Role of miRNA in Cardiovascular Diseases in Children—Systematic Review
Abstract
1. Introduction
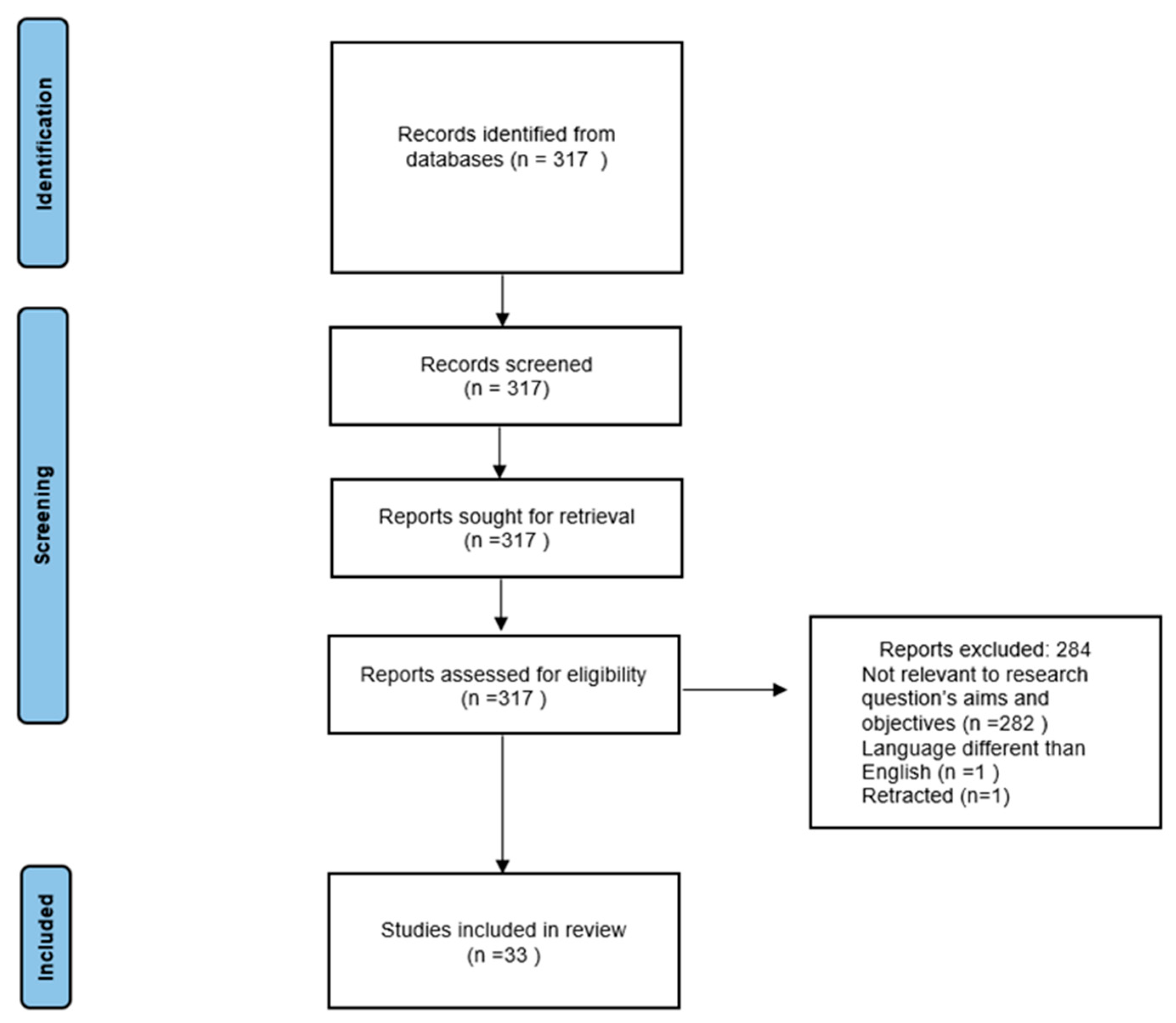
2. Materials and Methods
3. Results
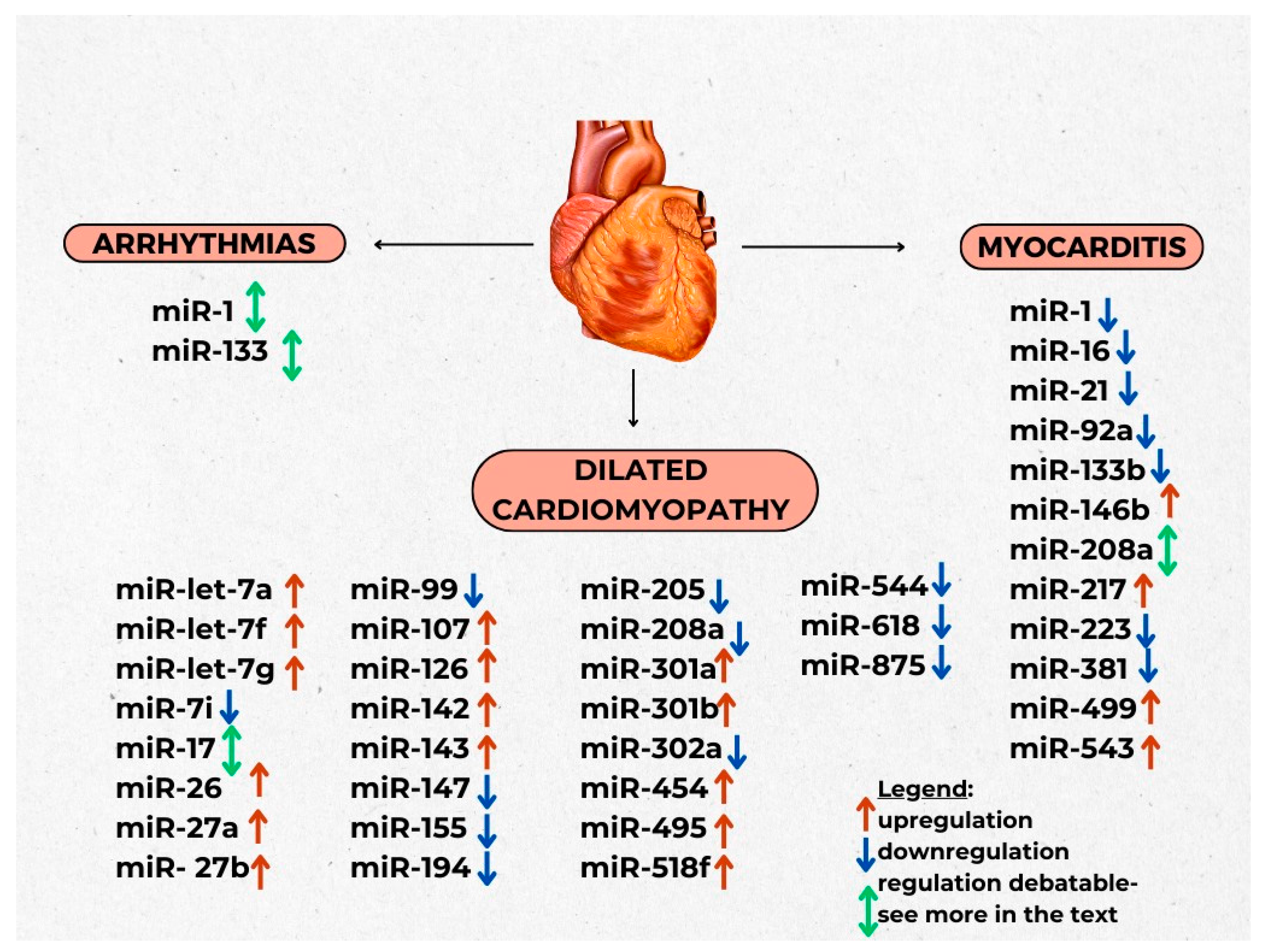
3.1. Arrhytmias
3.2. Myocarditis
3.3. Cardiomyopathies
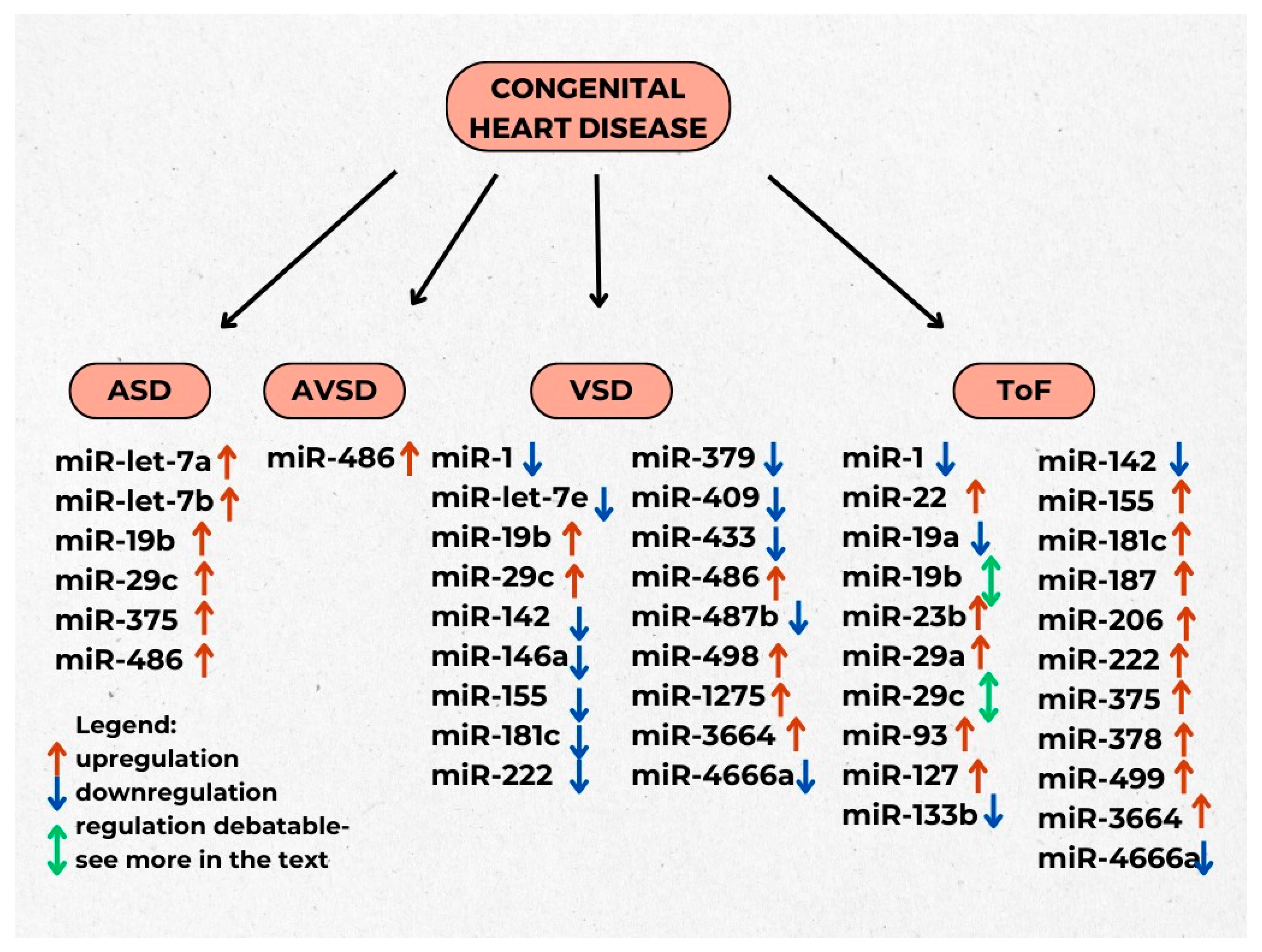
3.4. Congenital Heart Diseases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/The-Top-10-Causes-Of-Death?Fbclid=Iwar2llcq33xsxbhssptiozzuynislhja4oroomf3cqs-Svkzfutpy20x7heo (accessed on 9 December 2020).
- Lee, R.C.; Feinbaum, R.L.; Ambrost, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Tastsoglou, S.; Miliotis, M.; Kavakiotis, I.; Alexiou, A.; Gkotsi, E.C.; Lambropoulou, A.; Lygnos, V.; Kotsira, V.; Maroulis, V.; Zisis, D.; et al. Plasmir: A Manual Collection of Circulating Micrornas of Prognostic and Diagnostic Value. Cancers 2021, 13, 3680. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, L.M. MiRNA Activation Is an Endogenous Gene Expression Pathway. RNA Biol. 2018, 15, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Singh, M.; Kumar, N.; Singh, S.R. The Emerging Roles of MicroRNAs in Stem Cell Aging. In Advances in Experimental Medicine and Biology; Springer LLC: New York, NY, USA, 2018; Volume 1056, pp. 11–26. [Google Scholar]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila MicroRNA Mir-14 Suppresses Cell Death and Is Required for Normal Fat Metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Jordan-Alejandre, E.; Campos-Parra, A.D.; Castro-López, D.L.; Silva-Cázares, M.B. Potential MiRNA Use as a Biomarker: From Breast Cancer Diagnosis to Metastasis. Cells 2023, 12, 525. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Moric-Janiszewska, E.; Smolik, S.; Morka, A.; Szydłowski, L.; Kapral, M. Expression Levels of Serum Circulating MicroRNAs in Pediatric Patients with Ventricular and Supraventricular Arrhythmias. Adv. Med. Sci. 2021, 66, 411–417. [Google Scholar] [CrossRef]
- Hailu, F.T.; Karimpour-Fard, A.; Toni, L.S.; Bristow, M.R.; Miyamoto, S.D.; Stauffer, B.L.; Sucharov, C.C. Integrated Analysis of MiRNA–MRNA Interaction in Pediatric Dilated Cardiomyopathy. Pediatr. Res. 2022, 92, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Jat, K.R.; Lodha, R.; Kabra, S.K. Arrhythmias in Children. Indian J. Pediatr. 2011, 78, 211–218. [Google Scholar] [CrossRef]
- Sun, L.; Sun, S.; Zeng, S.; Li, Y.; Pan, W.; Zhang, Z. Expression of Circulating MicroRNA-1 and MicroRNA-133 in Pediatric Patients with Tachycardia. Mol. Med. Rep. 2015, 11, 4039–4046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Cao, Y.; Ma, X.J.; Wang, H.J.; Zhang, J.; Luo, X.; Chen, W.; Wu, Y.; Meng, Y.; Zhang, J.; et al. Roles of MiR-1-1 and MiR-181c in Ventricular Septal Defects. Int. J. Cardiol. 2013, 168, 1441–1446. [Google Scholar] [CrossRef]
- Su, X.; Liang, H.; Wang, H.; Chen, G.; Jiang, H.; Wu, Q.; Liu, T.; Liu, Q.; Yu, T.; Gu, Y.; et al. Over-Expression of MicroRNA-1 Causes Arrhythmia by Disturbing Intracellular Trafficking System. Sci. Rep. 2017, 7, 46259. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Xiao, J.; Wang, Z. Cellular Physiology Regulation of Human Cardiac Ion Channel Genes by MicroRNAs: Theoretical Perspective and Pathophysiological Implications. Cell. Physiol. Biochem. 2010, 25, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Zhang, Y.; Liang, H.; Li, X.; Cai, R.; Wang, L.; Du, W.; Zhang, R.; Li, J.; et al. Overexpression of MicroRNA-1 Causes Atrioventricular Block in Rodents. Int. J. Biol. Sci. 2013, 9, 445–462. [Google Scholar] [CrossRef]
- Wahl, C.M.; Schmidt, C.; Hecker, M.; Ullrich, N.D. Distress-Mediated Remodeling of Cardiac Connexin-43 in a Novel Cell Model for Arrhythmogenic Heart Diseases. Int. J. Mol. Sci. 2022, 23, 174. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, G.; Jia, D.; Dou, J.; Liu, X.; Guo, Z. Fibroblast-Derived Exosomal MiRNA-133 Promotes Cardiomyocyte-Like Differentiation. Acta Histochem. 2022, 124, 151931. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, X.; Lin, H.; Zhang, Y.; Lu, Y.; Wang, N.; Zhang, Y.; Yang, B.; Wang, Z. MicroRNA MiR-133 Represses HERG K+ Channel Expression Contributing to QT Prolongation in Diabetic Hearts. J. Biol. Chem. 2007, 282, 12363–12367. [Google Scholar] [CrossRef]
- Hedley, P.L.; Carlsen, A.L.; Christiansen, K.M.; Kanters, J.K.; Behr, E.R.; Corfield, V.A.; Christiansen, M. MicroRNAs in Cardiac Arrhythmia: DNA Sequence Variation of MiR-1 and MiR-133A in Long QT Syndrome. Scand. J. Clin. Lab. Investig. 2014, 74, 485–491. [Google Scholar] [CrossRef][Green Version]
- Cheng, W.L.; Kao, Y.H.; Chao, T.F.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. MicroRNA-133 Suppresses ZFHX3-Dependent Atrial Remodelling and Arrhythmia. Acta Physiol. 2019, 227, e13322. [Google Scholar] [CrossRef]
