Polyamine Signal through HCC Microenvironment: A Key Regulator of Mitochondrial Preservation and Turnover in TAMs
Abstract
1. Introduction
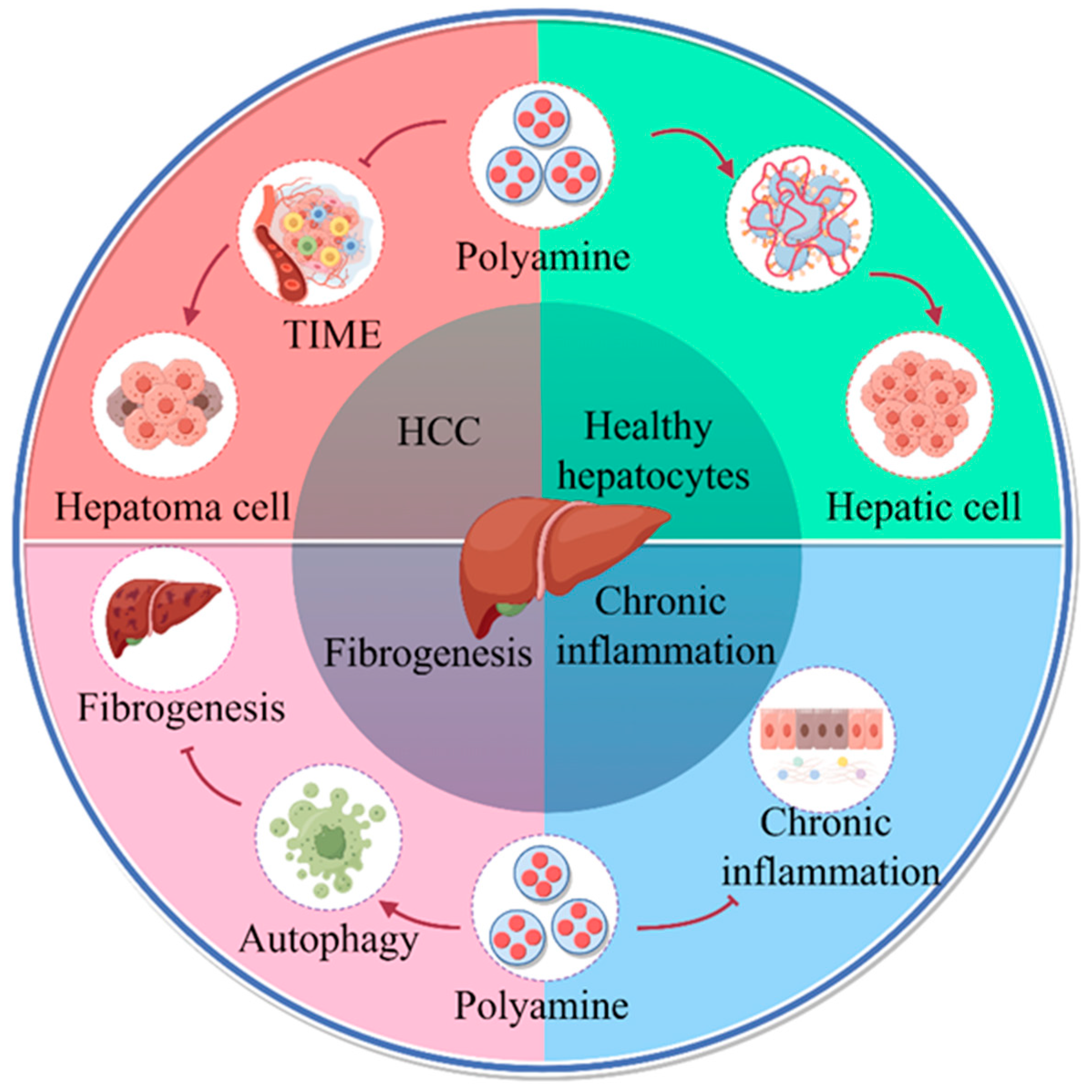
2. Polyamine Metabolism and HCC
2.1. Polyamine Metabolism Promotes the Progress of HCC
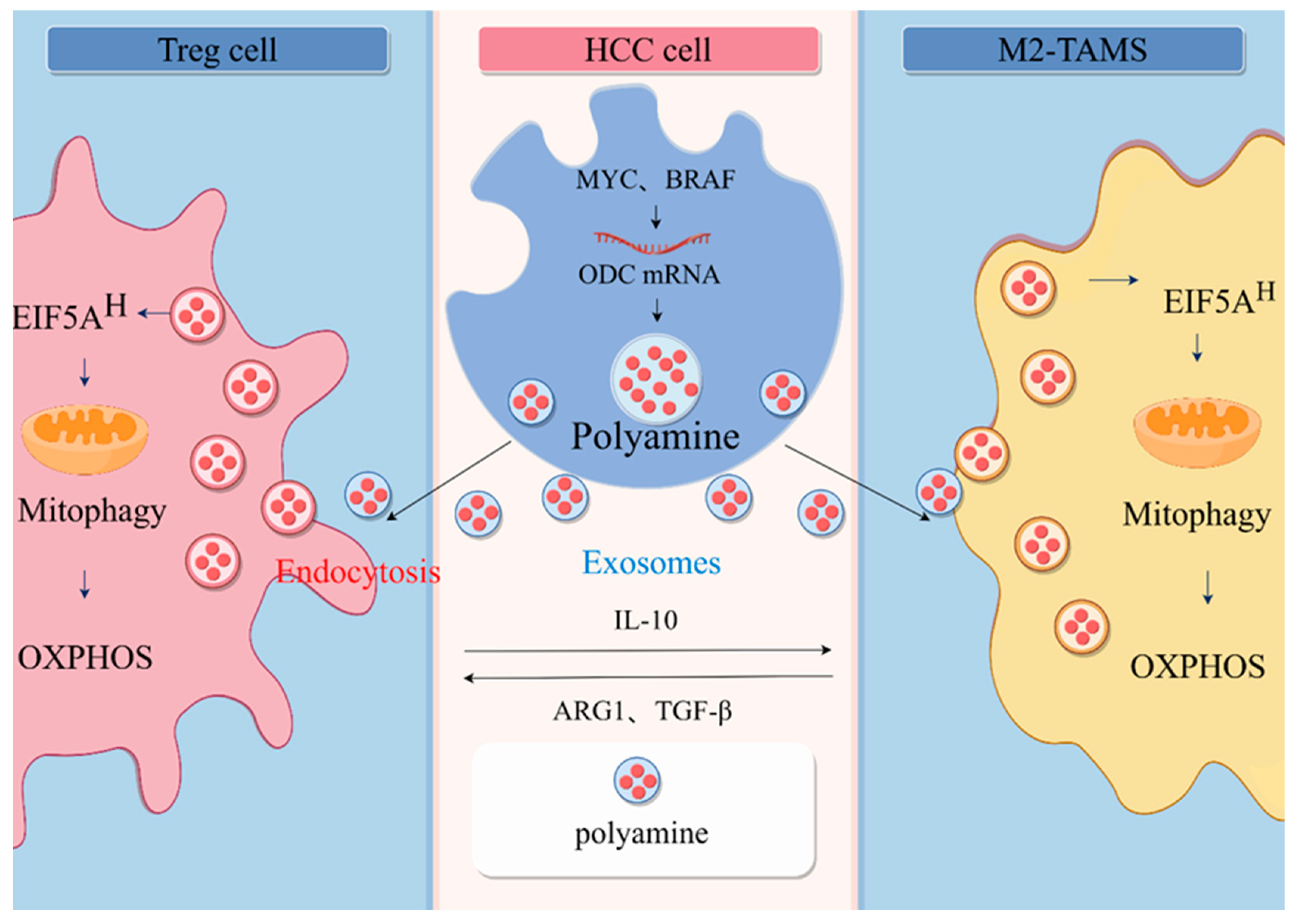
2.2. Involvement of Polyamines in Communication between HCC Cell and Immune Cells
3. Polyamines Promote Immunosuppressive Microenvironment by Regulating Mitochondrial Energy Metabolism
4. Mitochondrial Autophagy Is an Important Pathway for the Enhancement of Mitochondrial Function by Polyamine Metabolism
5. Future Research Directions
5.1. Polyamines as Biomarkers for Cancer
5.2. Polyamine Therapy
- (1)
- Inhibition of polyamine biosyntheses, such as ODC inhibitors, AdoMetDC inhibitors, SRM, and SMS inhibitors (Table 1).
