Systematic Review of Peptide CAQK: Properties, Applications, and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Assessing Risk of Bias
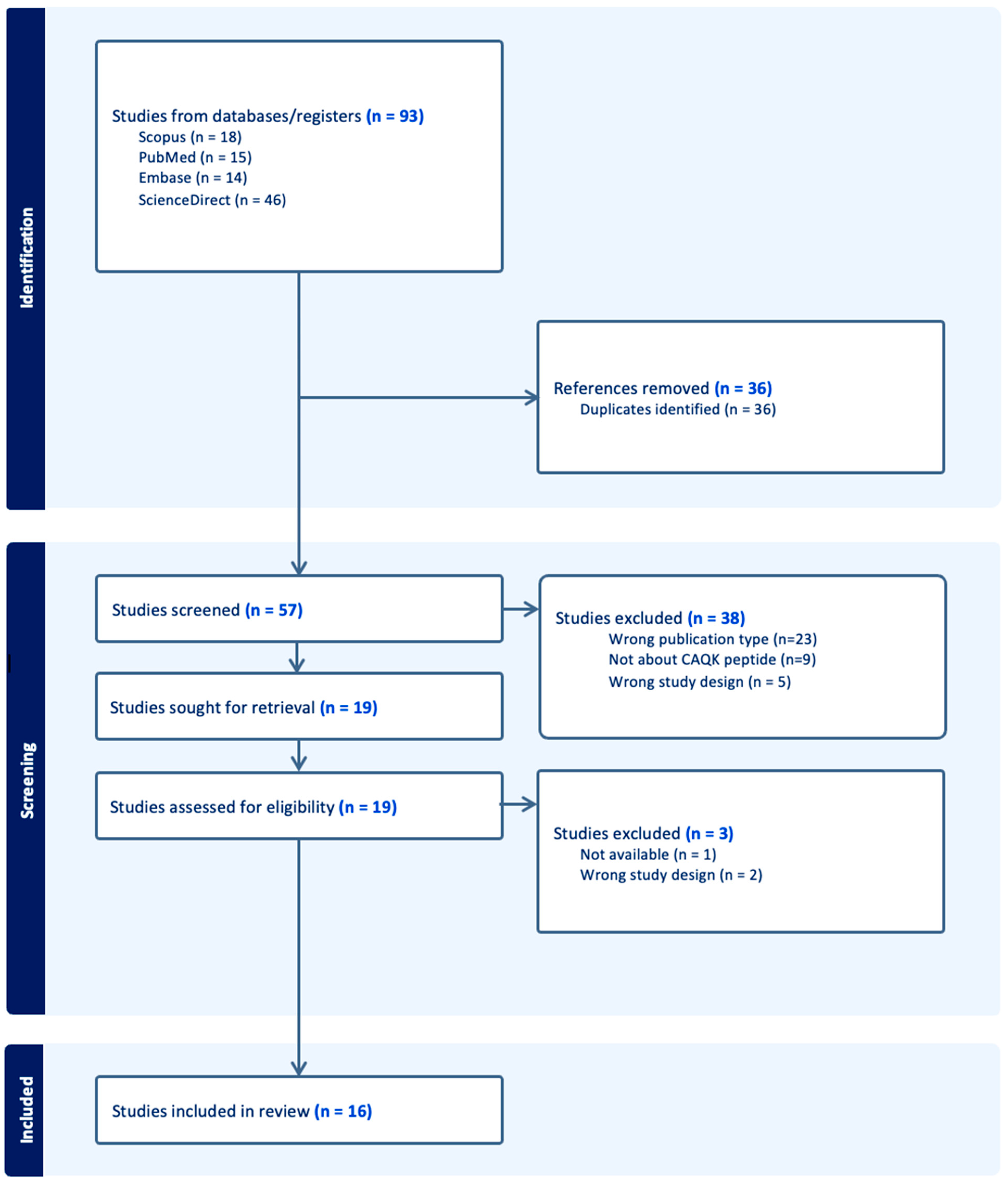
3. Results
3.1. Informational Flow
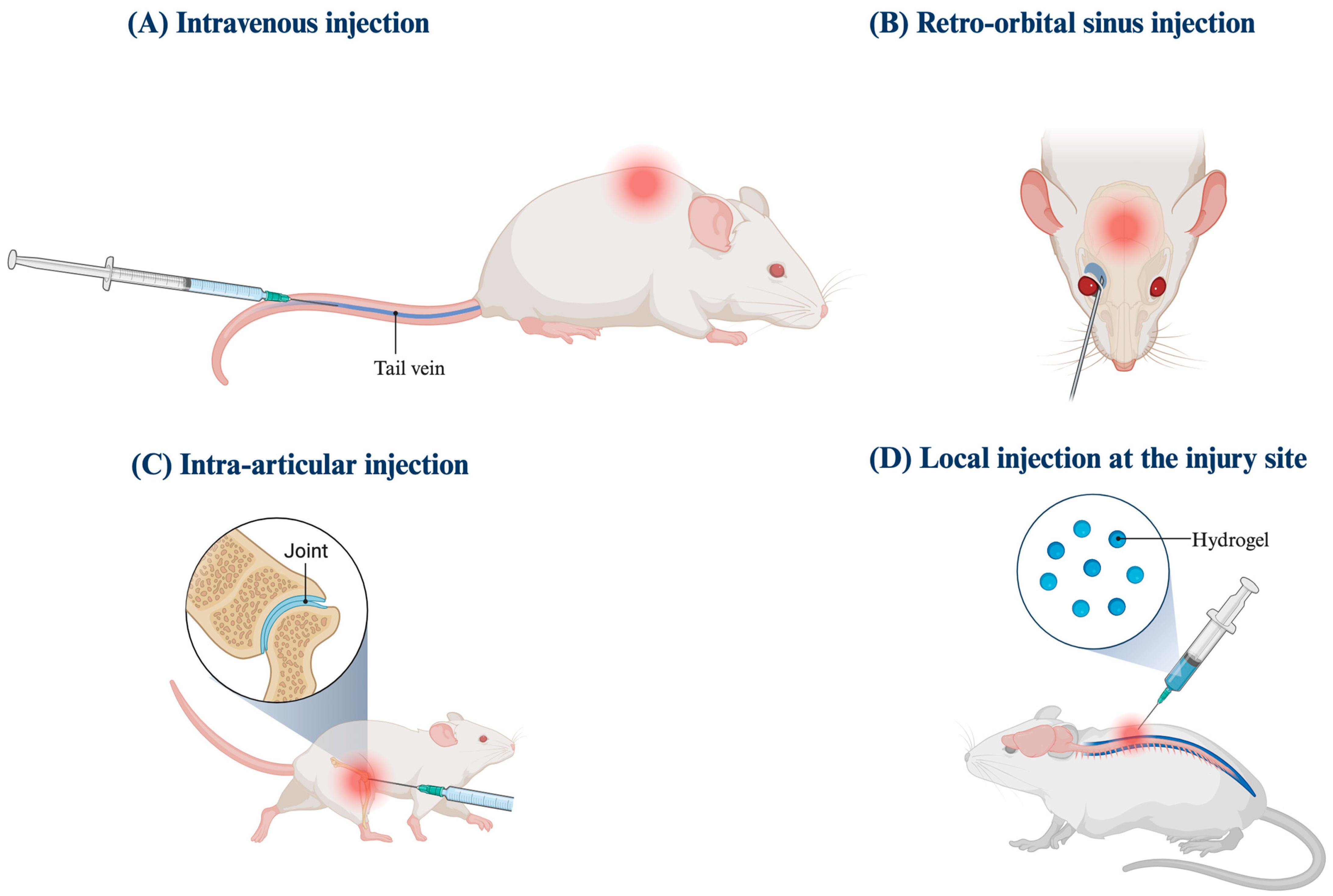
3.2. Animal Models
3.3. Human Correlations
3.4. Conjugation/Target and Therapeutics
