CsCuAO1 Associated with CsAMADH1 Confers Drought Tolerance by Modulating GABA Levels in Tea Plants
Abstract
1. Introduction
2. Results
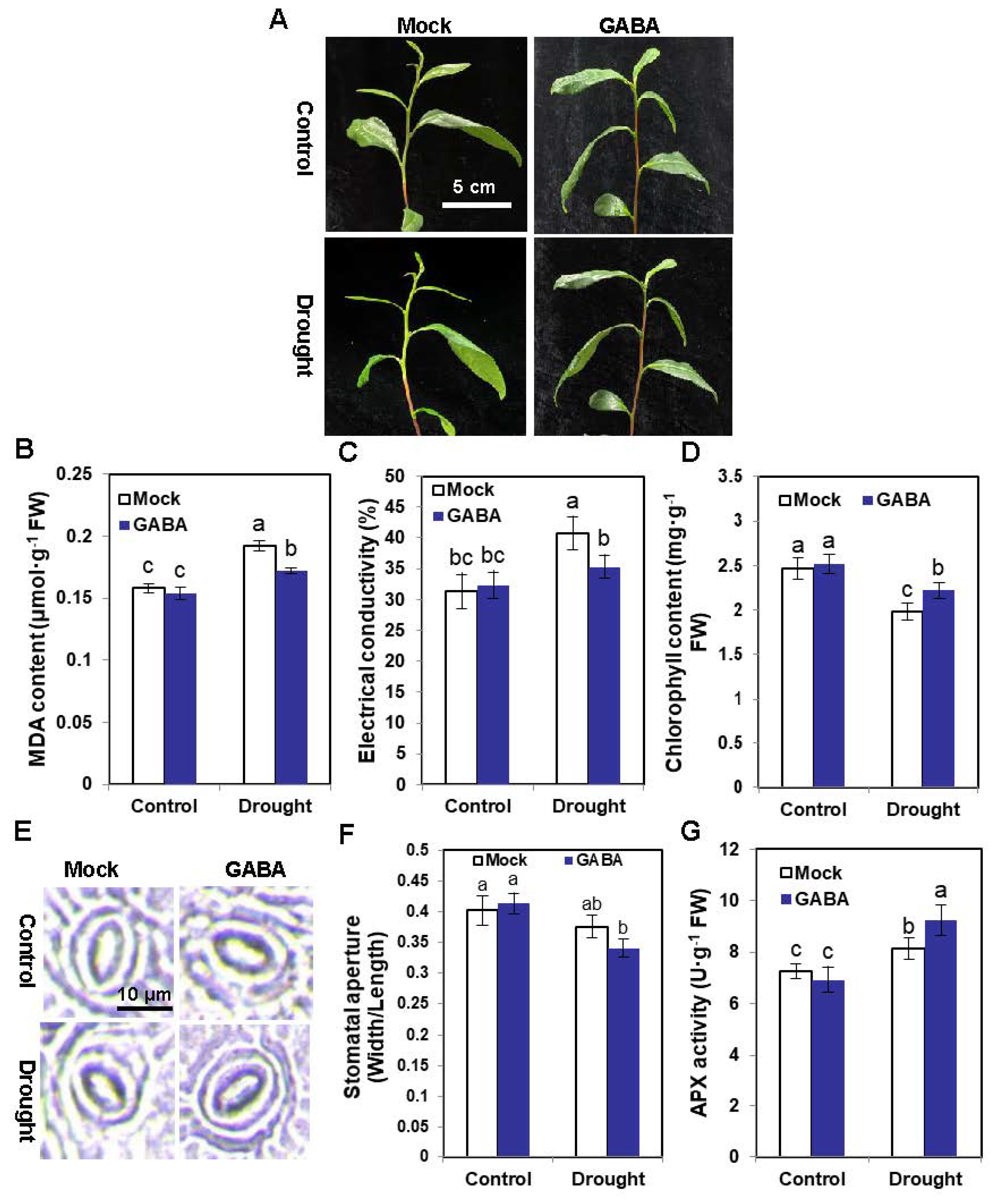
2.1. Exogenous GABA Supply Enhances the Drought Tolerance of Tea Plants
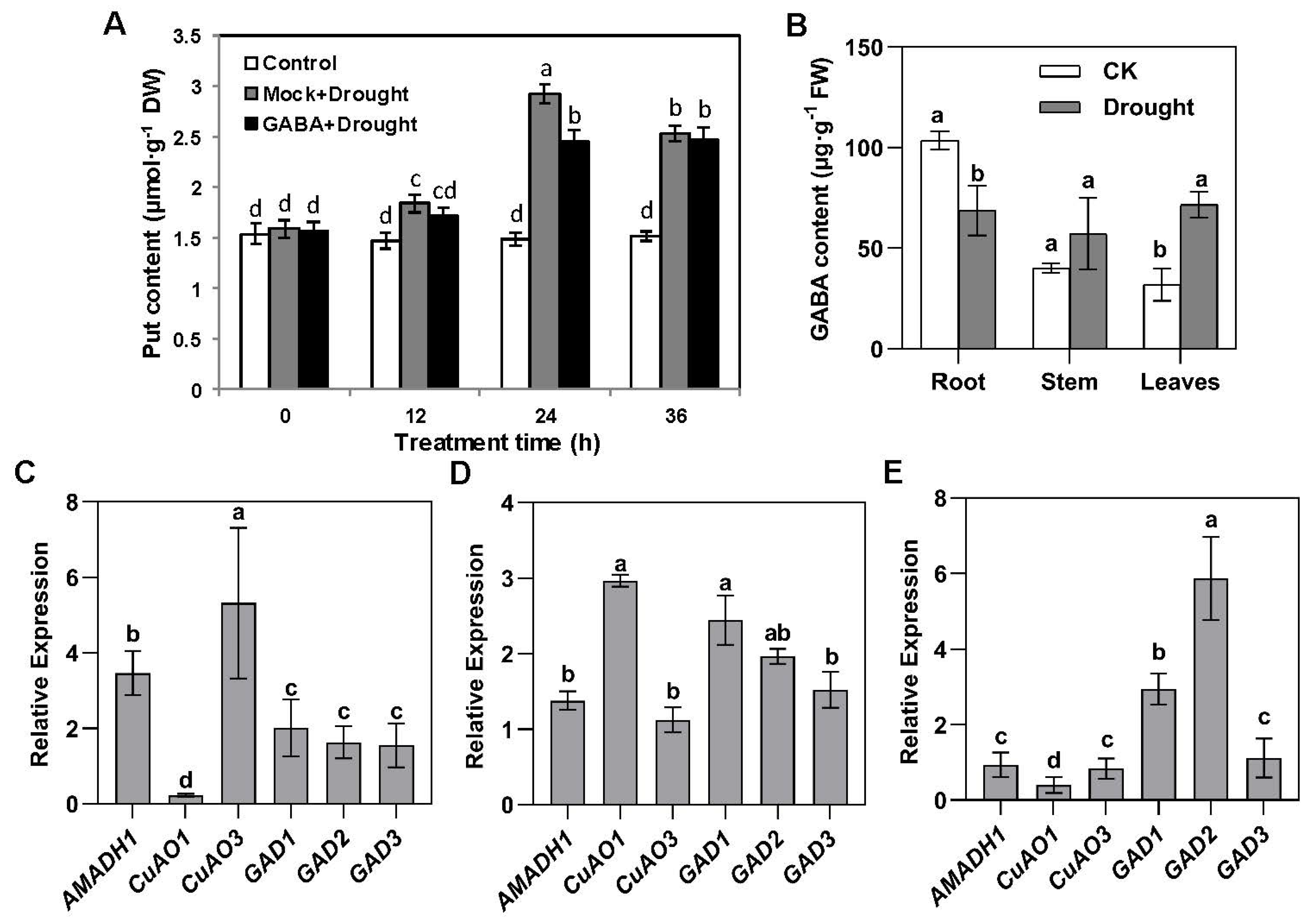
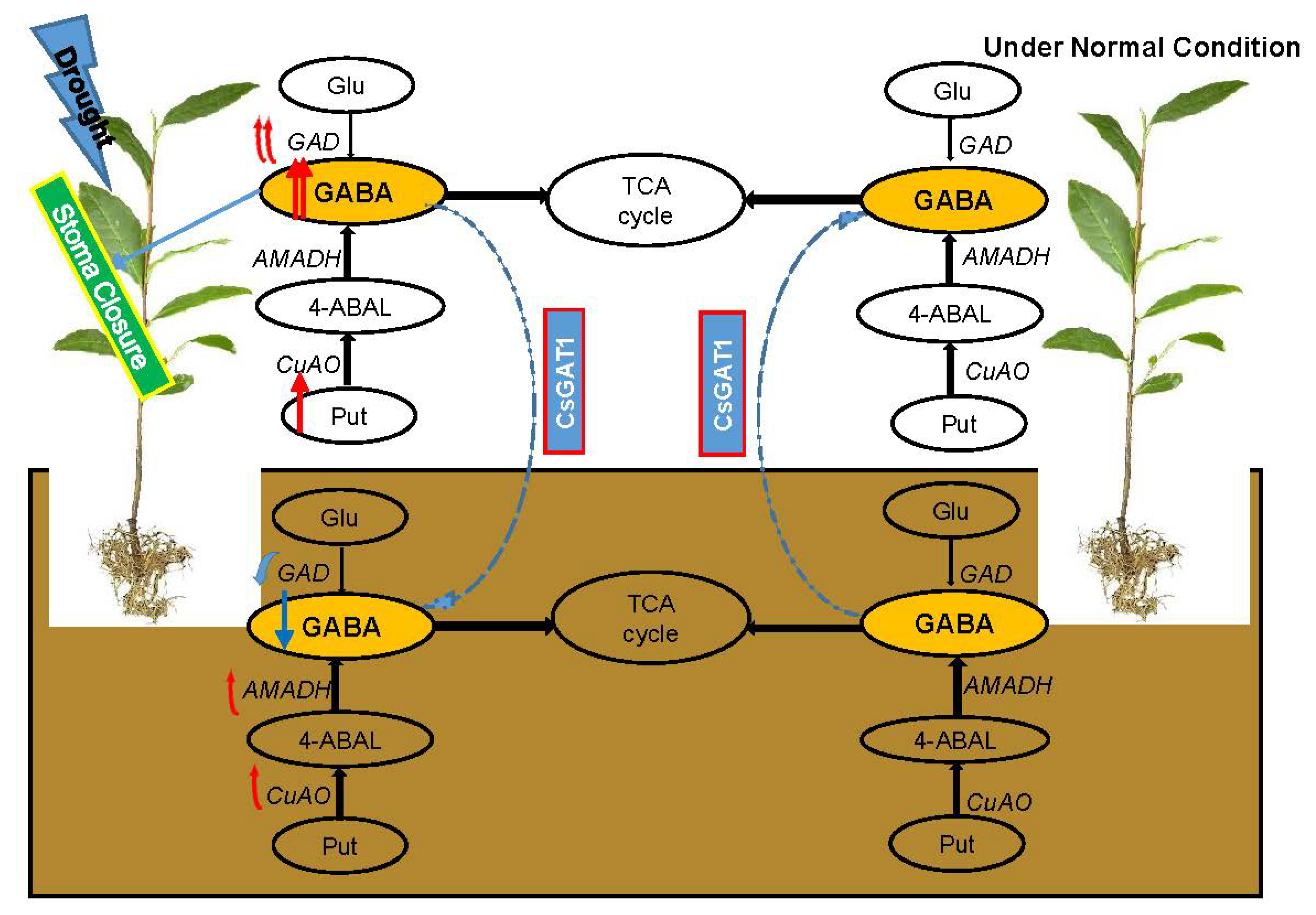
2.2. Drought Promotes the Transfer of GABA in Plants
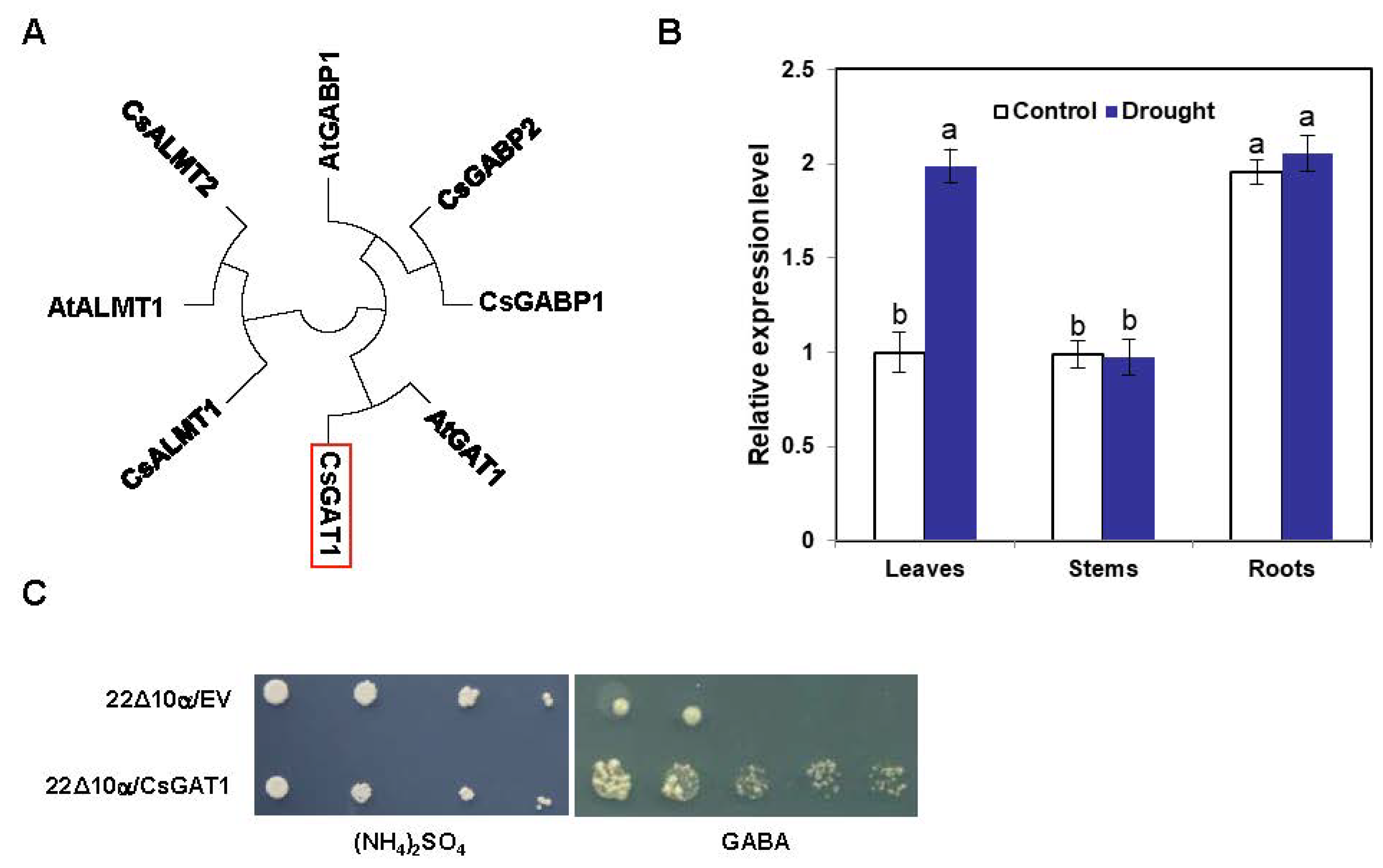
2.3. Identification and Characterization of CsGAT1
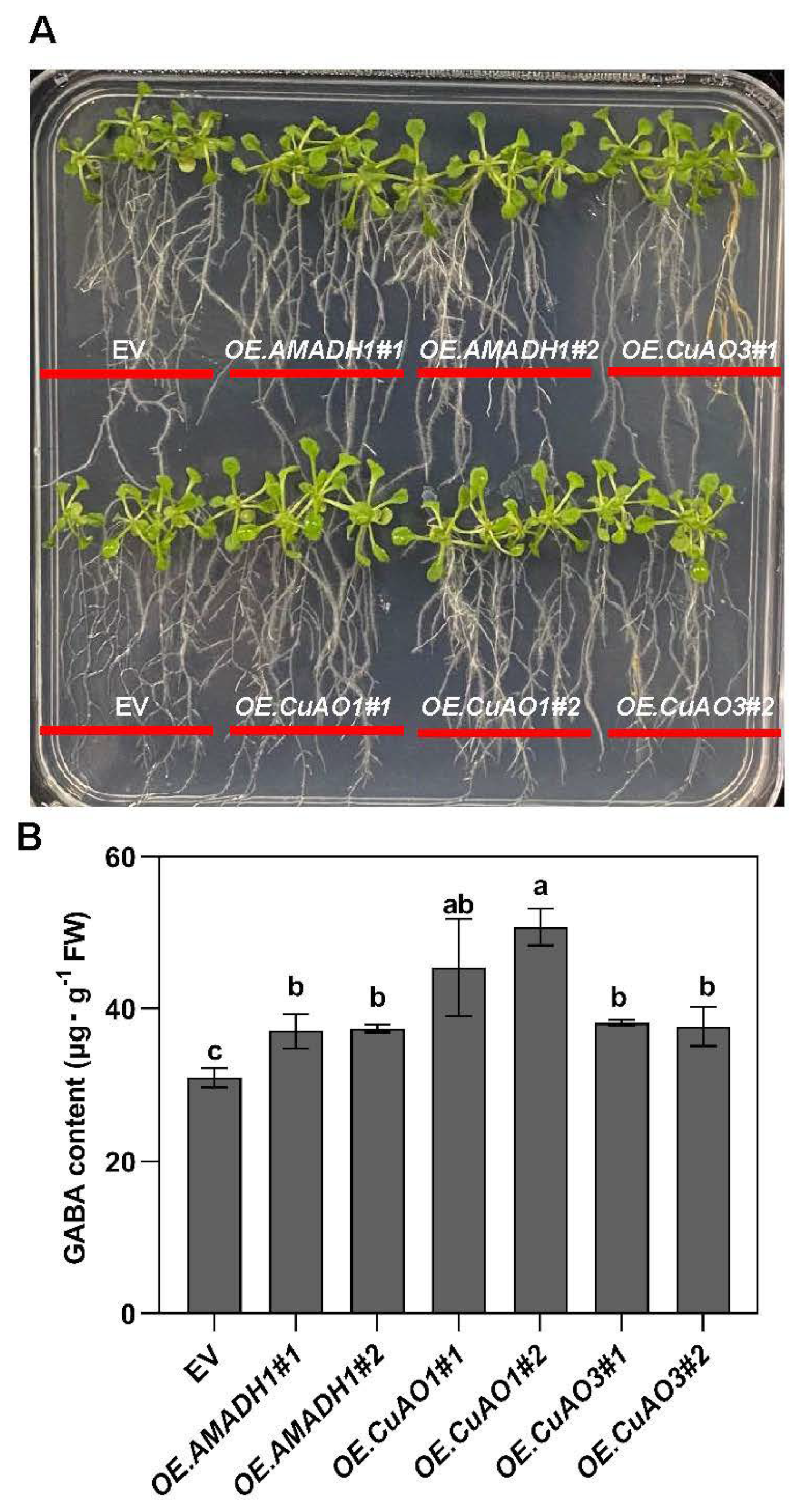
2.4. The Impact of Exogenous Putrescine on GABA Accumulation in CsAMADH1-, CsCuAO1-, and CsCuAO3-Overexpressing Arabidopsis Lines
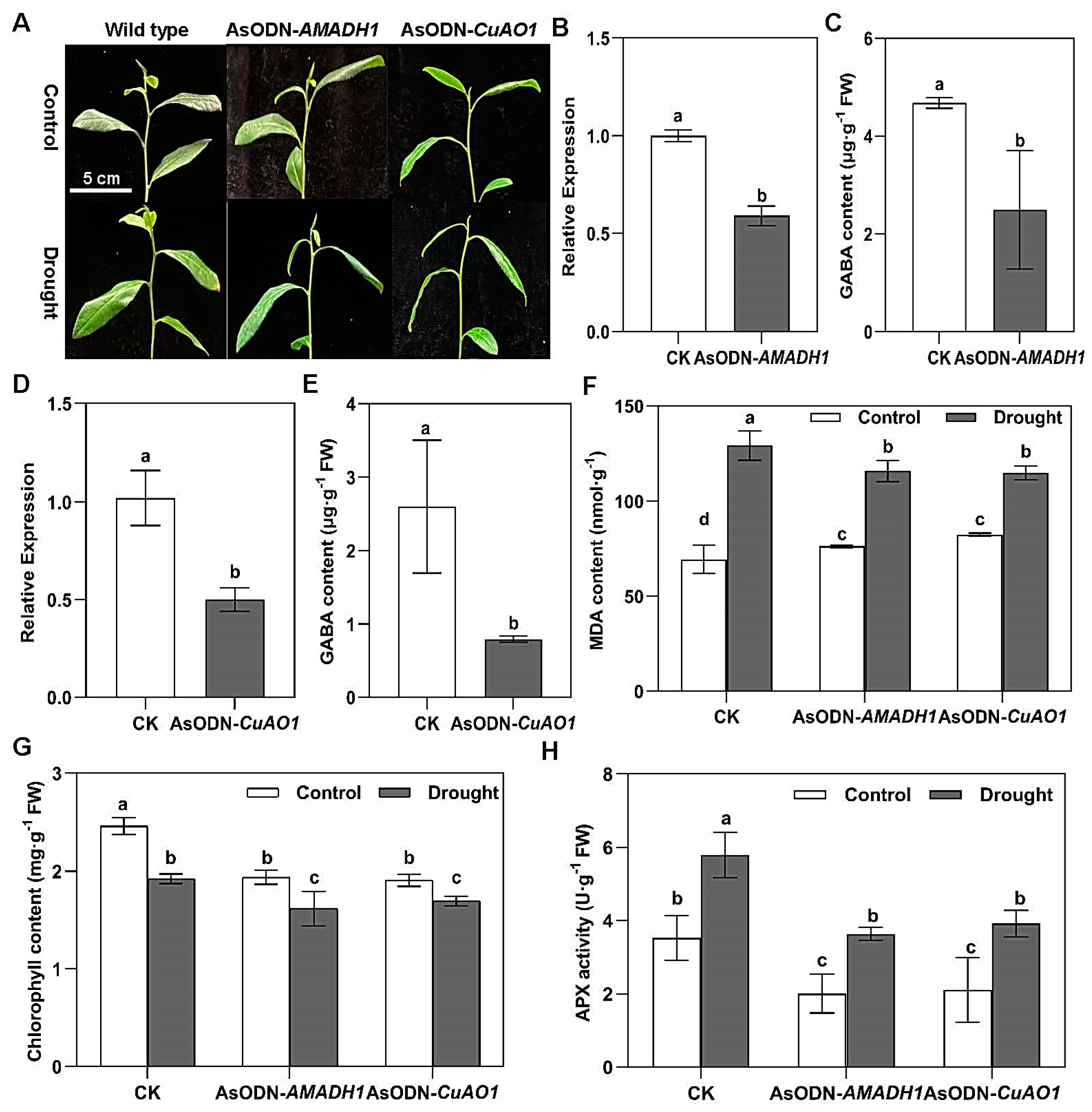
2.5. The Suppression of Putrescine-Derived GABA-Responsive Genes Reduces the Drought Tolerance of Tea Plants
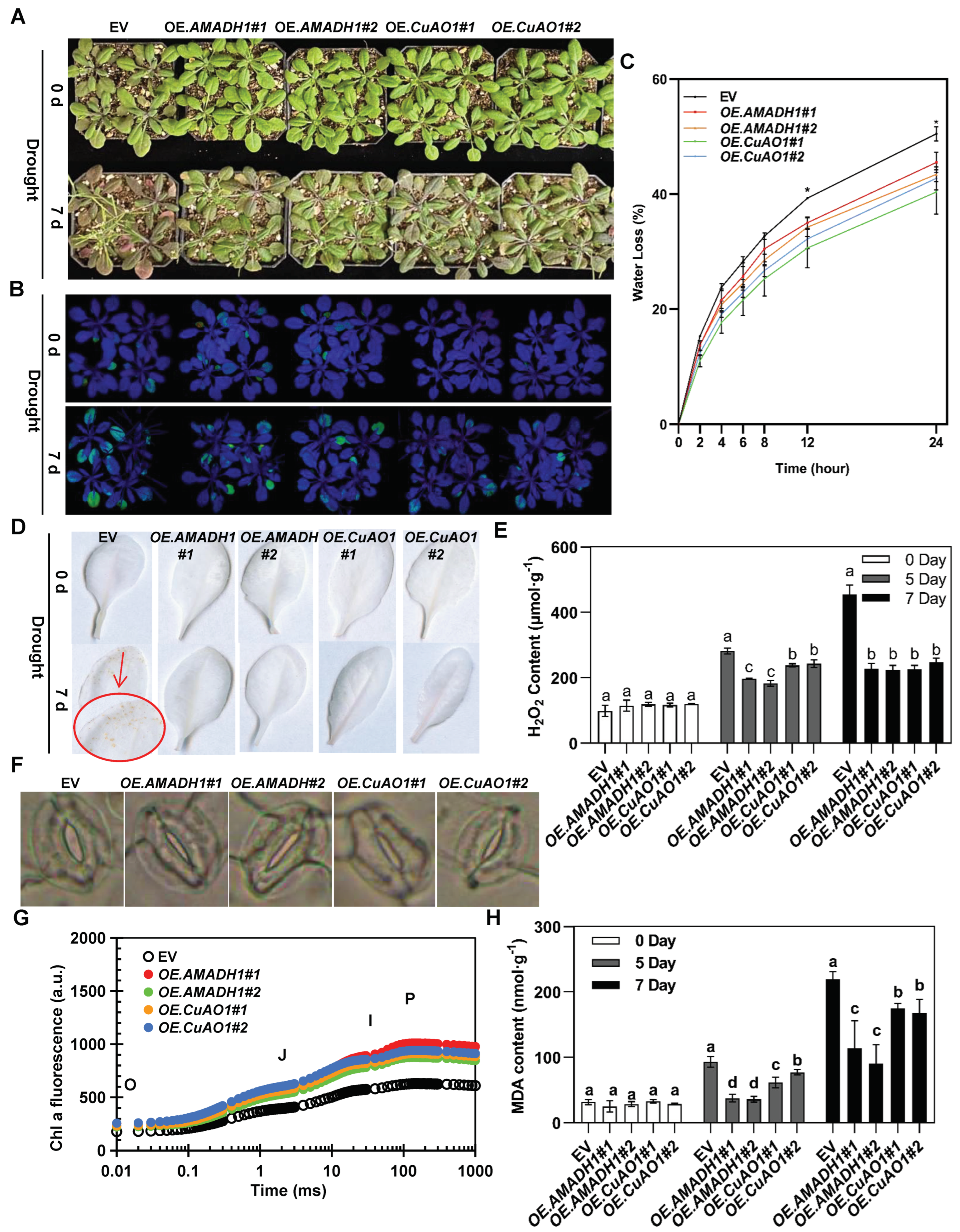
2.6. Overexpressing Arabidopsis Lines Exhibit High Tolerance to Drought
2.7. Transient Assay and Transgenic Analysis of CsCuAO1-CsAMADH1 Co-Expression
3. Discussion
3.1. CsCuAO1 and CsAMADH1 Encode Functional Proteins
3.2. GABA Contributes to Drought Stress Tolerance through Affecting ROS Scavenging and Stomatal Closure in Tea Plants
3.3. GAT1 Acts as a GABA Transporter in Tea Plants
