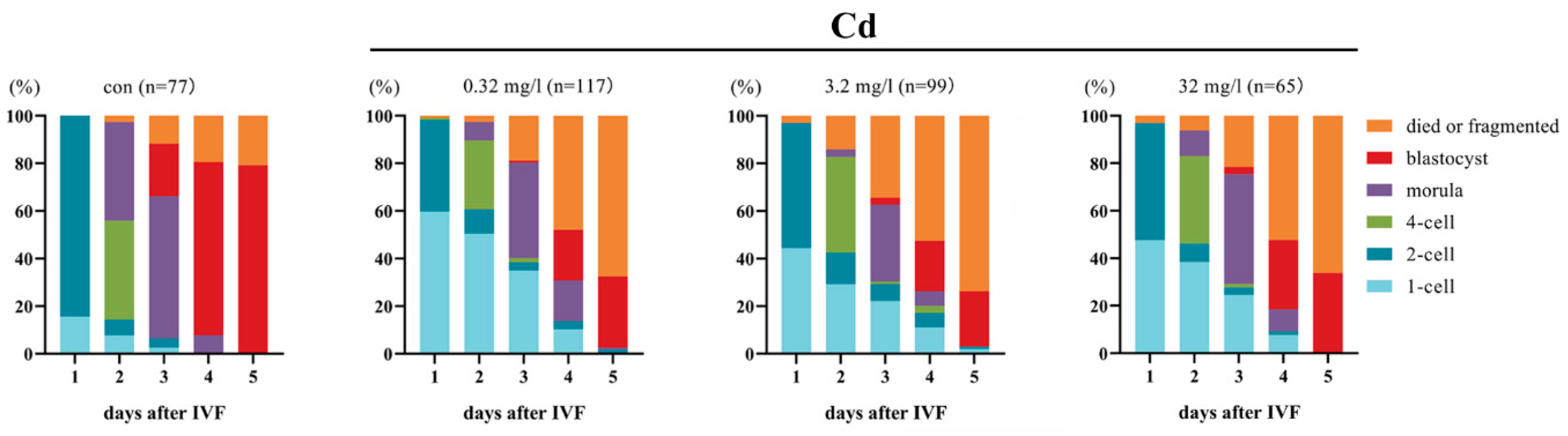
2.3. Cadmium Exposure during Pregnancy Impairs the Embryonic Development in F1 Mice
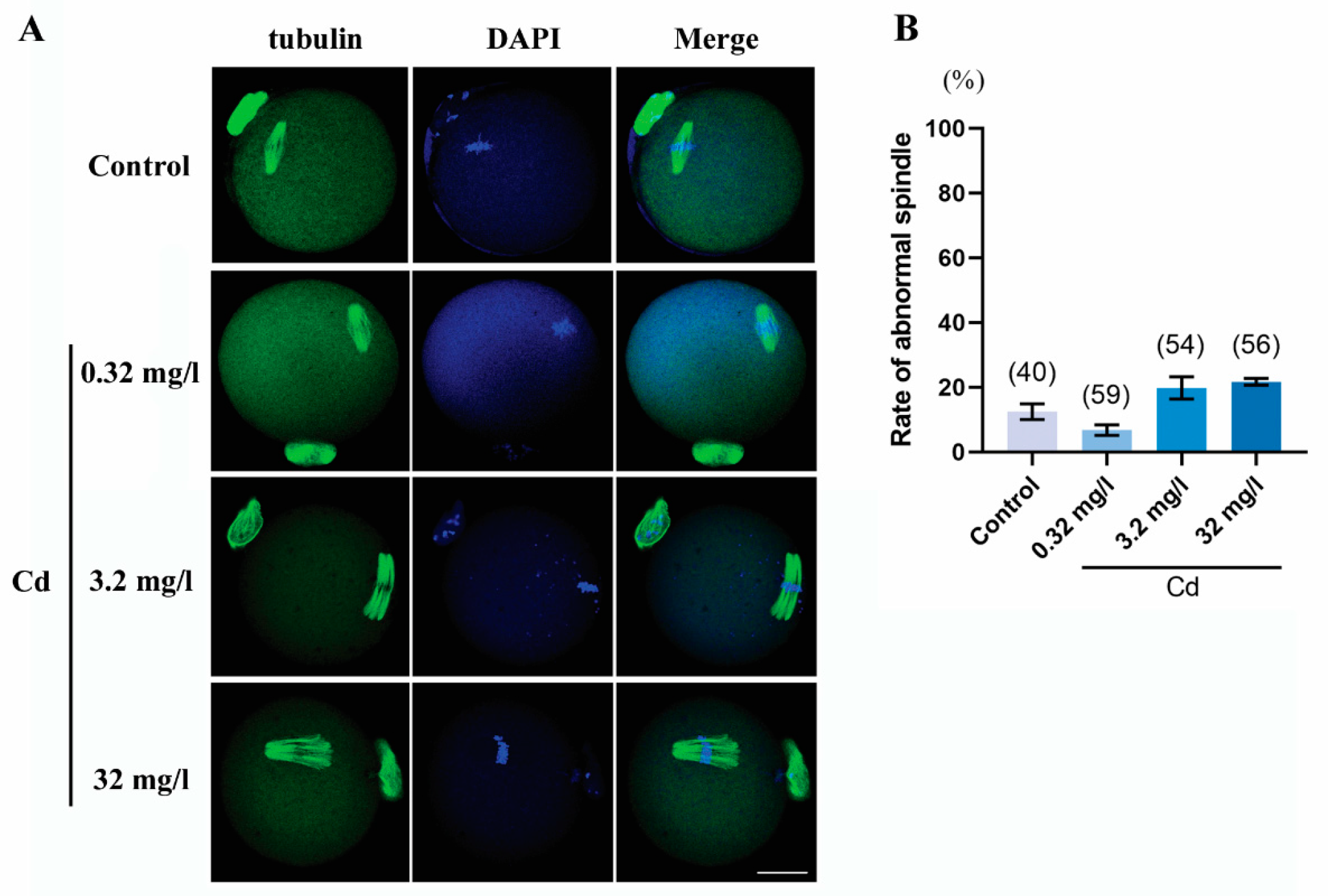
2.4. Cadmium Exposure during Pregnancy Did Not Affect Meiotic Spindle Morphology in F1 Mouse Oocytes
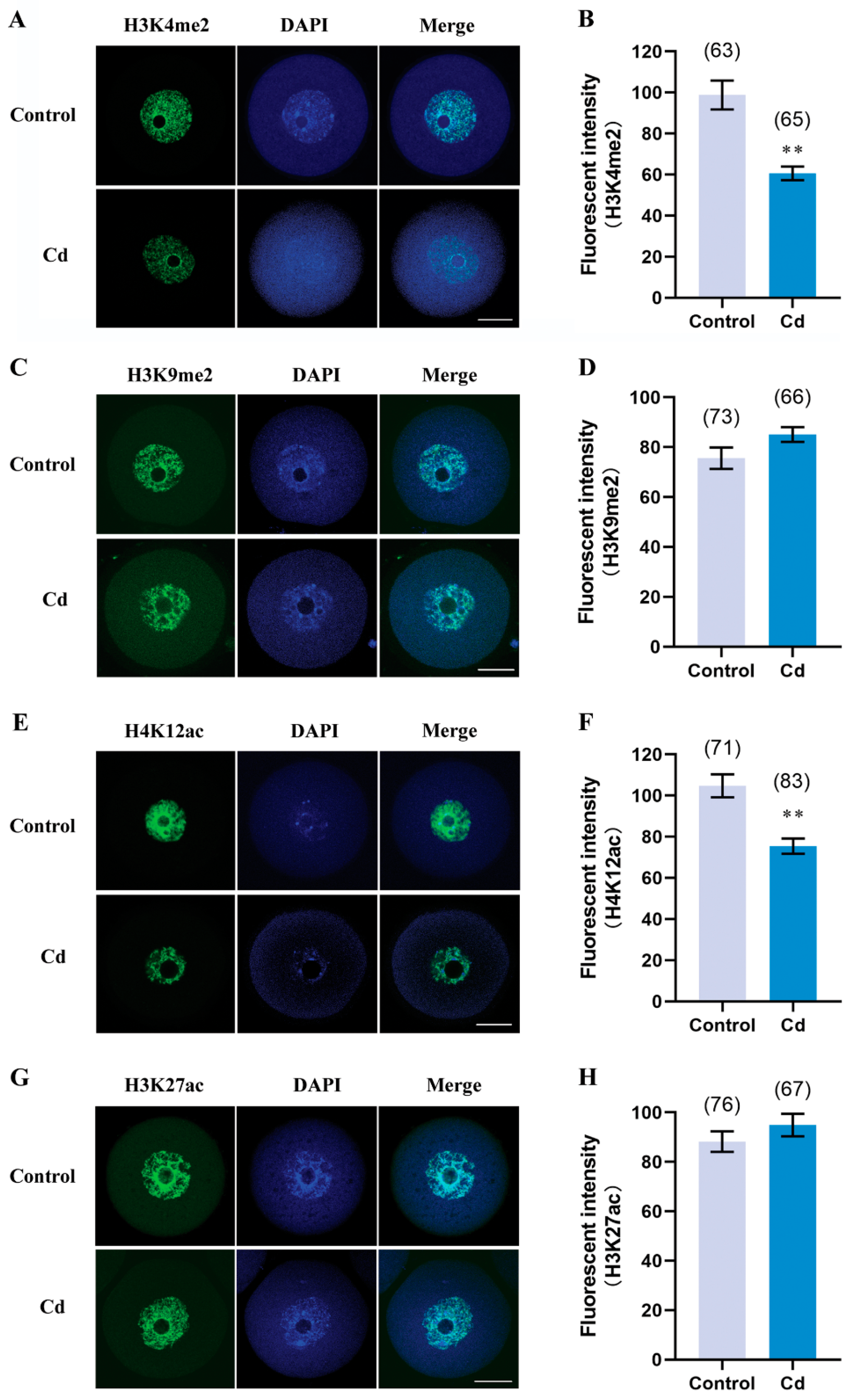
2.5. Cadmium Exposure during Pregnancy Decreased H3K4me2 and H4K12ac in F1 Mouse Oocytes
2.6. Cadmium Exposure during Pregnancy Altered DNA Methylation of H19 in F1 Mouse Oocytes
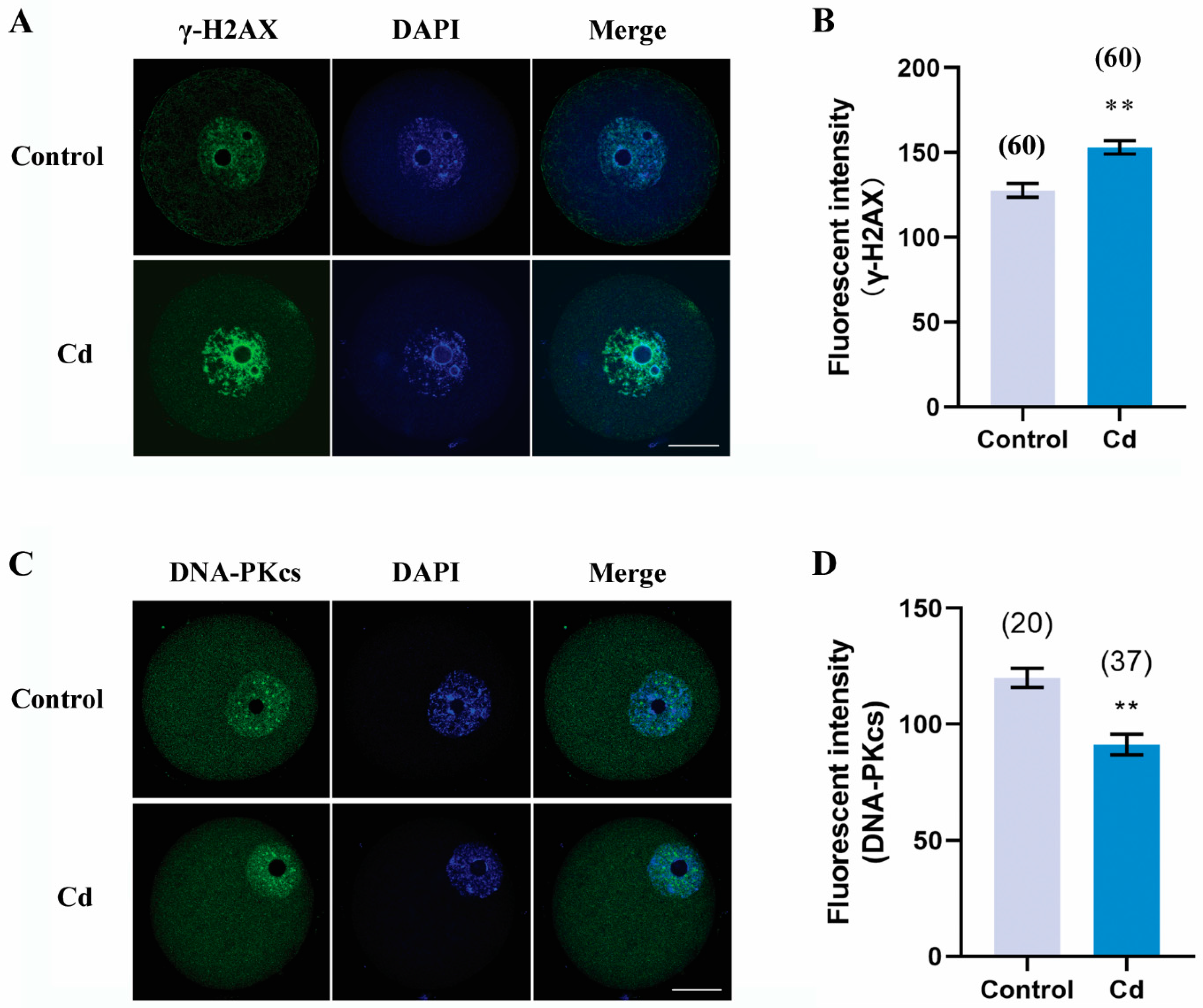
2.7. Cadmium Exposure during Pregnancy Caused DNA Damage and Decreased DNA Repair in F1 Mouse Oocytes
2.8. Cadmium Exposure during Pregnancy Did Not Affect the ROS Level and the Mitochondrial Activity in F1 Mouse Oocytes
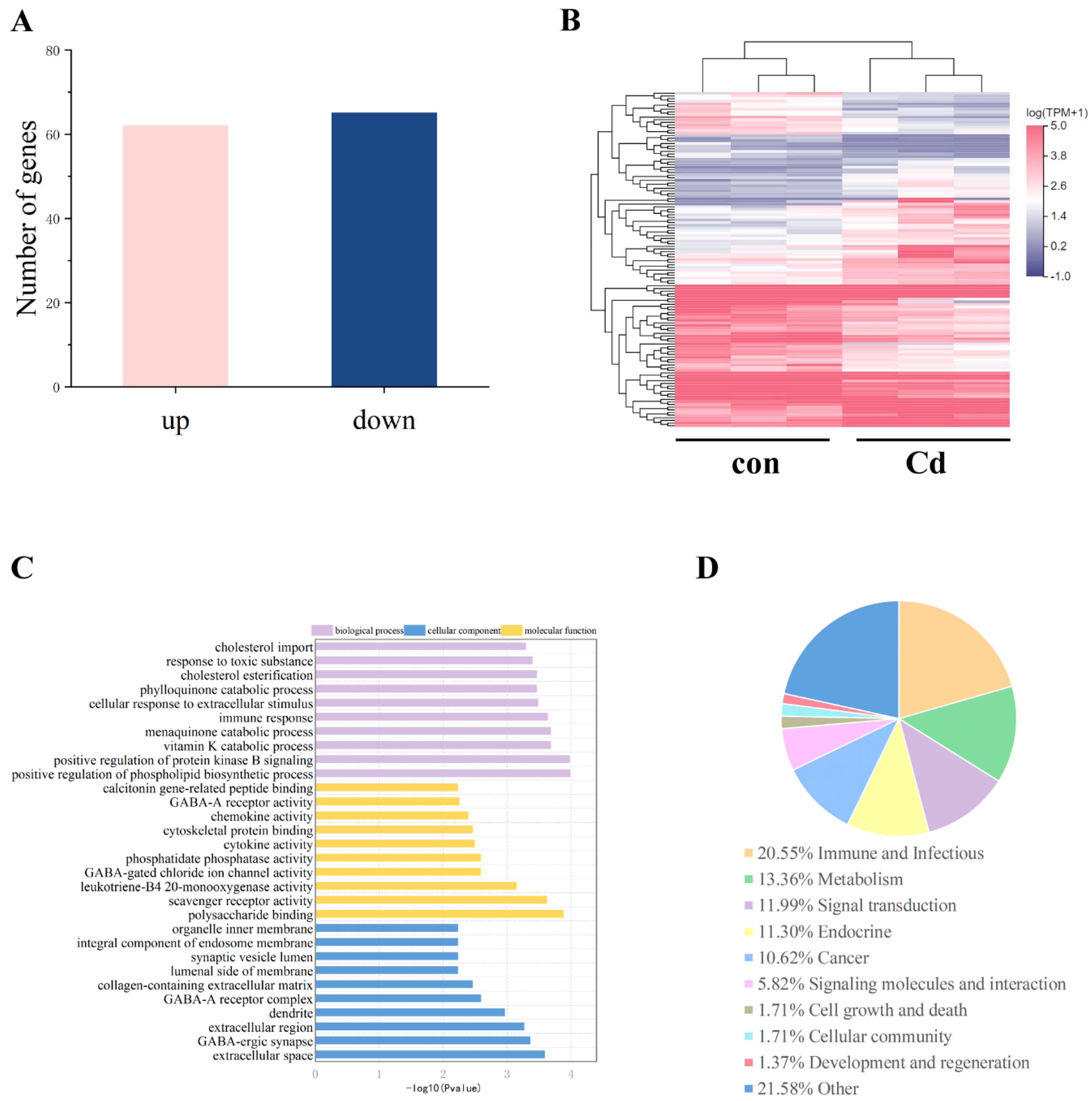
2.9. Cadmium Exposure during Pregnancy Altered the Transcriptome in F1 Mouse Ovaries
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Experimental Design
4.3. Organ Coefficient, MII-Oocyte Collection, and In Vitro Fertilization (IVF)
4.4. Analysis of Meiotic Spindles
4.5. GV-Oocyte Collection and In Vitro Maturation
4.6. Immunofluorescence Staining and Confocal Microscopy Analysis
4.7. DNA Methylation Analysis
4.8. ROS Measurement and Mitochondria Staining
4.9. RNA-Seq and Bioinformatics Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berglund, M.; Larsson, K.; Grander, M.