3.5. Efficacy
3.6. Dosing
3.7. Cellular Level
3.8. Functional Level
3.9. Side Effects
4. Discussion
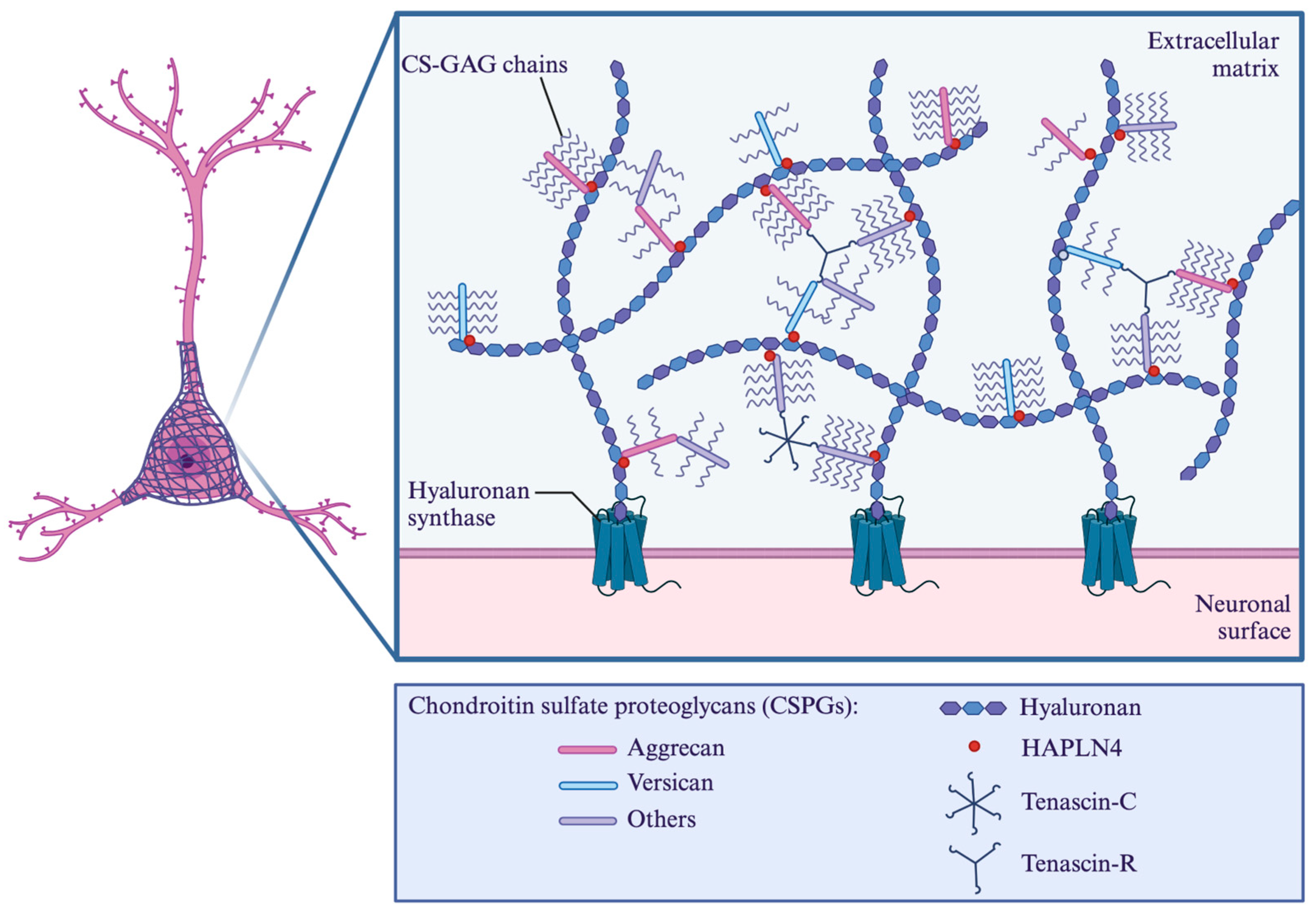
4.1. Origin
4.2. Protein Specificity
4.3. CAQK Applications
4.4. Benefits/Side Effects
4.5. Dosing
4.6. Human Targeting
4.7. Assessment and Risk of Bias
4.8. Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Over 1 in 3 People Affected by Neurological Conditions, the Leading Cause of Illness and Disability Worldwide. March 2024. Available online: https://www.who.int/news/item/14-03-2024-over-1-in-3-people-affected-by-neurological-conditions--the-leading-cause-of-illness-and-disability-worldwide (accessed on 29 July 2024).