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating MicroRNAs: A Potential Biomarker for Cardiac Damage, Inflammatory Response, and Left Ventricular Function Recovery in Pediatric Viral Myocarditis. J. Cardiovasc. Transl. Res. 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; Goretti, E.; Nazarov, P.V.; Azuaje, F.; Gilson, G.; Corsten, M.F.; Schroen, B.; Lair, M.L.; Heymans, S.; et al. Use of Circulating MicroRNAs to Diagnose Acute Myocardial Infarction. Clin. Chem. 2012, 58, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bei, Y.; Shen, S.; Huang, P.; Shi, J.; Zhang, J.; Sun, Q.; Chen, Y.; Yang, Y.; Xu, T.; et al. MiR-21-3p Controls Sepsis-Associated Cardiac Dysfunction via Regulating SORBS2. J. Mol. Cell. Cardiol. 2016, 94, 43–53. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 Reflect Myocardial Damage in Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 Prevents Excessive Inflammation and Cardiac Dysfunction after Myocardial Infarction through Targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Tao, L.; Li, X. MicroRNA-21-3p/Rcan1 Signaling Axis Affects Apoptosis of Cardiomyocytes of Sepsis Rats. Gen. Physiol. Biophys. 2023, 42, 217–227. [Google Scholar] [CrossRef]
- Li, Y.; Sun, G.; Wang, L. MiR-21 Participates in LPS-Induced Myocardial Injury by Targeting Bcl-2 and CDK6. Inflamm. Res. 2022, 71, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Sun, H.; Yu, Z.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. MicroRNA-381 Protects Myocardial Cell Function in Children and Mice with Viral Myocarditis via Targeting Cyclooxygenase-2 Expression. Exp. Ther. Med. 2018, 15, 5510–5516. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Lu, R.; Liu, Q.; Hong, S.; Zhang, Z.; Hu, G. MicroRNA-381-3p Signatures as a Diagnostic Marker in Patients with Sepsis and Modulates Sepsis-Steered Cardiac Damage and Inflammation by Binding HMGB1. Bioengineered 2021, 12, 11936–11946. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Dong, W.; Peng, W.; Yang, J. MiR-381 Negatively Regulates Cardiomyocyte Survival by Suppressing Notch Signaling. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 610–619. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Yan, H.; Li, X.; Ding, C.; Wang, Q.; Lu, Z. Protective Effect of MicroRNA-381 against Inflammatory Damage of Endothelial Cells during Coronary Heart Disease by Targeting CXCR4. Mol. Med. Rep. 2020, 21, 1439–1448. [Google Scholar] [CrossRef]
- Xia, K.; Zhang, Y.; Sun, D. MiR-217 and MiR-543 Downregulation Mitigates Inflammatory Response and Myocardial Injury in Children with Viral Myocarditis by Regulating the SIRT1/AMPK/NF-κB Signaling Pathway. Int. J. Mol. Med. 2020, 45, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Staszel, T.; Zapała, B.; Polus, A.; Sadakierska-Chudy, A.; Kieć-Wilk, B.; Stępień, E.; Wybrańska, I.; Chojnacka, M.; Dembińska-Kieć, A. Role of MicroRNAs in Endothelial Cell Pathophysiology. Pol. Arch. Med. Wewn. 2011, 121, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, T.; Cui, H.; Zhang, Y. Analysis of the Indicating Value of Cardiac Troponin I, Tumor Necrosis Factor-α, Interleukin-18, Mir-1 and Mir-146b for Viral Myocarditis among Children. Cell. Physiol. Biochem. 2016, 40, 1325–1333. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Zhao, C.; Chen, C.; Kong, Q.; Cai, Z.; Li, D. MiR-1/133 Attenuates Cardiomyocyte Apoptosis and Electrical Remodeling in Mice with Viral Myocarditis. Cardiol. J. 2020, 27, 285–294. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wu, W.F.; Xue, Y.; Gao, M.; Yan, Y.; Kong, Q.; Pang, Y.; Yang, F. MicroRNA-21 and -146b Are Involved in the Pathogenesis of Murine Viral Myocarditis by Regulating TH-17 Differentiation. Arch. Virol. 2013, 158, 1953–1963. [Google Scholar] [CrossRef]
- Di, Y.-F.; Li, D.-C.; Shen, Y.-Q.; Wang, C.-L.; Zhang, D.-Y.; Shang, A.-Q.; Hu, T. MiR-146b Protects Cardiomyocytes Injury in Myocardial Ischemia/Reperfusion by Targeting Smad4. Am. J. Transl. Res. 2017, 9, 656–663. [Google Scholar]
- Chouvarine, P.; Legchenko, E.; Geldner, J.; Riehle, C.; Hansmann, G. Hypoxia Drives Cardiac MiRNAs and Inflammation in the Right and Left Ventricle. J. Mol. Med. 2019, 97, 1427–1438. [Google Scholar] [CrossRef]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 Represses Endothelial Activation by Inhibiting pro-Inflammatory Pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Sun, H.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. Overexpression of MicroRNA-133b Reduces Myocardial Injuries in Children with Viral Myocarditis by Targeting Rab27B Gene. Cell. Mol. Biol. 2017, 6, 80–86. [Google Scholar] [CrossRef]
- Gumus, G.; Giray, D.; Bobusoglu, O.; Tamer, L.; Karpuz, D.; Hallioglu, O. MicroRNA Values in Children with Rheumatic Carditis: A Preliminary Study. Rheumatol. Int. 2018, 38, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yan, B.; Zhang, J.; Zhao, P.; Jing, Y.; Yu, J.; Hui, J.; Lu, Q. Mesenchymal Stem Cells-Derived Extracellular Vesicles-Shuttled MicroRNA-223-3p Suppress Lipopolysaccharide-Induced Cardiac Inflammation, Pyroptosis, and Dysfunction. Int. Immunopharmacol. 2022, 110, 108910. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, B.L.; Russell, G.; Nunley, K.; Miyamoto, S.D.; Sucharov, C.C. MiRNA Expression in Pediatric Failing Human Heart. J. Mol. Cell. Cardiol. 2013, 57, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, X.; Zhang, M.; Zheng, Y.; Zheng, X.; Yang, Q.; Li, J.; Gu, N.; Zhang, M.; Sun, Y.; et al. MicroRNA-223-3p Modulates Dendritic Cell Function and Ameliorates Experimental Autoimmune Myocarditis by Targeting the NLRP3 Inflammasome. Mol. Immunol. 2020, 117, 73–83. [Google Scholar] [CrossRef]