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Drought Stress Treatments
4.3. Gene Expression Analysis
4.4. Functional Verification of Transgenic Arabidopsis Plants
4.5. Gene silencing of CsAMADH1 and CsCuAO1 in Tea Plants
4.6. Determination of GABA Contents
4.7. Putrescine Content Determination
4.8. Determining Electric Conductivity
4.9. Determination of MDA and Chlorophyll Contents
4.10. Measurement of Stomatal Aperture
4.11. Determination of Photosynthetic Parameters and Chlorophyll Fluorescence Parameters
4.12. Detection of Oxidative Damage in Plants
4.13. Determination of Soil Water Content and Free Water Loss
4.14. Transient Assay in N. benthamiana Leaves
4.15. Pollen Hybridization and Functional Verification of Transgenic Arabidopsis
4.16. Phylogenetic Tree Construction
4.17. Yeast Spot Experiment
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, M.A.K.; Ač, A.; Klem, K.; Urban, O. A meta-analysis of the interactive effects of UV and drought on plants. Plant Cell Environ. 2022, 45, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ban, Q.; Hao, J.; Zhu, X.; Cheng, Y.; Mao, J.; Lin, M.; Xia, E.; Li, Y. Genome-wide characterization of the C-repeat binding factor (CBF) gene family involved in the response to abiotic stresses in tea plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 921. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Buscaill, P.; Rivas, S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Liu, C.; Wang, H.; Li, Y.; Xie, Y.; Ma, F.; Liang, J.; Li, C. Exogenous GABA improves the resistance of apple seedlings to long-term drought stress by enhancing GABA shunt and secondary cell wall biosynthesis. Tree Physiol. 2022, 42, 2563–2577. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahmon, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132. [Google Scholar] [CrossRef]
- Razik, E.S.A.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2020, 172, 505–527. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- AL-Quraan, N.A. GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: Insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol. Plant. 2015, 37, 86. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Dai, Y.; Cao, S. Exogenous calcium chloride (CaCl2) promotes γ-aminobutyric acid (GABA) accumulation in fresh-cut pears. Postharvest Biol. Technol. 2021, 174, 111446. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, B.S.; Hwang, B.K. Pepper arginine decarboxylase is required for polyamine and γ-aminobutyric acid signaling in cell death and defense response. Plant Physiol. 2013, 162, 2067–2083. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wu, X.; Xing, Z.; Li, Q.; Duan, Y.; Fang, W.; Zhu, X. γ-Aminobutyric acid (GABA) accumulation in tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 2017, 65, 3013–3018. [Google Scholar] [CrossRef]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, Y.; Cao, Y.; Chen, Y.; Zou, Z.; Li, F.; Shen, Q.; Yang, X.; Ma, Y.; Fang, W.; et al. CsCuAOs and CsAMADH1 Are Required for Putrescine-Derived γ-Aminobutyric Acid Accumulation in Tea. Foods 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.M.; Stegemann, T.; Sievert, C.; Kruse, L.H.; Kaltenegger, E.; Girreser, U.; Çiçek, S.S.; Nimtz, M.; Ober, D. Insights into polyamine metabolism: Homospermidine is double-oxidized in two discrete steps by a single copper-containing amine oxidase in pyrrolizidine alkaloid biosynthesis. Plant Cell 2022, 34, 2364–2382. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Delis, C.; Dimou, M.; Flemetakis, E.; Aivalakis, G.; Katinakis, P. A root- and hypocotyl-specific gene coding for copper-containing amine oxidase is related to cell expansion in soybean seedlings. J. Exp. Bot. 2006, 57, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Sites and regulation