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Castano, A.; Esteban, M.; Angerer, J.; Koch, H.M.; et al. Exposure determinants of cadmium in European mothers and their children. Environ. Res. 2015, 141, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Liu, Y.; Zhang, J.H.; Liu, Y.F.; Cao, J.Q.; Huang, Z.T.; Yuan, Y.; Bian, J.C.; Liu, Z.P. Cadmium Exposure of Female Mice Impairs the Meiotic Maturation of Oocytes and Subsequent Embryonic Development. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 164, 289–299. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Z.; Yang, F.; Zhu, M.; Cao, J.; Chen, J.; Lin, Y.; Guo, S.; Li, J.; Liu, Z. Cadmium disturbs epigenetic modification and induces DNA damage in mouse preimplantation embryos. Ecotoxicol. Environ. Saf. 2021, 219, 112306. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, Y.C.; Wang, J.X.; Zhu, H.D.; Liu, Q.F.; Fan, Y.G.; Wang, N.F.; Zhao, J.H.; Liu, H.S.; Ou-Yang, L.; et al. Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: A study on healthy pregnant women in China. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2004, 39, 2507–2515. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Zhu, H.L.; Nan, Y.; Cao, X.L.; Shi, X.T.; Yi, S.J.; Feng, Y.J.; Zhang, C.; Gao, L.; Chen, Y.H.; et al. Maternal cadmium exposure during late pregnancy causes fetal growth restriction via inhibiting placental progesterone synthesis. Ecotoxicol. Environ. Saf. 2020, 187, 109879. [Google Scholar] [CrossRef]