- Gou, W.; Zhang, Z.; Yang, C.; Li, Y. MiR-223/Pknox1 Axis Protects Mice from CVB3-Induced Viral Myocarditis by Modulating Macrophage Polarization. Exp. Cell Res. 2018, 366, 41–48. [Google Scholar] [CrossRef]
- Szemraj, J.; Masiarek, K.; Majos, A.; Szemraj-Rogucka, Z.M. Circulating MicroRNAs as Biomarkers for Myocardial Fibrosis in Patients with Left Ventricular Non-Compaction Cardiomyopathy. Arch. Med. Sci. 2019, 15, 376–384. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; Hoppe, P.; Kuhn, C.; Zille, E.; Frank, J.; Frey, N.; Frank, D. MicroRNA MiR-301a Is a Novel Cardiac Regulator of Cofilin-2. PLoS ONE 2017, 12, e0183901. [Google Scholar] [CrossRef]
- Zhen, L.-X.; Gu, Y.-Y.; Zhao, Q.; Zhu, H.-F.; Lü, J.-H.; Li, S.-J.; Xu, Z.; Li, L.; Yu, Z.-R. MiR-301a Promotes Embryonic Stem Cell Differentiation to Cardiomyocytes. World J. Stem Cells 2019, 11, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Maruyama, S.; Sano, S.; Accorsi, A.; Girgenrath, M.; Walsh, K.; Naya, F.J. MiR-410 and MiR-495 Are Dynamically Regulated in Diverse Cardiomyopathies and Their Inhibition Attenuates Pathological Hypertrophy. PLoS ONE 2016, 11, e0151515. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chen, Y.; Li, F. Attenuation of MicroRNA-495 Derepressed PTEN to Effectively Protect Rat Cardiomyocytes from Hypertrophy. Cardiology 2018, 139, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Su, Y.-L.; Shi, J.-Y.; Lu, Q.; Chen, C. MicroRNA-17-5p Promotes Cardiac Hypertrophy by Targeting Mfn2 to Inhibit Autophagy. Cardiovasc. Toxicol. 2021, 21, 759–771. [Google Scholar] [CrossRef]
- Callis, T.E.; Pandya, K.; Hee, Y.S.; Tang, R.H.; Tatsuguchi, M.; Huang, Z.P.; Chen, J.F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a Is a Regulator of Cardiac Hypertrophy and Conduction in Mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-H.; Li, J.-L.; Li, X.-Y.; Wang, S.-X.; Jiao, Z.-H.; Li, S.-Q.; Liu, J.; Ding, J. MiR-208a in Cardiac Hypertrophy and Remodeling. Front. Cardiovasc. Med. 2021, 8, 773314. [Google Scholar] [CrossRef] [PubMed]
- Enes Coşkun, M.; Kervancioǧlu, M.; Öztuzcu, S.; Yilmaz Coşkun, F.; Ergün, S.; Başpinar, O.; Kilinç, M.; Temel, L.; Coşkun, M.Y. Plasma MicroRNA Profiling of Children with Idiopathic Dilated Cardiomyopathy. Biomarkers 2016, 21, 56–61. [Google Scholar] [CrossRef]
- Fayez, A.G.; Esmaiel, N.N.; Salem, S.M.; Ashaat, E.A.; El-Saiedi, S.A.; El Ruby, M.O. MiR-454-3p and MiR-194-5p Targeting Cardiac Sarcolemma Ion Exchange Transcripts Are Potential Noninvasive Diagnostic Biomarkers for Childhood Dilated Cardiomyopathy in Egyptian Patients. Egypt. Heart J. 2022, 74, 65. [Google Scholar] [CrossRef]
- Toro, R.; Blasco-Turrión, S.; Morales-Ponce, F.J.; Gonzalez, P.; Martínez-Camblor, P.; López-Granados, A.; Brugada, R.; Campuzano, O.; Pérez-Serra, A.; Rosa Longobardo, F.; et al. Plasma MicroRNAs as Biomarkers for Lamin A/C-Related Dilated Cardiomyopathy. J. Mol. Med. 2018, 96, 845–856. [Google Scholar] [CrossRef]
- Mahjoub, S.; Mehri, S.; Bousaada, R.; Ouarda, F.; Zaroui, A.; Zouari, B.; Mechmeche, R.; Hammami, M.; Ben Arab, S. Association of ACE I/D Polymorphism in Tunisian Patients with Dilated Cardiomyopathy. JRAAS-J. Renin-Angiotensin-Aldosterone Syst. 2010, 11, 187–191. [Google Scholar] [CrossRef]
- Nair, N.; Kumar, S.; Gongora, E.; Gupta, S. Circulating MiRNA as Novel Markers for Diastolic Dysfunction. Mol. Cell. Biochem. 2013, 376, 33–40. [Google Scholar] [CrossRef]
- Li, X.; Luo, R.; Mo, X.; Jiang, R.; Kong, H.; Hua, W.; Wu, X. Polymorphism of ZBTB17 Gene Is Associated with Idiopathic Dilated Cardiomyopathy: A Case Control Study in a Han Chinese Population. Eur. J. Med. Res. 2013, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Ruppert, V.; Ackermann, S.; Richter, A.; Perrot, A.; Sperling, S.R.; Posch, M.G.; Maisch, B.; Pankuweit, S. Novel Mutations in the Sarcomeric Protein Myopalladin in Patients with Dilated Cardiomyopathy. Eur. J. Hum. Genet. 2013, 21, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Redwood, C.; Robinson, P. Alpha-Tropomyosin Mutations in Inherited Cardiomyopathies. J. Muscle Res. Cell Motil. 2013, 34, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, F.; Zugck, C.; Rauch, G.J.; Ivandic, B.; Weichenhan, D.; Müller-Bardorff, M.; Meder, B.; Mokhtari, N.E.E.; Regitz-Zagrosek, V.; Hetzer, R.; et al. HBEGF, SRA1, and IK: Three Cosegregating Genes as Determinants of Cardiomyopathy. Genome Res. 2009, 19, 395–403. [Google Scholar] [CrossRef]