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Saf. Res. 2012, 43, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, P.; Wei, X.; Yang, Y.; Al Mamun, A.; Zhang, X.; Zhu, Y.; Mo, T.; Zhang, H.; Jiang, C.; et al. Barrier-penetrating liposome targeted delivery of basic fibroblast growth factor for spinal cord injury repair. Mater. Today Bio. 2023, 18, 100546. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zeng, S.; Liu, X.; Shi, H.; Zhang, R.; Wang, B.; Zhou, C.; Yu, T. Synthesis and Characterization of a Silica-Based Drug Delivery System for Spinal Cord Injury Therapy. Nano-Micro Lett. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.S.; Omay, S.B.; Sheth, K.N.; Zhou, J. Nanoparticle-based drug delivery for the treatment of traumatic brain injury. Expert Opin. Drug Deliv. 2023, 20, 55–73. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ciciriello, A.J.; Dumont, C.M.; Pearson, R.M. Nanoparticle-Based Delivery to Treat Spinal Cord Injury—A Mini-Review. AAPS PharmSciTech 2021, 22, 101. [Google Scholar] [CrossRef]
- Lindsay, S.L.; McCanney, G.A.; Willison, A.G.; Barnett, S.C. Multi-target approaches to CNS repair: Olfactory mucosa-derived cells and heparan sulfates. Nat. Rev. Neurol. 2020, 16, 229–240. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Mann, A.P.; Scodeller, P.; Hussain, S.; Joo, J.; Kwon, E.; Braun, G.B.; Mölder, T.; She, Z.-G.; Kotamraju, V.R.; Ranscht, B.; et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 2016, 7, 11980. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Liang, C.; Wang, C.; Zhou, X.; Ying, L.; Tao, Y.; Xu, H.; Shu, J.; Huang, X.; et al. Scar Tissue-Targeting Polymer Micelle for Spinal Cord Injury Treatment. Small 2020, 16, e1906415. [Google Scholar] [CrossRef]
- Li, T.; Higgins, J.P.T.; Deeks, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5. 2019. Available online: https://training.cochrane.org/handbook (accessed on 9 October 2024).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Xu, H.; Zhao, Y.; Li, Z.; Li, J.; Wang, H.; Zhuge, D.; Guo, X.; Xu, H.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, H.; Gou, X.; Wu, X.; Zhang, S.; Deng, G.; Chen, Q.; Wu, P.; Zhao, H.; Gou, X.; et al. Targeted delivery of polypeptide nanoparticle for treatment of traumatic brain injury. Int. J. Nanomed. 2019, 14, 4059–4069. [Google Scholar] [CrossRef] [PubMed]
- Abi-Ghanem, C.; Jonnalagadda, D.; Chun, J.; Kihara, Y.; Ranscht, B. CAQK, a peptide associating with extracellular matrix components targets sites of demyelinating injuries. Front. Cell Neurosci. 2022, 16, 908401. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jing, P.; Yang, L.; Wan, Y.; Du, X.; Wei, J.; Zhou, M.; Liu, Z.; Lin, Y.; Zhong, Z. CAQK modification enhances the targeted accumulation of metformin-loaded nanoparticles in rats with spinal cord injury. Nanomedicine 2022, 41, 102526. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.; Wang, J.; Ye, H.; Wan, Y.; Du, X.; Sun, X.; Zhou, M.; Lin, Y.; Jing, P.; et al. Nanoformulated metformin enhanced the treatment of spinal cord injury. Chem. Eng. J. 2022, 446, 137227. [Google Scholar] [CrossRef]
- Rong, Y.; Wang, Z.; Tang, P.; Wang, J.; Ji, C.; Chang, J.; Zhu, Y.; Ye, W.; Bai, J.; Liu, W.; et al. Engineered extracellular vesicles for delivery of siRNA promoting targeted repair of traumatic spinal cord injury. Bioact. Mater. 2023, 23, 328–342. [Google Scholar] [CrossRef]
- Waggoner, L.E.; Kang, J.; Zuidema, J.M.; Vijayakumar, S.; Hurtado, A.A.; Sailor, M.J.; Kwon, E.J. Porous Silicon Nanoparticles Targeted to the Extracellular Matrix for Therapeutic Protein Delivery in Traumatic Brain Injury. Bioconjugate Chem. 2022, 33, 1685–1697. [Google Scholar] [CrossRef]