- (2)
- (3)
- Polyamine blockade therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shlomai, A.; de Jong, Y.P. Virus associated malignancies: The role of viral hepatitis in hepatocellular carcinoma. Semin. Cancer Biol. 2014, 26, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Long, X. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology 2018, 7, e1440166. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.X. Hepatocellular carcinoma and macrophage interaction induced tumor immunosuppression via Treg requires TLR4 signaling. World J. Gastroenterol. 2012, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Zeng, H. Metabolism as a guiding force for immunity. Nat. Cell Biol. 2019, 21, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Zhang, D. Mitochondrial Quality Control in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 713721. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2212613120. [Google Scholar] [CrossRef]
- Mi, Y.; Tang, M. NMAAP1 regulated macrophage polarizion into M1 type through glycolysis stimulated with BCG. Int. Immunopharmacol. 2023, 126, 111257. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Mondanelli, G.; Bianchi, R. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity 2017, 46, 233–244. [Google Scholar] [CrossRef]
- Pietrocola, F.; Pol, J. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016, 30, 147–160. [Google Scholar] [CrossRef]
- Yoshida, R.; Hayaishi, O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. USA 1978, 75, 3998–4000. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.B.; Zamarin, D. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Buck, M.D. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Baixauli, F. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.e20. [Google Scholar] [CrossRef] [PubMed]
- Berod, L.; Friedrich, C. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, e1802896. [Google Scholar] [CrossRef]
- Shen, M.; Shen, Y. Roles of Macrophages and Exosomes in Liver Diseases. Front. Med. 2020, 7, 583691. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X. Exosomes in the tumor microenvironment of cholangiocarcinoma: Current status and future perspectives. J. Transl. Med. 2022, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Bosc, C.; Broin, N. Autophagy regulates fatty acid availability for oxidative phosphorylation through mitochondria-endoplasmic reticulum contact sites. Nat. Commun. 2020, 11, 4056. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Li, W. Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res. 2017, 77, 2938–2951. [Google Scholar] [CrossRef]
- Bian, X.; Shi, D. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation. Clin. Transl. Med. 2021, 11, e352. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Li, L.; Mao, Y. p53 regulation of ammonia metabolism through urea cycle controls polyamine biosynthesis. Nature 2019, 567, 253–256. [Google Scholar] [CrossRef]
- Kubo, S.; Tamori, A. Relationship of polyamine metabolism to degree of malignancy of human hepatocellular carcinoma. Oncol. Rep. 1998, 5, 1385–1388. [Google Scholar] [CrossRef]
- Roy, U.K.; Rial, N.S. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol. Carcinog. 2008, 47, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chi, H. Revolutionizing anti-tumor therapy: Unleashing the potential of B cell-derived exosomes. Front. Immunol. 2023, 14, 1188760. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Schrum, A.G. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.Y.; Zolp, A. Polyamine Immunometabolism: Central Regulators of Inflammation, Cancer and Autoimmunity. Cells 2022, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zheng, C. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, X. Spermidine protects against acute kidney injury by modulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway. Mol. Med. 2022, 28, 103. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Ochoa, A.C. Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity. Front. Immunol. 2017, 8, 93. [Google Scholar] [CrossRef]
- Halaby, M.J.; McGaha, T.L. Amino Acid Transport and Metabolism in Myeloid Function. Front. Immunol. 2021, 12, 695238. [Google Scholar] [CrossRef]
- Park, M.H.; Cooper, H.L. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. USA 1981, 78, 2869–2873. [Google Scholar] [CrossRef]