- Seeger, T.S.; Frank, D.; Rohr, C.; Will, R.; Just, S.; Grund, C.; Lyon, R.; Luedde, M.; Koegl, M.; Sheikh, F.; et al. Myozap, a Novel Intercalated Disc Protein, Activates Serum Response Factor-Dependent Signaling and Is Required to Maintain Cardiac Function In Vivo. Circ. Res. 2010, 106, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, T.; Niu, X.; Hu, L.; Cheng, J.; Guo, D.; Ren, H.; Zhao, R.; Ji, Z.; Liu, P.; et al. ADSC-Derived Exosomes Attenuate Myocardial Infarction Injury by Promoting MiR-205-Mediated Cardiac Angiogenesis. Biol. Direct 2023, 18, 6. [Google Scholar] [CrossRef]
- Brody, M.J.; Hacker, T.A.; Patel, J.R.; Feng, L.; Sadoshima, J.; Tevosian, S.G.; Balijepalli, R.C.; Moss, R.L.; Lee, Y. Ablation of the Cardiac-Specific Gene Leucine-Rich Repeat Containing 10 (Lrrc10) Results in Dilated Cardiomyopathy. PLoS ONE 2012, 7, e51621. [Google Scholar] [CrossRef]
- Stillitano, F.; Lonardo, G.; Zicha, S.; Varro, A.; Cerbai, E.; Mugelli, A.; Nattel, S. Molecular Basis of Funny Current (If) in Normal and Failing Human Heart. J. Mol. Cell. Cardiol. 2008, 45, 289–299. [Google Scholar] [CrossRef]
- Duboscq-Bidot, L.; Charron, P.; Ruppert, V.; Fauchier, L.; Richter, A.; Tavazzi, L.; Arbustini, E.; Wichter, T.; Maisch, B.; Komajda, M.; et al. Mutations in the ANKRD1 Gene Encoding CARP Are Responsible for Human Dilated Cardiomyopathy. Eur. Heart J. 2009, 30, 2128–2136. [Google Scholar] [CrossRef]
- Ramasamy, S.; Velmurugan, G.; Rekha, B.; Anusha, S.; Shanmugha Rajan, K.; Shanmugarajan, S.; Ramprasath, T.; Gopal, P.; Tomar, D.; Karthik, K.V.; et al. Egr-1 Mediated Cardiac MiR-99 Family Expression Diverges Physiological Hypertrophy from Pathological Hypertrophy. Exp. Cell Res. 2018, 365, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; Romito, A.; Borel, C.; Gehrig, C.; Gagnebin, M.; Falconnet, E.; Izzo, A.; Altucci, L.; Banfi, S.; Antonarakis, S.E.; et al. Cardiomyogenesis Is Controlled by the MiR-99a/Let-7c Cluster and Epigenetic Modifications. Stem Cell Res. 2014, 12, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, Z.; Jin, Y.; Dragas, D.; Zhang, L.; Adjei, B.S.; Wang, A.; Dai, Y.; Zhou, X. MicroRNA-99 Family Members Suppress Homeobox A1 Expression in Epithelial Cells. PLoS ONE 2013, 8, e80625. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Tabuchi, T.; Nakamura, M. A Cellular MicroRNA, Let-7i, Is a Novel Biomarker for Clinical Outcome in Patients with Dilated Cardiomyopathy. J. Card. Fail. 2011, 17, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.D.; Karimpour-Fard, A.; Peterson, V.; Auerbach, S.R.; Stenmark, K.R.; Stauffer, B.L.; Sucharov, C.C. Circulating MicroRNA as a Biomarker for Recovery in Pediatric Dilated Cardiomyopathy. J. Heart Lung Transplant. 2015, 34, 724–733. [Google Scholar] [CrossRef]
- Tili, E.; Croce, C.M.; Michaille, J.J. MiR-155: On the Crosstalk between Inflammation and Cancer. Int. Rev. Immunol. 2009, 28, 264–284. [Google Scholar] [CrossRef]
- Seok, H.Y.; Chen, J.; Kataoka, M.; Huang, Z.P.; Ding, J.; Yan, J.; Hu, X.; Wang, D.Z. Loss of MicroRNA-155 Protects the Heart from Pathological Cardiac Hypertrophy. Circ. Res. 2014, 114, 1585–1595. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhao, C.M.; Li, Z.G.; Wang, Y.M.; Miao, W.; Wu, X.J.; Wang, W.J.; Liu, C.; Wang, D.; Wang, K.; et al. MiR-218 Involvement in Cardiomyocyte Hypertrophy Is Likely through Targeting REST. Int. J. Mol. Sci. 2016, 17, 848. [Google Scholar] [CrossRef]
- Small, E.M.; Sutherland, L.B.; Rajagopalan, K.N.; Wang, S.; Olson, E.N. Microrna-218 Regulates Vascular Patterning by Modulation of Slit-Robo Signaling. Circ. Res. 2010, 107, 1336–1344. [Google Scholar] [CrossRef]
- Hassel, D.; Dahme, T.; Erdmann, J.; Meder, B.; Huge, A.; Stoll, M.; Just, S.; Hess, A.; Ehlermann, P.; Weichenhan, D.; et al. Nexilin Mutations Destabilize Cardiac Z-Disks and Lead to Dilated Cardiomyopathy. Nat. Med. 2009, 15, 1281–1288. [Google Scholar] [CrossRef]
- Hu, Q.L.; Xu, Z.P.; Lan, Y.F.; Li, B. MiR-636 Represses Cell Survival by Targeting CDK6/Bcl-2 in Cervical Cancer. Kaohsiung J. Med. Sci. 2020, 36, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Misbah, M.; Kumar, M.; Lee, K.H.; Shen, S.C. Identification of Novel MiRNAs, Targeting Genes, Signaling Pathway, and the Small Molecule for Overcoming Oxaliplatin Resistance of Metastatic Colorectal Cancer. BioMed Res. Int. 2022, 2022, 3825760. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, C.; Chen, X. MiR-636 Inhibits EMT, Cell Proliferation and Cell Cycle of Ovarian Cancer by Directly Targeting Transcription Factor Gli2 Involved in Hedgehog Pathway. Cancer Cell Int. 2021, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, X.; Lv, P.; Wang, F. MiR-639 Promotes the Proliferation and Invasion of Breast Cancer Cell In Vitro. Cancer Cell Int. 2014, 14, 39. [Google Scholar] [CrossRef][Green Version]