of polyamine catabolism in the tobacco plant: Correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol. 2005, 138, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Ruangnam, S.; Wanchana, S.; Phoka, N.; Saeansuk, C.; Mahatheeranont, S.; Hoop, S.J.; Toojinda, T.; Vanavichit, A.; Arikit, S. A deletion of the gene encoding amino aldehyde dehydrogenase enhances the “pandan-like” aroma of winter melon (Benincasa hispida) and is a functional marker for the development of the aroma. Theor. Appl. Genet. 2017, 130, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Trobacher, C.P.; Shelp, B.J. NAD+-aminoaldehyde dehydrogenase candidates for 4-aminobutyrate (GABA) and β-alanine production during terminal oxidation of polyamines in apple fruit. FEBS Lett. 2015, 589, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Arikit, S.; Yoshihashi, T.; Wanchana, S.; Uyen, T.T.; Huong, N.T.T.; Wongpornchai, S.; Vanavichit, A. Deficiency in the amino aldehyde dehydrogenase encoded by GmAMADH2, the homologue of rice Os2AP, enhances 2-acetyl-1-pyrroline biosynthesis in soybeans (Glycine max L.). Plant Biotechnol. J. 2011, 9, 75–87. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Cao, S. Effects of exogenous calcium chloride (CaCl2) and ascorbic acid (AsA) on the γ-aminobutyric acid (GABA) metabolism in shredded carrots. Postharvest Biol. Technol. 2019, 152, 111–117. [Google Scholar] [CrossRef]