- Zhu, H.L.; Dai, L.M.; Xiong, Y.W.; Shi, X.T.; Liu, W.B.; Fu, Y.T.; Zhou, G.X.; Zhang, S.; Gao, L.; Zhang, C.; et al. Gestational exposure to environmental cadmium induces placental apoptosis and fetal growth restriction via Parkin-modulated MCL-1 degradation. J. Hazard. Mater. 2022, 424 Pt A, 127268. [Google Scholar] [CrossRef]
- Chatterjee, M.; Kortenkamp, A. Cadmium exposures and deteriorations of cognitive abilities: Estimation of a reference dose for mixture risk assessments based on a systematic review and confidence rating. Environ. Health A Glob. Access Sci. Source 2022, 21, 69. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, Z.; Zhu, Y.I.; Li, S.; Zhang, L.; Luo, Y. Effects of paternal cadmium exposure on the sperm quality of male rats and the neurobehavioral system of their offspring. Exp. Ther. Med. 2015, 10, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Hu, X.; Yang, P.; Sun, L.; Gu, W.; Zhang, M. The effects of cadmium on the development of Drosophila and its transgenerational inheritance effects. Toxicology 2021, 462, 152931. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mu, Y.; Xu, L.; Han, X.; Gu, W.; Zhang, M. Transgenerational inheritance of wing development defects in Drosophila melanogaster induced by cadmium. Ecotoxicol. Environ. Saf. 2023, 250, 114486. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, Y.; Lv, L.; Wang, D.; Yang, G.; Jin, Y.; Wang, Q. Transgenerational effects of co-exposure to cadmium and carbofuran on zebrafish based on biochemical and transcriptomic analyses. J. Hazard. Mater. 2022, 439, 129644. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Q.; Sun, X.; Li, Y. The environment and female reproduction: Potential mechanism of cadmium poisoning to the growth and development of ovarian follicle. Ecotoxicol. Environ. Saf. 2022, 244, 114029. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Cao, Y.; Zhao, H.; Song, R.; Zou, H.; Gu, J.; Yuan, Y.; Bian, J.; Zhu, J.; et al. The effect of P2X7 on cadmium-induced osteoporosis in mice. J. Hazard. Mater. 2021, 405, 124251. [Google Scholar] [CrossRef]
- Yang, J.L.; Chen, S.; Xi, J.F.; Lin, X.Y.; Xue, R.Y.; Ma, L.Q.; Zhou, D.; Li, H.B. Sex-dependent effects of rice cadmium exposure on body weight, gut microflora, and kidney metabolomics based on a mouse model. Sci. Total Environ. 2024, 908, 168498. [Google Scholar] [CrossRef]
- Gliga, A.R.; Malin Igra, A.; Hellberg, A.; Engstrom, K.; Raqib, R.; Rahman, A.; Vahter, M.; Kippler, M.; Broberg, K. Maternal exposure to cadmium during pregnancy is associated with changes in DNA methylation that are persistent at 9 years of age. Environ. Int. 2022, 163, 107188. [Google Scholar] [CrossRef]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef]
- Wang, R.; Sang, P.; Guo, Y.; Jin, P.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2023, 405 Pt A, 134666. [Google Scholar] [CrossRef]
- Tuffour, A.; Adebayiga Kosiba, A.; Addai Peprah, F.; Gu, J.; Zhou, Y.; Shi, H. Cadmium-induced stress: A close look at the relationship between autophagy and apoptosis. Toxicol. Sci. Off. J. Soc. Toxicol. 