- Wang, B.; Chang, M.; Zhang, R.; Wo, J.; Wu, B.; Zhang, H.; Zhou, Z.; Li, Z.; Zhang, F.; Zhong, C.; et al. Spinal cord injury target-immunotherapy with TNF-α autoregulated and feedback-controlled human umbilical cord mesenchymal stem cell derived exosomes remodelled by CRISPR/Cas9 plasmid. Biomater. Adv. 2022, 133, 112624. [Google Scholar] [CrossRef]
- Xu, J.; Shi, C.; Yuan, F.; Ding, Y.; Xie, Y.; Liu, Y.; Zhu, F.; Lu, H.; Duan, C.; Hu, J.; et al. Targeted transplantation of engineered mitochondrial compound promotes functional recovery after spinal cord injury by enhancing macrophage phagocytosis. Bioact. Mater. 2024, 32, 427–444. [Google Scholar] [CrossRef]
- Xu, P.; Li, T.-T.; Wang, B.-C.; Yi, Y.-J.; Zhang, W.-C.; Sun, G.-D.; Zhang, Y.; Li, Z.-Z. Supramolecular assemblies with spatio-temporal sequential drug delivery capability treat spinal cord injury via neuroprotection and immunoregulation. J. Control Release 2023, 360, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, T.; Shi, H.; Hong, J.; Jin, X.; Cao, L.; Wang, J.; Lin, Y.; Pan, Z.; Wang, S.; et al. Cartilage-targeting mRNA-lipid nanoparticles rescue perifocal apoptotic chondrocytes for integrative cartilage repair. Chem. Eng. J. 2023, 465, 142841. [Google Scholar] [CrossRef]
- Zare, L.; Rezaei, S.; Esmaeili, E.; Khajeh, K.; Javan, M. Targeted drug delivery into glial scar using CAQK peptide in a mouse model of multiple sclerosis. Brain Commun. 2023, 5, fcad325. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Mapping of vascular ZIP codes by phage display. Methods Enzymol. 2012, 503, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Brain extracellular matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef]
- Asher, R.A.; Morgenstern, D.A.; Shearer, M.C.; Adcock, K.H.; Pesheva, P.; Fawcett, J.W. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 2002, 22, 2225–2236. [Google Scholar] [CrossRef]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Okabe, M.; Ikawa, M.; Kominami, K.; Nakanishi, T.; Nishimune, Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997, 407, 313–319. [Google Scholar] [CrossRef]
- Joo, J.; Liu, X.; Kotamraju, V.R.; Ruoslahti, E.; Nam, Y.; Sailor, M.J. Gated Luminescence Imaging of Silicon Nanoparticles. ACS Nano. 2015, 9, 6233–6241. [Google Scholar] [CrossRef]
- Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 2013, 4, 2326. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Kim, Y.-M.; Hong, L.T.A.; Kim, H.S.; Kim, S.H.; Jin, X.; Hwang, D.H.; Kwon, M.J.; Song, S.-C.; Kim, B.G. Dual-functional hydrogel system for spinal cord regeneration with sustained release of arylsulfatase B alleviates fibrotic microenvironment and promotes axonal regeneration. Biomaterials 2022, 284, 121526. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, H.; Tan, Z.; Hou, Y.; Pang, M.; Chen, S.; Xiao, L.; Yuan, Q.; Liu, B.; Rong, L.; et al. Remodeling Microenvironment for Endogenous Repair through Precise Modulation of Chondroitin Sulfate Proteoglycans Following Spinal Cord Injury. Small 2023, 19, e2205012. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Nandi, S.; Garg, S.; Ghosh, S.; Samat, R.; Ghosh, S. Targeting Chondroitin Sulfate Proteoglycans: An Emerging Therapeutic Strategy to Treat CNS Injury. ACS Chem. Neurosci. 2020, 11, 231–232. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Lee, D.-H.; Rötger, C.; Appeldoorn, C.C.; Reijerkerk, A.; Gladdines, W.; Gaillard, P.J.; Linker, R.A. Glutathione PEGylated liposomal methylprednisolone (2B3-201) attenuates CNS inflammation and degeneration in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 274, 96–101. [Google Scholar] [CrossRef]