- Zeng, J.; Ye, Z. Targeted inhibition of eIF5A(hpu) suppresses tumor growth and polarization of M2-like tumor-associated macrophages in oral cancer. Cell Death Dis. 2023, 14, 579. [Google Scholar] [CrossRef]
- Alexander, E.T.; Minton, A. A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget 2017, 8, 84140–84152. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wang, C. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 2021, 184, 4168–4185.e21. [Google Scholar] [CrossRef] [PubMed]
- Carriche, G.M.; Almeida, L. Regulating T-cell differentiation through the polyamine spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348.e11. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.; Choi, B.S. L-arginine deprivation impairs Leishmania major-specific T-cell responses. Eur. J. Immunol. 2009, 39, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Han, Q. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 2019, 8, 1601479. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, X. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci. 2022, 113, 1968–1983. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liang, H.X. Overexpressed Tumor Suppressor Exosomal miR-15a-5p in Cancer Cells Inhibits PD1 Expression in CD8+T Cells and Suppresses the Hepatocellular Carcinoma Progression. Front. Oncol. 2021, 11, 622263. [Google Scholar] [CrossRef]
- Moriyama, Y.; Hatano, R. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183208. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Juge, N.; Moriyama, S. Plasmodium falciparum chloroquine resistance transporter is a H+-coupled polyspecific nutrient and drug exporter. Proc. Natl. Acad. Sci. USA 2015, 112, 3356–3361. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, M.; Miyaji, T. Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 2014, 4, 6836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xiao, R. Spermine on Endothelial Extracellular Vesicles Mediates Smoking-Induced Pulmonary Hypertension Partially through Calcium-Sensing Receptor. Arterioscler Thromb. Vasc. Biol. 2019, 39, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Puhka, M.; Takatalo, M. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics 2017, 7, 3824–3841. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Casero, R.A. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 2012, 42, 711–723. [Google Scholar] [CrossRef] [PubMed]
- van Veen, S.; Martin, S. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, X. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci. Adv. 2020, 6, eabc4275. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Sasaki, R.; Kanda, T. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef]
- Sezai, S.; Sakurabayashi, S. Hepatic arterial and portal venous oxygen content and extraction in liver cirrhosis. Liver 1993, 13, 31–35. [Google Scholar] [CrossRef]
- Tan, A.S.; Baty, J.W. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015, 21, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.; Ipseiz, N. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity 2021, 54, 2531–2546.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F. RAGE-mediated T cell metabolic reprogramming shapes T cell inflammatory response after stroke. J. Cereb. Blood Flow Metab. 2022, 42, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Mehla, K.; Singh, P.K. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Cluxton, D.; Petrasca, A. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front. Immunol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Martínez-Méndez, D.; Mendoza, L. Continuous Modeling of T CD4 Lymphocyte Activation and Function. Front. Immunol. 2021, 12, 743559. [Google Scholar] [CrossRef]
- Zhou, J.; Pang, J. Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression. Nat. Commun. 2022, 13, 5202. [Google Scholar] [CrossRef]
- Schroeder, S.; Hofer, S.J. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef]
- Puleston, D.J.; Zhang, H. Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife 2014, 3, e03706. [Google Scholar] [CrossRef]
- Liu, R.; Li, X. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef]