- Lei, S.; Shen, F.; Chen, J.; Feng, J.; Cai, W.; Shen, L.; Hu, Z.; Xu, B. MiR-639 Promoted Cell Proliferation and Cell Cycle in Human Thyroid Cancer by Suppressing CDKN1A Expression. Biomed. Pharmacother. 2016, 84, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, J.; Hou, L.; Guo, X. Circ_0001955 Acts as a MiR-646 Sponge to Promote the Proliferation, Metastasis and Angiogenesis of Hepatocellular Carcinoma. Dig. Dis. Sci. 2022, 67, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, W.M.; Zhang, H.; Li, Y.Q.; Peng, Y.; Wang, J.; Liu, G.N.; Huang, X.T.; Zhao, J.J.; Li, G.; et al. MiR-646 Inhibited Cell Proliferation and EMT-Induced Metastasis by Targeting FOXK1 in Gastric Cancer. Br. J. Cancer 2017, 117, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; You, H.Z.; Yang, X.Y.; Yuan, H.; Li, Y.L.; Liu, W.X.; Jin, M.; Du, J. Circulating MicroRNA Signature for the Diagnosis of Childhood Dilated Cardiomyopathy. Sci. Rep. 2018, 8, 724. [Google Scholar] [CrossRef]
- Sharma, S.; Liu, J.; Wei, J.; Yuan, H.; Zhang, T.; Bishopric, N.H. Repression of MiR-142 by P300 and MAPK Is Required for Survival Signalling via Gp130 during Adaptive Hypertrophy. EMBO Mol. Med. 2012, 4, 617–632. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, M.; Hu, S.; Wang, S.; Wang, L.; Tu, X.; Huang, C.; Jiang, H.; Wu, G. Overexpression of MiR-142-3p Improves Mitochondrial Function in Cardiac Hypertrophy. Biomed. Pharmacother. 2018, 108, 1347–1356. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, Y.; Zhang, H.; Xie, D.; Nie, W.; Shi, K. Inhibition of MicroRNA-143-3p Attenuates Myocardial Hypertrophy by Inhibiting Inflammatory Response. Cell Biol. Int. 2018, 42, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Noda, A.; Ueda, J.; Ogata, T.; Matsuyama, R.; Nishizawa, Y.; Qiao, S.; Iwata, S.; Ito, M.; Fujihara, Y.; et al. Forced Expression of MiR-143 and -145 in Cardiomyocytes Induces Cardiomyopathy with a Reductive Redox Shift. Cell. Mol. Biol. Lett. 2020, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Deacon, D.C.; Nevis, K.R.; Cashman, T.J.; Zhou, Y.; Zhao, L.; Washko, D.; Guner-Ataman, B.; Burns, C.G.; Burns, C.E. The MiR-143-Adducin3 Pathway Is Essential for Cardiac Chamber Morphogenesis. Development 2010, 137, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Velasco, E.; Galiano-Torres, J.; Jodar-Garcia, A.; Aranega, A.E.; Franco, D. MiR-27 and MiR-125 Distinctly Regulate Muscle-Enriched Transcription Factors in Cardiac and Skeletal Myocytes. BioMed Res. Int. 2015, 2015, 391306. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Torres, F.; Aranega, A.E.; Franco, D. Identification of Regulatory Elements Directing MiR-23a-MiR-27a-MiR-24-2 Transcriptional Regulation in Response to Muscle Hypertrophic Stimuli. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, F.; Luo, S.; Zhao, W.; Yang, T.; Zhang, G.; Gao, M.; Lu, R.; Shu, Y.; Mu, W.; et al. Let-7a Is an Antihypertrophic Regulator in the Heart via Targeting Calmodulin. Int. J. Biol. Sci. 2017, 13, 22–31. [Google Scholar] [CrossRef]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A Signature of Circulating MicroRNAs Differentiates Takotsubo Cardiomyopathy from Acute Myocardial Infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.A.; et al. Global, Regional, and National Burden of Congenital Heart Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef]
- Rohit, M.; Shrivastava, S. Acyanotic and Cyanotic Congenital Heart Diseases. Indian J. Pediatr. 2018, 85, 454–460. [Google Scholar] [CrossRef]
- Hu, C.; Huang, S.; Wu, F.; Ding, H. MicroRNA-219-5p Participates in Cyanotic Congenital Heart Disease Progression by Regulating Cardiomyocyte Apoptosis. Exp. Ther. Med. 2020, 21, 36. [Google Scholar] [CrossRef]
- Song, Y.; Higgins, H.; Guo, J.; Harrison, K.; Schultz, E.N.; Hales, B.J.; Moses, E.K.; Goldblatt, J.; Pachter, N.; Zhang, G. Clinical Significance of Circulating MicroRNAs as Markers in Detecting and Predicting Congenital Heart Defects in Children. J. Transl. Med. 2018, 16, 42. [Google Scholar] [CrossRef]
- Samani, A.; Hightower, R.M.; Reid, A.L.; English, K.G.; Lopez, M.A.; Scott Doyle, J.; Conklin, M.J.; Schneider, D.A.; Bamman, M.M.; Widrick, J.J.; et al. MiR-486 Is Essential for Muscle Function and Suppresses a Dystrophic Transcriptome. Life Sci. Alliance 2022, 5, e202101215. [Google Scholar] [CrossRef]
- Lange, S.; Banerjee, I.; Carrion, K.; Serrano, R.; Habich, L.; Kameny, R.; Lengenfelder, L.; Dalton, N.; Meili, R.; Börgeson, E.; et al. MiR-486 Is Modulated by Stretch and Increases Ventricular Growth. JCI Insight 2019, 4, e125507. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Q.; Li, L.; Wang, X.; Lu, Y.; Liang, J. MiR-486 Regulates Cardiomyocyte Apoptosis by P53-Mediated BCL-2 Associated Mitochondrial Apoptotic Pathway. BMC Cardiovasc. Disord. 2017, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ji, L.; Liu, L.; Liu, Y.; Hou, H.; Yu, K.; Sun, Q.; Zhao, Z. Characterization of Circulating MicroRNA Expression in Patients with a Ventricular Septal Defect. PLoS ONE 2014, 9, e106318. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.Y.; Tatsuguchi, M.; Callis, T.E.; He, A.; Pu, W.T.; Wang, D.Z. MiR-155 Inhibits Expression of the MEF2A Protein to Repress Skeletal Muscle Differentiation. J. Biol. Chem. 2011, 286, 35339–35346. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S. Circulating MicroRNA as a Novel Biomarker for Pulmonary Arterial Hypertension Due to Congenital Heart Disease. Pediatr. Cardiol. 2017, 38, 86–94. [Google Scholar] [CrossRef]