- Herrnstein, R.J. Placebo effect in the rat. Science 1962, 138, 677–678. [Google Scholar] [CrossRef]
- Dours-Zimmermann, M.T.; Zimmermann, D.R. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J. Biol. Chem. 1994, 269, 32992–32998. [Google Scholar] [CrossRef]
- Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012, 24, 3747–3756. [Google Scholar] [CrossRef]

| Bias Domain | Source of Bias | AP Mann [10] | Q Wang [14] | G Sun [5] | P Wu [15] | J Wang [11] | C Abi-Ghanem [16] | T Li [17] | T Li [18] | Y Rong [19] | LE Waggoner [20] | B Wang [21] | F Wu [3] | J Xu [22] | P Xu [23] | X Yu [24] | L Zare [25] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection Bias | Random Sequence Generation | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Allocation Concealment | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Performance Bias | Blinding of participants and personnel | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Detection Bias | Blinding of outcome assessment | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Attrition Bias | Incomplete outcome data | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Reporting Bias | Selective reporting | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Other Bias | Anything else, ideally prespecified | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Reference | Injury Model | Mechanism of Injury | Route of Administration | Treatment Frequency | Nanoparticle | Therapeutic Molecule | CAQK Concentration | Cellular Impact | Functional Impact | Adverse Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. AP Mann et al., 2016 USA [10] | TBI | Penetrating injuryBlunt cortical impact | Tail vein | 6 h and 24 h PI | Porous silicon nanoparticles (PSiNPs)Silver nanoparticles | siRNA | 300 μg of CAQK-PSiNPs/siRNA | Gene downregulation | N/A | N/A |
| 2. P Wu et al., 2019 China [15] | TBI | Controlled cortical impact | Tail vein | ~PI, Once | Activatable protein nanoparticle (APNP) | Tat-NR2B9c (TN) | N/A | Neuroprotective | Improved EPM | Not tested |
| 3. LE Waggoner et al., 2022 USA [20] | TBI | Controlled cortical impact | Tail vein | 2 h PI, Once | Porous silicon nanoparticle (PSiNPs) | Brain-derived neurotrophic factor | 10 μg of CAQK | Neuroprotective | N/A | N/A |
| 4. L Zare et al., 2023 Iran [25] | TBI | Demyelination | Tail vein | 6d PI, Once | Porous silicon nanoparticle (PSiNPs) | Methylprednisolone (MP) | 4.84 μg of CAQK-PSiNPs/MP | Anti-inflammatoryNeuroprotective | N/A | N/A |
| 5. C Abi-Ghanem et al., 2022 USA [16] | TBISCI | Demyelination | Retro-orbital plexusTail vein | 24 h or 5d PI, Once | None | None | 1 nmol/μL of FAM-CAQK | N/A | N/A | N/A |
| 6. Q Wang et al., 2018 China & USA [14] | SCI | Weight drop | Local administration using hydrogel | ~PI, Once | Liposome (LIP) | Docetaxel (DTX)Brain-derived neurotrophic factor (GFs) | 10 μg of CAQK-LIP/GFs & DTX | Neuroprotective | Improved BBB and footprint test | N/A |
| 7. G Sun et al., 2019 China [5] | SCI | Weight drop | Tail vein | 1d PI, Once | Mesoporous silica nanoparticles (MSN) | Arctigenin (ARC-G) | 0.5 mg of CAQK-MSN/ARC-G(on uninjured model) | Anti-inflammatoryNeuroprotective | Improved MEP | No adverse effects in heart, liver, spleen, lung, or kidneyCBC/BMP normal |