- Shi, L.; Lim, J.Y. Substrate stiffness enhances human regulatory T cell induction and metabolism. Biomaterials 2023, 292, 121928. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, X. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qiu, Q. ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts. Sci. Rep. 2016, 6, 24700. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Liang, Y. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy 2021, 17, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, J. CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy 2022, 18, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H. PINK1-Mediated Mitophagy Promotes Oxidative Phosphorylation and Redox Homeostasis to Induce Drug-Tolerant Persister Cancer Cells. Cancer Res. 2023, 83, 398–413. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, D. FBXL4 mutations cause excessive mitophagy via BNIP3/BNIP3L accumulation leading to mitochondrial DNA depletion syndrome. Cell Death Differ. 2023, 30, 2351–2363. [Google Scholar] [CrossRef]
- Miolo, G.; Muraro, E. Pharmacometabolomics study identifies circulating spermidine and tryptophan as potential biomarkers associated with the complete pathological response to trastuzumab-paclitaxel neoadjuvant therapy in HER-2 positive breast cancer. Oncotarget 2016, 7, 39809–39822. [Google Scholar] [CrossRef]
- Liu, R.; Li, P. Plasma N-acetylputrescine, cadaverine and 1,3-diaminopropane: Potential biomarkers of lung cancer used to evaluate the efficacy of anticancer drugs. Oncotarget 2017, 8, 88575–88585. [Google Scholar] [CrossRef]
- Xu, H.; Liu, R. Polyamine Metabolites Profiling for Characterization of Lung and Liver Cancer Using an LC-Tandem MS Method with Multiple Statistical Data Mining Strategies: Discovering Potential Cancer Biomarkers in Human Plasma and Urine. Molecules 2016, 21, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Onishi, H. Prognostic significance of urine N1, N12-diacetylspermine in patients with non-small cell lung cancer. Anticancer Res. 2014, 34, 3053–3059. [Google Scholar] [PubMed]
- Takahashi, Y.; Sakaguchi, K. Urinary N1, N12-diacetylspermine is a non-invasive marker for the diagnosis and prognosis of non-small-cell lung cancer. Br. J. Cancer 2015, 113, 1493–1501. [Google Scholar] [CrossRef]
- Niemi, R.J.; Roine, A.N. Urinary Polyamines as Biomarkers for Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Bitonti, A.J.; Bacchi, C.J. Catalytic irreversible inhibition of Trypanosoma brucei brucei ornithine decarboxylase by substrate and product analogs and their effects on murine trypanosomiasis. Biochem. Pharmacol. 1985, 34, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef]
- Hessels, J.; Kingma, A.W. Microbial flora in the gastrointestinal tract abolishes cytostatic effects of alpha-difluoromethylornithine in vivo. Int. J. Cancer 1989, 43, 1155–1164. [Google Scholar] [CrossRef]
- Levêque, J.; Burtin, F. The gastrointestinal polyamine source depletion enhances DFMO induced polyamine depletion in MCF-7 human breast cancer cells in vivo. Anticancer Res. 1998, 18, 2663–2668. [Google Scholar]
- Gerner, E.W.; Bruckheimer, E. Cancer pharmacoprevention: Targeting polyamine metabolism to manage risk factors for colon cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef]
- LoGiudice, N.; Le, L. Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases. Med. Sci. 2018, 6, 12. [Google Scholar] [CrossRef]
- Sholler, G.L.S.; Ferguson, W. Maintenance DFMO Increases Survival in High Risk Neuroblastoma. Sci. Rep. 2018, 8, 14445. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Kraveka, J.M. A subset analysis of a phase II trial evaluating the use of DFMO as maintenance therapy for high-risk neuroblastoma. Int. J. Cancer 2020, 147, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Bassiri, H.; Benavides, A. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl. Pediatr. 2015, 4, 226–238. [Google Scholar]
- Levin, V.A.; Ictech, S.E. Clinical importance of eflornithine (α-difluoromethylornithine) for the treatment of malignant gliomas. CNS Oncol. 2018, 7, Cns16. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Simoneau, A.R. Chemoprevention of prostate cancer with the polyamine synthesis inhibitor difluoromethylornithine. Recent Results Cancer Res. 2014, 202, 115–120. [Google Scholar] [PubMed]