- Li, X.; Xiang, D.; Shu, Y.; Hu, K.; Zhang, Y.; Li, Y. MicroRNA-204 as an Indicator of Severity of Pulmonary Hypertension in Children with Congenital Heart Disease Complicated with Pulmonary Hypertension. Med. Sci. Monit. 2019, 25, 10173–10179. [Google Scholar] [CrossRef]
- Jin, Y.; Ai, L.; Chai, X.; Tang, P.; Zhang, W.; Yang, L.; Hu, Y.; Xu, Y.; Li, S. Maternal Circulating Exosomal MiRNAs as Non-Invasive Biomarkers for the Prediction of Fetal Ventricular Septal Defect. Front. Genet. 2021, 12, 717208. [Google Scholar] [CrossRef]
- Zhu, S.; Cao, L.; Zhu, J.; Kong, L.; Jin, J.; Qian, L.; Zhu, C.; Hu, X.; Li, M.; Guo, X.; et al. Identification of Maternal Serum MicroRNAs as Novel Non-Invasive Biomarkers for Prenatal Detection of Fetal Congenital Heart Defects. Clin. Chim. Acta 2013, 424, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, F.; Mi, Y.; Wang, F.; Cai, K.; Yang, X.; Zhang, R.; Liu, L.; Zhang, Y.; Wang, Y.; et al. Aberrant Expression of MiR-29b-3p Influences Heart Development and Cardiomyocyte Proliferation by Targeting NOTCH2. Cell Prolif. 2020, 53, e12764. [Google Scholar] [CrossRef]
- Wang, L.; Song, G.; Liu, M.; Chen, B.; Chen, Y.; Shen, Y.; Zhu, J.; Zhou, X. MicroRNA-375 Overexpression Influences P19 Cell Proliferation, Apoptosis and Differentiation through the Notch Signaling Pathway. Int. J. Mol. Med. 2016, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Wang, D.Z. MiR-22 in Cardiac Remodeling and Disease. Trends Cardiovasc. Med. 2014, 24, 267–272. [Google Scholar] [CrossRef]
- Gu, H.; Chen, L.; Xue, J.; Huang, T.; Wei, X.; Liu, D.; Ma, W.; Cao, S.; Yuan, Z. Expression Profile of Maternal Circulating MicroRNAs as Non-Invasive Biomarkers for Prenatal Diagnosis of Congenital Heart Defects. Biomed. Pharmacother. 2019, 109, 823–830. [Google Scholar] [CrossRef]
- You, G.; Zu, B.; Wang, B.; Fu, Q.; Li, F. Identification of MiRNA–MRNA–TFs Regulatory Network and Crucial Pathways Involved in Tetralogy of Fallot. Front. Genet. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, L.; Han, L.; Zhao, Y.; Jing, H.; Song, W.; Shi, H. Role of MicroRNA-93 I in Pathogenesis of Left Ventricular Remodeling via Targeting Cyclin-D1. Med. Sci. Monit. 2017, 23, 3981–3988. [Google Scholar] [CrossRef][Green Version]
- Wo, Y.; Guo, J.; Li, P.; Yang, H.; Wo, J. Long Non-Coding RNA CHRF Facilitates Cardiac Hypertrophy through Regulating Akt3 via MiR-93. Cardiovasc. Pathol. 2018, 35, 29–36. [Google Scholar] [CrossRef]
- Ektesabi, A.M.; Mori, K.; Tsoporis, J.; Walsh, C.; Mai, S.; Hu, P.; DosSantos, C. Regulation of Mir-187b in Endotoxemic Primary Cardiomyocytes and Septic Murine Hearts Treated with Mesenchymal Stromal/Stem Cells. Can. J. Cardiol. 2019, 35, S48. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, J.J.; Xu, F.; Ma, X.J.; Wu, Y.; Li, W.C.; Wang, H.J.; Huang, G.Y.; Ma, D. MicroRNA Deregulation in Right Ventricular Outflow Tract Myocardium in Nonsyndromic Tetralogy of Fallot. Can. J. Cardiol. 2013, 29, 1695–1703. [Google Scholar] [CrossRef]
- Grunert, M.; Appelt, S.; Dunkel, I.; Berger, F.; Sperling, S.R. Altered MicroRNA and Target Gene Expression Related to Tetralogy of Fallot. Sci. Rep. 2019, 9, 19063. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, G.; Zhu, Z.; Chen, H.; Fu, Q. Sexual Difference of Small RNA Expression in Tetralogy of Fallot. Sci. Rep. 2018, 8, 12847. [Google Scholar] [CrossRef]
- Bittel, D.C.; Kibiryeva, N.; Marshall, J.A.; O’Brien, J.E. MicroRNA-421 Dysregulation Is Associated with Tetralogy of Fallot. Cells 2014, 3, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Xiao, Q.R.; Yang, Y.; Xu, J.L.; Zhang, F.; Liu, C.M.; Zhang, Z.M.; Lu, Y.Q.; Huang, N.P. MicroRNA-34a Modulates the Notch Signaling Pathway in Mice with Congenital Heart Disease and Its Role in Heart Development. J. Mol. Cell. Cardiol. 2018, 114, 300–308. [Google Scholar] [CrossRef]
- Sánchez-Gómez, M.C.; García-Mejía, K.A.; Pérez-Díaz Conti, M.; Díaz-Rosas, G.; Palma-Lara, I.; Sánchez-Urbina, R.; Klünder-Klünder, M.; Botello-Flores, J.A.; Balderrábano-Saucedo, N.A.; Contreras-Ramos, A. MicroRNAs Association in the Cardiac Hypertrophy Secondary to Complex Congenital Heart Disease in Children. Pediatr. Cardiol. 2017, 38, 991–1003. [Google Scholar] [CrossRef]
- Zloto, K.; Tirosh-Wagner, T.; Bolkier, Y.; Bar-Yosef, O.; Vardi, A.; Mishali, D.; Paret, G.; Nevo-Caspi, Y. Preoperative MiRNA-208a as a Predictor of Postoperative Complications in Children with Congenital Heart Disease Undergoing Heart Surgery. J Cardiovasc. Transl. Res. 2020, 13, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Li, H.; Su, Z.; Wang, J.; Zhang, H. Down-Regulation of MicroRNA-184 Contributes to the Development of Cyanotic Congenital Heart Diseases. Int. J. Clin. Exp. Pathol. 2015, 8, 14221–14227. [Google Scholar] [PubMed]