- Meyer, A.; Eskandari, S.; Grallath, S.; Rentsch, D. AtGAT1, a high affinity transporter for gamma-aminobutyric acid in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 7197–7204. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.P.; Chen, C.L.; Yin, W.; Fu, X.Z.; Liu, J.H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914. [Google Scholar] [CrossRef]
- Han, J.; Gu, L.; Wen, J.; Sun, Y. Inference of photosynthetic capacity parameters from Chlorophyll a Fluorescence is affected by redox state of PSII reaction centers. Plant Cell Environ. 2022, 45, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-Activated Malate Transporters Can Facilitate GABA Transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Besnard, J.; Pratelli, R.; Zhao, C.S.; Sonawala, U.; Collakova, E.; Pilot, G.; Okumoto, S. UMAMIT14 is an amino acid exporter involved in phloem unloading in Arabidopsis roots. J. Exp. Bot. 2016, 67, 6385–6397. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhao, M.; Gao, T.; Jing, T.; Zhang, N.; Wang, J.; Zhang, X.; Huang, J.; Schwab, W.; Song, C. Amplification of early drought responses caused by volatile cues emitted from neighboring tea plants. Hortic. Res. 2021, 8, 243. [Google Scholar] [CrossRef]
- Zhu, X.J.; Li, Q.H.; Hu, J.Y.; Wang, M.L.; Li, X.H. Molecular Cloning and Characterization of Spermine Synthesis Gene Associated with Cold Tolerance in Tea Plant (Camellia sinensis). Appl. Biochem. Biotechnol. 2015, 17, 1055–1068. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Ferroni, L.; Zivcak, M.; Kovar, M.; Colpo, A.; Pancaldi, S.; Allakhverdiev, S.I.; Brestič, M. Fast chlorophyll a fluorescence induction (OJIP) phenotyping of chlorophyll-deficient wheat suggests that an enlarged acceptor pool size of Photosystem I helps compensate for a deregulated photosynthetic electron flow. J. Photochem. Photobiol. B 2022, 234, 112549. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, J.; Dong, C.; Li, F.; Qiao, S.; Lin, S.; Yang, T.; Wu, Y.; Bao, S.; Lucas, W.J.; et al. CsAlaDC and CsTSI work coordinately to determine theanine biosynthesis in tea plants (Camellia sinensis L.) and confer high levels of theanine accumulation in a non-tea plant. Plant Biotechnol. J. 2021, 19, 2395–2397. [Google Scholar] [CrossRef]