2023, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Wu, T.; Jiang, X.; Jia, H.; Qing, S.; An, Q.; Zhang, Y.; Su, J. Reproductive toxicity of acute Cd exposure in mouse: Resulting in oocyte defects and decreased female fertility. Toxicol. Appl. Pharmacol. 2019, 379, 114684. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Castle, V.; Taketo, T. Interplay between Caspase 9 and X-linked Inhibitor of Apoptosis Protein (XIAP) in the oocyte elimination during fetal mouse development. Cell Death Dis. 2019, 10, 790. [Google Scholar] [CrossRef]
- Ene, A.C.; Park, S.; Edelmann, W.; Taketo, T. Caspase 9 is constitutively activated in mouse oocytes and plays a key role in oocyte elimination during meiotic prophase progression. Dev. Biol. 2013, 377, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Pandya, C.; Gupta, S.; Gupta, S. Biochemical and molecular effects of gestational and lactational coexposure to lead and cadmium on ovarian steroidogenesis are associated with oxidative stress in F1 generation rats. J. Biochem. Mol. Toxicol. 2010, 24, 384–394. [Google Scholar] [CrossRef]
- Dong, F.; Li, J.; Lei, W.L.; Wang, F.; Wang, Y.; Ouyang, Y.C.; Hou, Y.; Wang, Z.B.; Schatten, H.; Sun, Q.Y. Chronic cadmium exposure causes oocyte meiotic arrest by disrupting spindle assembly checkpoint and maturation promoting factor. Reprod. Toxicol. 2020, 96, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pizzaia, D.; Nogueira, M.L.; Mondin, M.; Carvalho, M.E.A.; Piotto, F.A.; Rosario, M.F.; Azevedo, R.A. Cadmium toxicity and its relationship with disturbances in the cytoskeleton, cell cycle and chromosome stability. Ecotoxicology 2019, 28, 1046–1055. [Google Scholar] [CrossRef]