- Zhang, Y.; Peng, B.; Han, Y. MiR-182 Alleviates the Development of Cyanotic Congenital Heart Disease by Suppressing HES1. Eur. J. Pharmacol. 2018, 836, 18–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, W.K.; Jian, Z.; Zhao, L.; Liu, C.C.; Wang, Y.; Xiao, Y. Bin Downregulation of MicroRNA-199a-5p Protects Cardiomyocytes in Cyanotic Congenital Heart Disease by Attenuating Endoplasmic Reticulum Stress. Mol. Med. Rep. 2017, 16, 2992–3000. [Google Scholar] [CrossRef]
- Sucharov, C.C.; Sucharov, J.; Karimpour-Fard, A.; Nunley, K.; Stauffer, B.L.; Miyamoto, S.D. Micro-RNA Expression in Hypoplastic Left Heart Syndrome. J. Card. Fail. 2015, 21, 83–88. [Google Scholar] [CrossRef]
- Smolka, C.; Schlösser, D.; Koentges, C.; Tarkhnishvili, A.; Gorka, O.; Pfeifer, D.; Bemtgen, X.; Asmussen, A.; Groß, O.; Diehl, P.; et al. Cardiomyocyte-Specific MiR-100 Overexpression Preserves Heart Function under Pressure Overload in Mice and Diminishes Fatty Acid Uptake as Well as ROS Production by Direct Suppression of Nox4 and CD36. FASEB J. 2021, 35, e21956. [Google Scholar] [CrossRef]
- Wong, L.L.; Wee, A.S.Y.; Lim, J.Y.; Ng, J.Y.X.; Chong, J.P.C.; Liew, O.W.; Lilyanna, S.; Martinez, E.C.; Ackers-Johnson, M.A.; Vardy, L.A.; et al. Natriuretic Peptide Receptor 3 (NPR3) Is Regulated by MicroRNA-100. J. Mol. Cell. Cardiol. 2015, 82, 13–21. [Google Scholar] [CrossRef]
- Chen, A.; Li, G.; Chen, L.; Guo, J.; Liu, Y. Downregulation of MicroRNA-100 Protects H2O2-Induced Apoptosis in Neonatal Cardiomyocytes. Int. J. Clin. Exp. Pathol. 2015, 8, 5491–5496. [Google Scholar] [PubMed]
- Wang, L.; Tian, D.; Hu, J.; Xing, H.; Sun, M.; Wang, J.; Jian, Q.; Yang, H. MiRNA-145 Regulates the Development of Congenital Heart Disease Through Targeting FXN. Pediatr. Cardiol. 2016, 37, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Vecoli, C.; Mercuri, A.; Turchi, S.; Andreassi, M.G. Individual and Joint Effects of Genetic Polymorphisms in MicroRNA-Machinery Genes on Congenital Heart Disease Susceptibility. Cardiol. Young 2021, 31, 965–968. [Google Scholar] [CrossRef]
- Toro, R.; Pérez-Serra, A.; Mangas, A.; Campuzano, O.; Sarquella-Brugada, G.; Quezada-Feijoo, M.; Ramos, M.; Alcalá, M.; Carrera, E.; García-Padilla, C.; et al. MiR-16-5p Suppression Protects Human Cardiomyocytes against Endoplasmic Reticulum and Oxidative Stress-Induced Injury. Int. J. Mol. Sci. 2022, 23, 1036. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Pai, P.Y.; Kuo, C.H.; Ho, T.J.; Lin, J.Y.; Lin, D.Y.; Tsai, F.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. P53-Mediated MiR-18 Repression Activates HSF2 for IGF-IIR-Dependent Myocyte Hypertrophy in Hypertension-Induced Heart Failure. Cell Death Dis. 2017, 8, e2990. [Google Scholar] [CrossRef]
- Huang, G.-J.; Xie, X.-L.; Zou, Y. MiR-23b Targets GATA6 to down-Regulate IGF-1 and Promote the Development of Congenital Heart Disease. Acta Cardiol. 2022, 77, 375–384. [Google Scholar] [CrossRef]
- Icli, B.; Dorbala, P.; Feinberg, M.W. An Emerging Role for the MiR-26 Family in Cardiovascular Disease. Trends Cardiovasc. Med. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, X.; Fang, Y.; Nie, Y.; Cai, S.; Chen, Y.; Mo, D. Mir-127-3p Inhibits the Proliferation of Myocytes by Targeting KMT5a. Biochem. Biophys. Res. Commun. 2018, 503, 970–976. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Jiang, J.; Zhang, L.; Zhou, P.; Ren, H. MicroRNA-127-3p Regulates Myoblast Proliferation by Targeting Sept7. Biotechnol. Lett. 2020, 42, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, D.; Shi, H.; Zhang, Y.; Li, H.; Han, Q. MiR-195-5p Promotes Cardiomyocyte Hypertrophy by Targeting MFN2 and FBXW7. BioMed Res. Int. 2019, 2019, 1580982. [Google Scholar] [CrossRef]
- Cheng, X.; Du, J.; Shen, L.; Tan, Z.; Jiang, D.; Jiang, A.; Li, Q.; Tang, G.; Jiang, Y.; Wang, J.; et al. MiR-204-5p Regulates C2C12 Myoblast Differentiation by Targeting MEF2C and ERRγ. Biomed. Pharmacother. 2018, 101, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Liu, S.; Li, Y.; Dong, J.; Luo, J.; Liu, T.; Jin, Y.; Sun, Z. Short-Term Vagus Nerve Stimulation Reduces Myocardial Apoptosis by Downregulating MicroRNA-205 in Rats with Chronic Heart Failure. Mol. Med. Rep. 2017, 16, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Salant, G.M.; Tat, K.L.; Goodrich, J.A.; Kugel, J.F. MiR-206 Knockout Shows It Is Critical for Myogenesis and Directly Regulates Newly Identified Target MRNAs. RNA Biol. 2020, 17, 956–965. [Google Scholar] [CrossRef]
- Anderson, C.; Catoe, H.; Werner, R. MIR-206 Regulates Connexin43 Expression during Skeletal Muscle Development. Nucleic Acids Res. 2006, 34, 5863–5871. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Sun, X. The Functions of MicroRNA-208 in the Heart. Diabetes Res. Clin. Pract. 2020, 160, 108004. [Google Scholar] [CrossRef]
- Xu, F.; Yang, J.; Shang, J.; Lan, F.; Li, M.; Shi, L.; Shen, L.; Wang, Y.; Ge, J. MicroRNA-302d Promotes the Proliferation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibiting LATS2 in the Hippo Pathway. Clin. Sci. 2019, 133, 1387–1399. [Google Scholar] [CrossRef]