- Dong, C.; Li, F.; Yang, T.; Feng, L.; Zhang, S.; Li, F.; Li, W.; Xu, G.; Bao, S.; Wan, X.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Chen, Y.; Cheng, N.; Zhang, K.; Duan, Y.; Fang, S.; Shen, Q.; Yang, X.; Fang, W.; Zhu, X. CsCuAO1 Associated with CsAMADH1 Confers Drought Tolerance by Modulating GABA Levels in Tea Plants. Int. J. Mol. Sci. 2024, 25, 992. https://doi.org/10.3390/ijms25020992
Cao Y, Chen Y, Cheng N, Zhang K, Duan Y, Fang S, Shen Q, Yang X, Fang W, Zhu X. CsCuAO1 Associated with CsAMADH1 Confers Drought Tolerance by Modulating GABA Levels in Tea Plants. International Journal of Molecular Sciences. 2024; 25(2):992. https://doi.org/10.3390/ijms25020992
Chicago/Turabian StyleCao, Yu, Yiwen Chen, Nuo Cheng, Kexin Zhang, Yu Duan, Shimao Fang, Qiang Shen, Xiaowei Yang, Wanping Fang, and Xujun Zhu. 2024. "CsCuAO1 Associated with CsAMADH1 Confers Drought Tolerance by Modulating GABA Levels in Tea Plants" International Journal of Molecular Sciences 25, no. 2: 992. https://doi.org/10.3390/ijms25020992
APA StyleCao, Y., Chen, Y., Cheng, N., Zhang, K., Duan, Y., Fang, S., Shen, Q., Yang, X., Fang, W., & Zhu, X. (2024). CsCuAO1 Associated with CsAMADH1 Confers Drought Tolerance by Modulating GABA Levels in Tea Plants. International Journal of Molecular Sciences, 25(2), 992. https://doi.org/10.3390/ijms25020992