- Guo, A.H.; Kumar, S.; Lombard, D.B. Epigenetic mechanisms of cadmium-induced nephrotoxicity. Curr. Opin. Toxicol. 2022, 32, 100372. [Google Scholar] [CrossRef]
- Lawless, L.; Xie, L.; Zhang, K. The inter- and multi-generational epigenetic alterations induced by maternal cadmium exposure. Front. Cell Dev. Biol. 2023, 11, 1148906. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Q.; Sun, Q.Y. Histone modifications during mammalian oocyte maturation: Dynamics, regulation and functions. Cell Cycle 2010, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Gu, Y.L.; Jiang, Y.Q.; Yu, J.; Chen, C.; Shi, C.L.; Li, H. Photoaged Polystyrene Nanoplastics Result in Transgenerational Reproductive Toxicity Associated with the Methylation of Histone H3K4 and H3K9 in. Environ. Sci. Technol. 2023, 57, 19341–19351. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Liao, V.H. Transgenerational Reproductive Effects of Arsenite Are Associated with H3K4 Dimethylation and SPR-5 Downregulation in Caenorhabditis elegans. Environ. Sci. Technol. 2016, 50, 10673–10681. [Google Scholar] [CrossRef]
- Huang, J.; Li, T.; Ding, C.H.; Brosens, J.; Zhou, C.Q.; Wang, H.H.; Xu, Y.W. Insufficient histone-3 lysine-9 deacetylation in human oocytes matured in vitro is associated with aberrant meiosis. Fertil. Steril. 2012, 97, 178–184.e3. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Zhang, Y. CBP/p300 and HDAC activities regulate H3K27 acetylation dynamics and zygotic genome activation in mouse preimplantation embryos. EMBO J. 2022, 41, e112012. [Google Scholar] [CrossRef]
- Pierron, F.; Bureau du Colombier, S.; Moffett, A.; Caron, A.; Peluhet, L.; Daffe, G.; Lambert, P.; Elie, P.; Labadie, P.; Budzinski, H.; et al. Abnormal ovarian DNA methylation programming during gonad maturation in wild contaminated fish. Environ. Sci. Technol. 2014, 48, 11688–11695. [Google Scholar] [CrossRef] [PubMed]
- Soto-Palma, C.; Niedernhofer, L.J.; Faulk, C.D.; Dong, X. Epigenetics, DNA damage, and aging. J. Clin. Investig. 2022, 132, e158446. [Google Scholar] [CrossRef]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Rev. Mutat. Res. 2019, 780, 37–47. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, F.; Liao, H.; Chen, W.; Dai, X.; Peng, C.; Li, Z.; Wang, H.; Zhang, T.; Cao, H. Molybdenum and cadmium cause blood-testis barrier dysfunction through ROS-mediated NLRP3 inflammasome activation in sheep. Sci. Total Environ. 2024, 906, 167267. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Yamanobe, Y.; Nagahara, N.; Matsukawa, T.; Ito, T.; Niimori-Kita, K.; Chiba, M.; Yokoyama, K.; Takizawa, T. Sex differences in shotgun proteome analyses for chronic oral intake of cadmium in mice. PLoS ONE 2015, 10, e0121819. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Q.; Si, Y.J.; Cheng, L.Y.; Xu, B.Z.; Wang, Q.W.; Zhang, X.; Wang, H.; Liu, Z.P. Sodium fluoride disrupts DNA methylation of H19 and Peg3 imprinted genes during the early development of mouse embryo. Arch. Toxicol. 2014, 88, 241–248. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Guo, S.; Cao, J.; Zhao, H.; Ma, Y.; Zou, H.; Ju, H.; Liu, Z.; Li, J. Epigenetic Modifications Are Involved in Transgenerational Inheritance of Cadmium Reproductive Toxicity in Mouse Oocytes. Int. J. Mol. Sci. 2024, 25, 10996. https://doi.org/10.3390/ijms252010996
Zhu J, Guo S, Cao J, Zhao H, Ma Y, Zou H, Ju H, Liu Z, Li J. Epigenetic Modifications Are Involved in Transgenerational Inheritance of Cadmium Reproductive Toxicity in Mouse Oocytes. International Journal of Molecular Sciences. 2024; 25(20):10996. https://doi.org/10.3390/ijms252010996
Chicago/Turabian StyleZhu, Jiaqiao, Shuai Guo, Jiangqin Cao, Hangbin Zhao, Yonggang Ma, Hui Zou, Huiming Ju, Zongping Liu, and Junwei Li. 2024. "Epigenetic Modifications Are Involved in Transgenerational Inheritance of Cadmium Reproductive Toxicity in Mouse Oocytes" International Journal of Molecular Sciences 25, no. 20: 10996. https://doi.org/10.3390/ijms252010996
APA StyleZhu, J., Guo, S., Cao, J., Zhao, H., Ma, Y., Zou, H., Ju, H., Liu, Z., & Li, J. (2024). Epigenetic Modifications Are Involved in Transgenerational Inheritance of Cadmium Reproductive Toxicity in Mouse Oocytes. International Journal of Molecular Sciences, 25(20), 10996. https://doi.org/10.3390/ijms252010996