- Fang, Y.C.; Yeh, C.H. Inhibition of MIR-302 Suppresses Hypoxia-Reoxygenation-Induced H9c2 Cardiomyocyte Death by Regulating Mcl-1 Expression. Oxid. Med. Cell. Longev. 2017, 2017, 7968905. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, L.; Han, N.; Yin, G. MiR-375-3p Contributes to Hypoxia-Induced Apoptosis by Targeting Forkhead Box P1 (FOXP1) and Bcl2 like Protein 2 (Bcl2l2) in Rat Cardiomyocyte H9c2 Cells. Biotechnol. Lett. 2021, 43, 353–367. [Google Scholar] [CrossRef]
- Feng, H.; Wu, J.; Chen, P.; Wang, J.; Deng, Y.; Zhu, G.; Xian, J.; Huang, L.; Ouyang, W. MicroRNA-375-3p Inhibitor Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy by Promoting Lactate Dehydrogenase B Expression. J. Cell. Physiol. 2019, 234, 14198–14209. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.; Patel, A.; Sundaresan, N.R.; Gupta, M.P.; Solaro, R.J.; Nagalingam, R.S.; Gupta, M. A Novel Cardiomyocyte-Enriched MicroRNA, MiR-378, Targets Insulin-like Growth Factor 1 Receptor: Implications in Postnatal Cardiac Remodeling and Cell Survival. J. Biol. Chem. 2012, 287, 12913–12926. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 Suppresses Myocardial Fibrosis through a Paracrine Mechanism at the Early Stage of Cardiac Hypertrophy Following Mechanical Stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef]
- Fang, J.; Song, X.W.; Tian, J.; Chen, H.Y.; Li, D.F.; Wang, J.F.; Ren, A.J.; Yuan, W.J.; Lin, L. Overexpression of MicroRNA-378 Attenuates Ischemia-Induced Apoptosis by Inhibiting Caspase-3 Expression in Cardiac Myocytes. Apoptosis 2012, 17, 410–423. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Hu, Y.; Dorn, G.W. Regulation of Cardiac MicroRNAs by Cardiac MicroRNAs. Circ. Res. 2013, 113, 62–71. [Google Scholar] [CrossRef]
- Ling, T.Y.; Wang, X.L.; Chai, Q.; Lau, T.W.; Koestler, C.M.; Park, S.J.; Daly, R.C.; Greason, K.L.; Jen, J.; Wu, L.Q.; et al. Regulation of the SK3 Channel by MicroRNA-499—Potential Role in Atrial Fibrillation. Heart Rhythm 2013, 10, 1001–1009. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Zhang, C.; Sun, M.; Wang, W.; Chen, P.; Ma, K.; Zhang, Y.; Li, X.; Zhou, C. MiR-499 Protects Cardiomyocytes from H2O2-Induced Apoptosis via Its Effects on Pdcd4 and Pacs2. RNA Biol. 2014, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Han, Y.; Niu, L.; Li, J.; Chen, Y. MiR-499 Inhibited Hypoxia/Reoxygenation Induced Cardiomyocytes Injury by Targeting SOX6. Biotechnol. Lett. 2019, 41, 837–847. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Hu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Dorn, G.W. Direct and Indirect Involvement of MicroRNA-499 in Clinical and Experimental Cardiomyopathy. Circ. Res. 2012, 111, 521–531. [Google Scholar] [CrossRef]
- Yang, H.; Su, J.; Meng, W.; Chen, X.; Xu, Y.; Sun, B. Mir-518a-5p Targets Gzmb to Extenuate Vascular Endothelial Cell Injury Induced by Hypoxia-Reoxygenation and Thereby Improves Myocardial Ischemia. Int. Heart J. 2021, 62, 658–665. [Google Scholar] [CrossRef]
- Kang, T.; Xing, W.; Xi, Y.; Chen, K.; Zhan, M.; Tang, X.; Wang, Y.; Zhang, R.; Lei, M. MiR-543 Regulates Myoblast Proliferation and Differentiation of C2C12 Cells by Targeting KLF6. J. Cell. Biochem. 2020, 121, 4827–4837. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Jiang, M.; Li, J.; Tang, Y.; Zhou, L. MiR-543 in Human Mesenchymal Stem Cell–Derived Exosomes Promotes Cardiac Microvascular Endothelial Cell Angiogenesis after Myocardial Infarction through COL4A1. IUBMB Life 2021, 73, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Nymark, P.; Wijshoff, P.; Cavill, R.; Van Herwijnen, M.; Coonen, M.L.J.; Claessen, S.; Catalán, J.; Norppa, H.; Kleinjans, J.C.S.; Briedé, J.J. Extensive Temporal Transcriptome and MicroRNA Analyses Identify Molecular Mechanisms Underlying Mitochondrial Dysfunction Induced by Multi-Walled Carbon Nanotubes in Human Lung Cells. Nanotoxicology 2015, 9, 624–635. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasławska, M.; Grodzka, A.; Peczyńska, J.; Sawicka, B.; Bossowski, A.T. Role of miRNA in Cardiovascular Diseases in Children—Systematic Review. Int. J. Mol. Sci. 2024, 25, 956. https://doi.org/10.3390/ijms25020956
Pasławska M, Grodzka A, Peczyńska J, Sawicka B, Bossowski AT. Role of miRNA in Cardiovascular Diseases in Children—Systematic Review. International Journal of Molecular Sciences. 2024; 25(2):956. https://doi.org/10.3390/ijms25020956
Chicago/Turabian StylePasławska, Marta, Aleksandra Grodzka, Joanna Peczyńska, Beata Sawicka, and Artur Tadeusz Bossowski. 2024. "Role of miRNA in Cardiovascular Diseases in Children—Systematic Review" International Journal of Molecular Sciences 25, no. 2: 956. https://doi.org/10.3390/ijms25020956
APA StylePasławska, M., Grodzka, A., Peczyńska, J., Sawicka, B., & Bossowski, A. T. (2024). Role of miRNA in Cardiovascular Diseases in Children—Systematic Review. International Journal of Molecular Sciences, 25(2), 956. https://doi.org/10.3390/ijms25020956