- Ishmael, D.R.; Chen, W.R. A phase I human trial of mitoguazone and gemcitabine sequential bi-weekly treatment of cancer patients. Cancer Investig. 2003, 21, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wiernik, P.H.; Gordon, L.I. Evaluation of mitoguazone in patients with refractory chronic lymphocytic leukemia: A phase II study (P-H482) of the Eastern Cooperative Oncology Group. Leuk Lymphoma 1999, 35, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.A., 3rd; O’Bryan, R.M. Methyl-glyoxal bis guanyl hydrazone (methyl-GAG, MGBG) in advanced breast cancer. A Phase II trial of the Southwest Oncology Group. Investig. New Drugs 1984, 2, 71–73. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Moore, D.F. Phase II study of mitoguazone, cyclophosphamide, doxorubicin, vincristine and prednisone for patients with diffuse histologic subtypes of non-Hodgkin’s lymphoma: An Eastern Cooperative Oncology Group Study (PE481). Leuk Lymphoma 1998, 30, 601–607. [Google Scholar] [CrossRef]
- Pless, M.; Belhadj, K. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: Results from a phase II multicenter study. Clin. Cancer Res. 2004, 10, 1299–1305. [Google Scholar] [CrossRef]
- Siu, L.L.; Rowinsky, E.K. A phase I and pharmacokinetic study of SAM486A, a novel polyamine biosynthesis inhibitor, administered on a daily-times-five every-three-week schedule in patients with Advanced solid malignancies. Clin. Cancer Res. 2002, 8, 2157–2166. [Google Scholar] [PubMed]
- Paridaens, R.; Uges, D.R. A phase I study of a new polyamine biosynthesis inhibitor, SAM486A, in cancer patients with solid tumours. Br. J. Cancer 2000, 83, 594–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Coward, J.K. Stereospecific synthesis of (R)- and (S)-S-adenosyl-1,8-diamino-3-thiooctane, a potent inhibitor of polyamine biosynthesis. Comparison of asymmetric induction vs enantiomeric synthesis. J. Med. Chem. 1991, 34, 2094–2101. [Google Scholar] [CrossRef]
- Holm, I.; Persson, L. Effects of S-adenosyl-1,8-diamino-3-thio-octane and S-methyl-5′-methylthioadenosine on polyamine synthesis in Ehrlich ascites-tumour cells. Biochem. J. 1989, 261, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Wechter, R. Effect of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase, on polyamine metabolism in mammalian cells. Biochemistry 1989, 28, 8446–8453. [Google Scholar] [CrossRef] [PubMed]
- Woster, P.M.; Black, A.Y. Synthesis and biological evaluation of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase. J. Med. Chem. 1989, 32, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.W.; Bergeron, R.J. Regulation of polyamine biosynthetic activity by spermidine and spermine analogs--a novel antiproliferative strategy. Adv. Exp. Med. Biol. 1988, 250, 677–690. [Google Scholar] [PubMed]
- Xie, Y.; Murray-Stewart, T. Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J. Control Release 2017, 246, 110–119. [Google Scholar] [CrossRef]
- Goyal, L.; Supko, J.G. Phase 1 study of N(1),N(11)-diethylnorspermine (DENSPM) in patients with advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2013, 72, 1305–1314. [Google Scholar] [CrossRef]
- Hahm, H.A.; Ettinger, D.S. Phase I study of N(1),N(11)-diethylnorspermine in patients with non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 684–690. [Google Scholar]
- Streiff, R.R.; Bender, J.F. Phase 1 study of N1-N11-diethylnorspermine (DENSPM) administered TID for 6 days in patients with advanced malignancies. Investig. New Drugs 2001, 19, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Armstrong, D.K. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003, 9, 5922–5928. [Google Scholar] [PubMed]
- Casero, R.A., Jr.; Woster, P.M. Terminally alkylated polyamine analogues as chemotherapeutic agents. J. Med. Chem. 2001, 44, 1–26. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Von Hoff, D. A Phase Ib multicenter, dose-escalation study of the polyamine analogue PG-11047 in combination with gemcitabine, docetaxel, bevacizumab, erlotinib, cisplatin, 5-fluorouracil, or sunitinib in patients with advanced solid tumors or lymphoma. Cancer Chemother Pharmacol. 2021, 87, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hacker, A.; Marton, L.J. In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008, 63, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Ferrari, E. Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS ONE 2017, 12, e0175917. [Google Scholar]
- Murray Stewart, T.; Desai, A.A. A phase I dose-escalation study of the polyamine analog PG-11047 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2020, 85, 1089–1096. [Google Scholar] [CrossRef]