| 8. J Wang et al., 2020 China [11] | SCI | Weight drop | Intravenous | 2 h then every 2d PI | Polymeric micelle | Apocynin (APO) | 13.388 nmol/μL of CAQK | Anti-inflammatoryNeuroprotective | Improved BMS and footprint test | No adverse effects in heart, liver, spleen, lung, or kidney. |
| 9. T Li et al., 2022 China [17] | SCI | Weight drop | Intravenous | 6 h PI, Once | Zein-based spherical nanoparticles (NPs) | Metformin (MET) | 300 μg of CAQK | N/A | N/A | N/A |
| 10. T Li et al., 2022 China [18] | SCI | Weight drop | Tail vein | 6 h PI then daily for 7d | Zein-based spherical nanoparticles (NPs) | Metformin (MET) | N/A | Anti-inflammatoryNeuroprotective | Improved MEP | Liver and kidney damageMyelosupression that resolved |
| 11. Y Rong et al., 2022 China [19] | SCI | Weight drop | Tail vein | daily for 5d PI | Extracellular vesicles (EVs) | siRNA | 1 μg/μL CAQK-EVs | Anti-inflammatoryNeuroprotective | Improved MEP | No adverse effect |
| 12. B Wang et al., 2022 China [21] | SCI | Weight drop | Tail vein | ~PI, Once | PMSC-EVs (EXO) | CRISPR/Cas9 (@P) | 50 μg of CAQK-EXO/@P | Anti-inflammatoryNeuroprotective | Improved BMS and CatWalk | No adverse effects in heart, liver, spleen, lung, or kidneyCBC/BMP normal |
| 13. F Wu et al., 2023 China [3] | SCI | Weight drop | Tail vein | Weekly for 4 weeks | Dual-targeting liposome with R2KC peptide | Basic fibroblast growth factor | N/A | Anti-inflammatoryNeuroprotective | Improved BBB and footprint test | N/A |
| 14. J Xu et al., 2023 China [22] | SCI | Weight drop | Tail vein | 3d PI | Mitochondria (Mito) | Mitochondria (Mito) | 1 mg of Tpp-CAQK-Mito | Anti-inflammatoryNeuroprotective | Improved MEP, BMS, LSS, grid walking test | No adverse effect in heart/liver/spleen/lung/kidney |
| 15. P Xu et al., 2023 China [23] | SCI | Weight drop | Intravenous | 2 h then daily for 7d PI | HPAA-BM@CD-HPG-C | p38 inhibitorIGF-1 | N/A | Neuroprotective | Improved MEP and BMS | No adverse effects in heart, liver, spleen, lung, or kidneyCBC/BMP normal |
| 16. X Yu et al., 2023 China [24] | Knee joint | Patellar disconnection | Local | ~PI, Once | Cartilage targeting ionizable lipid nanoparticle | IGF-1 mRNA | N/A | Anti-apoptosis | N/A | No adverse effects in heart, liver, spleen, lung, or kidneyCBC/BMP normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, J.A., Jr.; Le, M.N.; Ratcliff, A.; Soufi, K.; Huang, K.; Vatoofy, S.; Ghaffari-Rafi, A.; Emerson, S.; Reynolds, E.; Pivetti, C.; et al. Systematic Review of Peptide CAQK: Properties, Applications, and Outcomes. Int. J. Mol. Sci. 2024, 25, 10990. https://doi.org/10.3390/ijms252010990
Castillo JA Jr., Le MN, Ratcliff A, Soufi K, Huang K, Vatoofy S, Ghaffari-Rafi A, Emerson S, Reynolds E, Pivetti C, et al. Systematic Review of Peptide CAQK: Properties, Applications, and Outcomes. International Journal of Molecular Sciences. 2024; 25(20):10990. https://doi.org/10.3390/ijms252010990
Chicago/Turabian StyleCastillo, Jose A., Jr., Michael Nhien Le, Amanda Ratcliff, Khadija Soufi, Kuanwei Huang, Sina Vatoofy, Arash Ghaffari-Rafi, Samuel Emerson, Elizabeth Reynolds, Christopher Pivetti, and et al. 2024. "Systematic Review of Peptide CAQK: Properties, Applications, and Outcomes" International Journal of Molecular Sciences 25, no. 20: 10990. https://doi.org/10.3390/ijms252010990
APA StyleCastillo, J. A., Jr., Le, M. N., Ratcliff, A., Soufi, K., Huang, K., Vatoofy, S., Ghaffari-Rafi, A., Emerson, S., Reynolds, E., Pivetti, C., Clark, K., Martin, A., Price, R., Kim, K., Wang, A., & Russo, R. (2024). Systematic Review of Peptide CAQK: Properties, Applications, and Outcomes. International Journal of Molecular Sciences, 25(20), 10990. https://doi.org/10.3390/ijms252010990