- Hayes, C.S.; DeFeo-Mattox, K. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids 2014, 46, 543–552. [Google Scholar] [CrossRef]
- Hayes, C.S.; Shicora, A.C. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol. Res. 2014, 2, 274–285. [Google Scholar] [CrossRef]
- Alexander, E.T.; Mariner, K. Polyamine Blocking Therapy Decreases Survival of Tumor-Infiltrating Immunosuppressive Myeloid Cells and Enhances the Antitumor Efficacy of PD-1 Blockade. Mol. Cancer Ther. 2020, 19, 2012–2022. [Google Scholar] [CrossRef]
- Gitto, S.B.; Pandey, V. Difluoromethylornithine Combined with a Polyamine Transport Inhibitor Is Effective against Gemcitabine Resistant Pancreatic Cancer. Mol. Pharm. 2018, 15, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.D.; Purgato, S. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci. Transl. Med. 2019, 11, eaau1099. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Gamble, L.D. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat. Commun. 2021, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.T.; El Naggar, O. Harnessing the polyamine transport system to treat BRAF inhibitor-resistant melanoma. Cancer Biol. Ther. 2021, 22, 225–237. [Google Scholar] [CrossRef]
- Muth, A.; Kamel, J. Development of polyamine transport ligands with improved metabolic stability and selectivity against specific human cancers. J. Med. Chem. 2013, 56, 5819–5828. [Google Scholar] [CrossRef]
- Qu, X.; Yu, J. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef]
- Qiu, D.M.; Wang, G.L. The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer 2014, 14, 327. [Google Scholar] [CrossRef]
| Drug | Target | Structure | Status |
|---|---|---|---|
| DFMO | ODC | 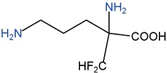 | Approved for treatment of human African trypanosomiasis and hirsutism; multiple ongoing cancer clinical trials, including chemoprevention trials [89,90,91,92,93,94,95] |
| MGBG | AdoMetDC | 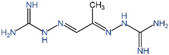 | Phases 1 and 2 completed [96,97,98,99] |
| SAM486A | AdoMetDC | 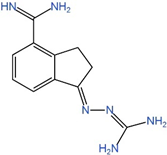 | Phases 1 and 2 completed [100,101,102] |
| AdoDATO | SRM |  | Preclinical use [103,104] |
| AdoDATAD | SMS | 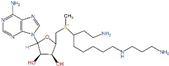 | Preclinical use [105,106] |
| Drug | Target | Structure | Status |
|---|---|---|---|
| BENSpm | ODC, AdoMetDC, SRM, SMS, SSAT, and/or SMOX |  | Phases 1 and 2 completed. Formulated into nanoparticles [108,109,110,111,112] |
| CPENSpm | ODC, AdoMetDC, SRM, SMS, SSAT, and/or SMOX |  | Preclinical use [113] |
| PG-11047 | ODC, AdoMetDC, SRM, SMS, SSAT, and/or SMOX |  | Phases 1, 1b and 2 completed. Formulated into nanopartides [114,115,116,117] |
| MDL72527 | PAOX and SMOX | 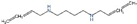 | Preclinical use [118] |
| Drug | Target | Structure | Status |
|---|---|---|---|
| Trimer44NMe | Polyamine transport | 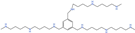 | Preclinical use [41,120,121] |
| AMXT 1501 | Polyamine transport | 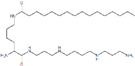 | Preclinical use [119,122,123], Phase 1 trial in combination with DFMO completed (NCT03536728) |
| MeN44Nap44NMe (AP) | Polyamine transport | 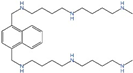 | Preclinical use [124,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yan, X.; Li, R.; Yuan, Y.; Wang, J.; Zhao, Y.; Fu, J.; Su, J. Polyamine Signal through HCC Microenvironment: A Key Regulator of Mitochondrial Preservation and Turnover in TAMs. Int. J. Mol. Sci. 2024, 25, 996. https://doi.org/10.3390/ijms25020996
Liu Q, Yan X, Li R, Yuan Y, Wang J, Zhao Y, Fu J, Su J. Polyamine Signal through HCC Microenvironment: A Key Regulator of Mitochondrial Preservation and Turnover in TAMs. International Journal of Molecular Sciences. 2024; 25(2):996. https://doi.org/10.3390/ijms25020996
Chicago/Turabian StyleLiu, Qingqing, Xiaoyu Yan, Runyuan Li, Yuan Yuan, Jian Wang, Yuanxin Zhao, Jiaying Fu, and Jing Su. 2024. "Polyamine Signal through HCC Microenvironment: A Key Regulator of Mitochondrial Preservation and Turnover in TAMs" International Journal of Molecular Sciences 25, no. 2: 996. https://doi.org/10.3390/ijms25020996
APA StyleLiu, Q., Yan, X., Li, R., Yuan, Y., Wang, J., Zhao, Y., Fu, J., & Su, J. (2024). Polyamine Signal through HCC Microenvironment: A Key Regulator of Mitochondrial Preservation and Turnover in TAMs. International Journal of Molecular Sciences, 25(2), 996. https://doi.org/10.3390/ijms25020996






