Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm
Abstract
1. Introduction
2. Pathological Features of Human TAAs
3. Rodent TAA Models
3.1. β-Aminopropionitrile (BAPN)-Induced TAA in Rodents
3.2. Angiotensin II Infusion-Induced TAA in Rodents
3.3. Elastase-Induced TAA in Rodents
3.4. Calcium Chloride-Induced TAA in Rodents
3.5. Combination of BAPN and Angiotensin II-Induced TAA in Rodents
3.6. Combination of High-Fat Diet and Angiotensin II-Induced TAA in Rodents
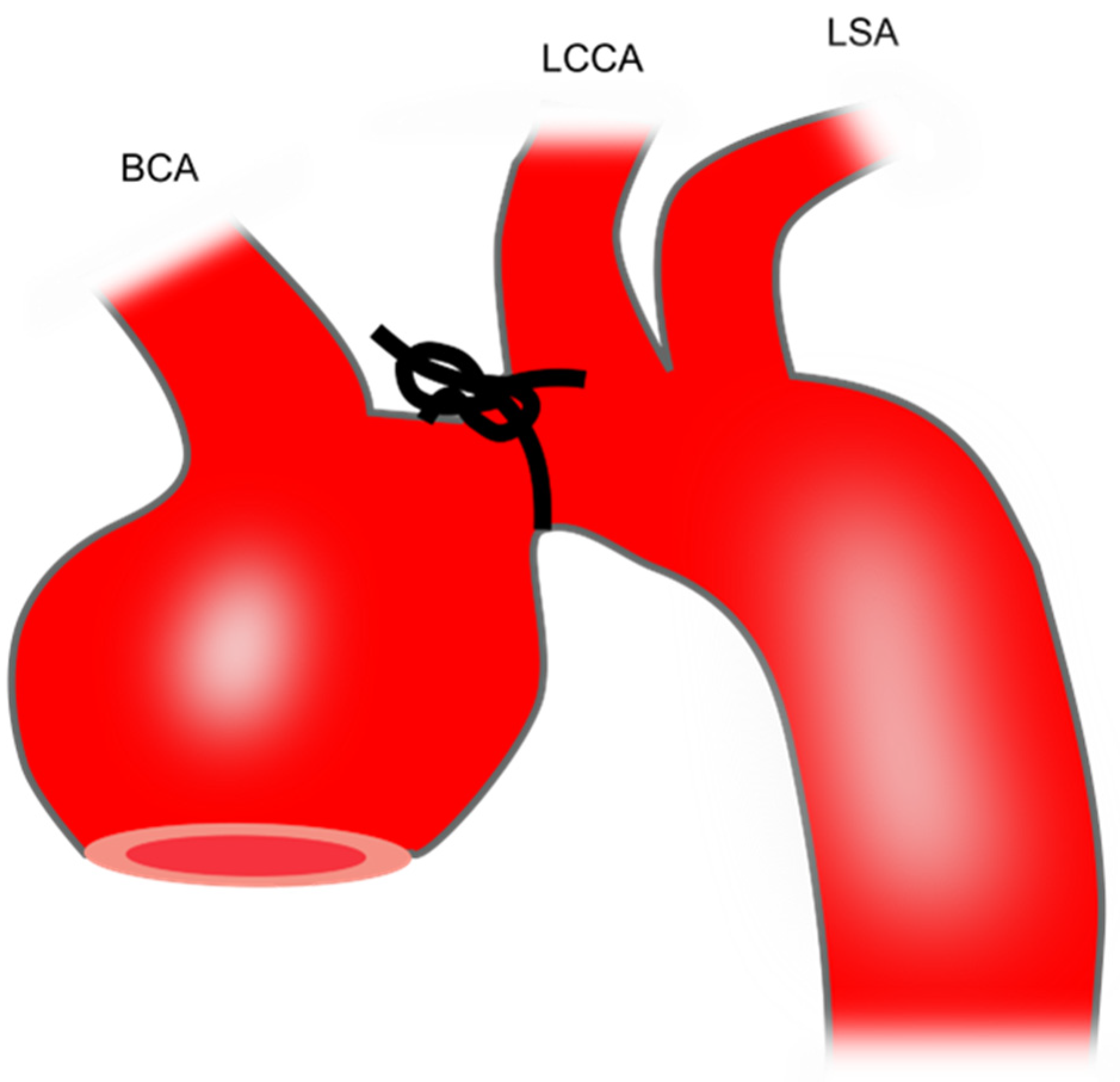
3.7. Transverse Aortic Constriction-Induced TAA in Rodents
3.8. Genetic TAA Models in Rodents
4. Porcine TAA Models
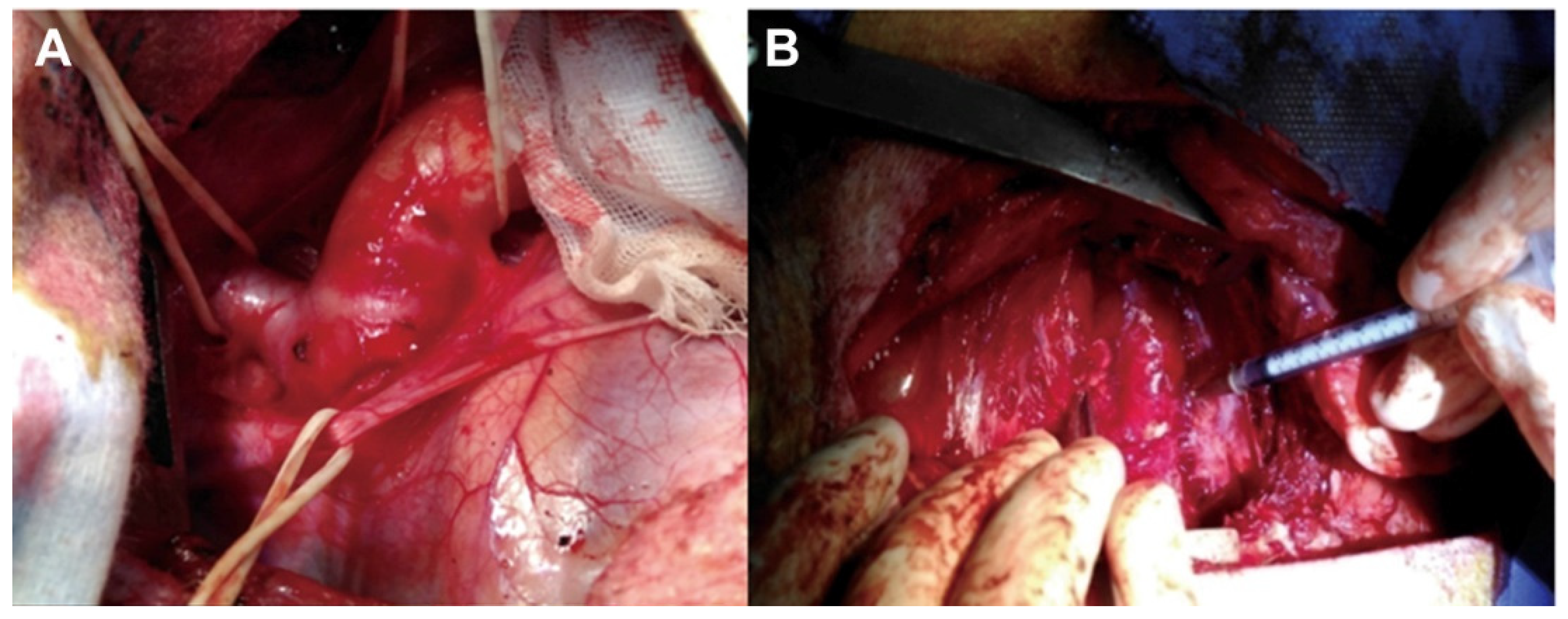
4.1. Intra-Adventitial Injection of Elastase
4.2. Intra-Adventitial Injections of Collagenase in Combination with Periadventitial Application of Calcium Chloride
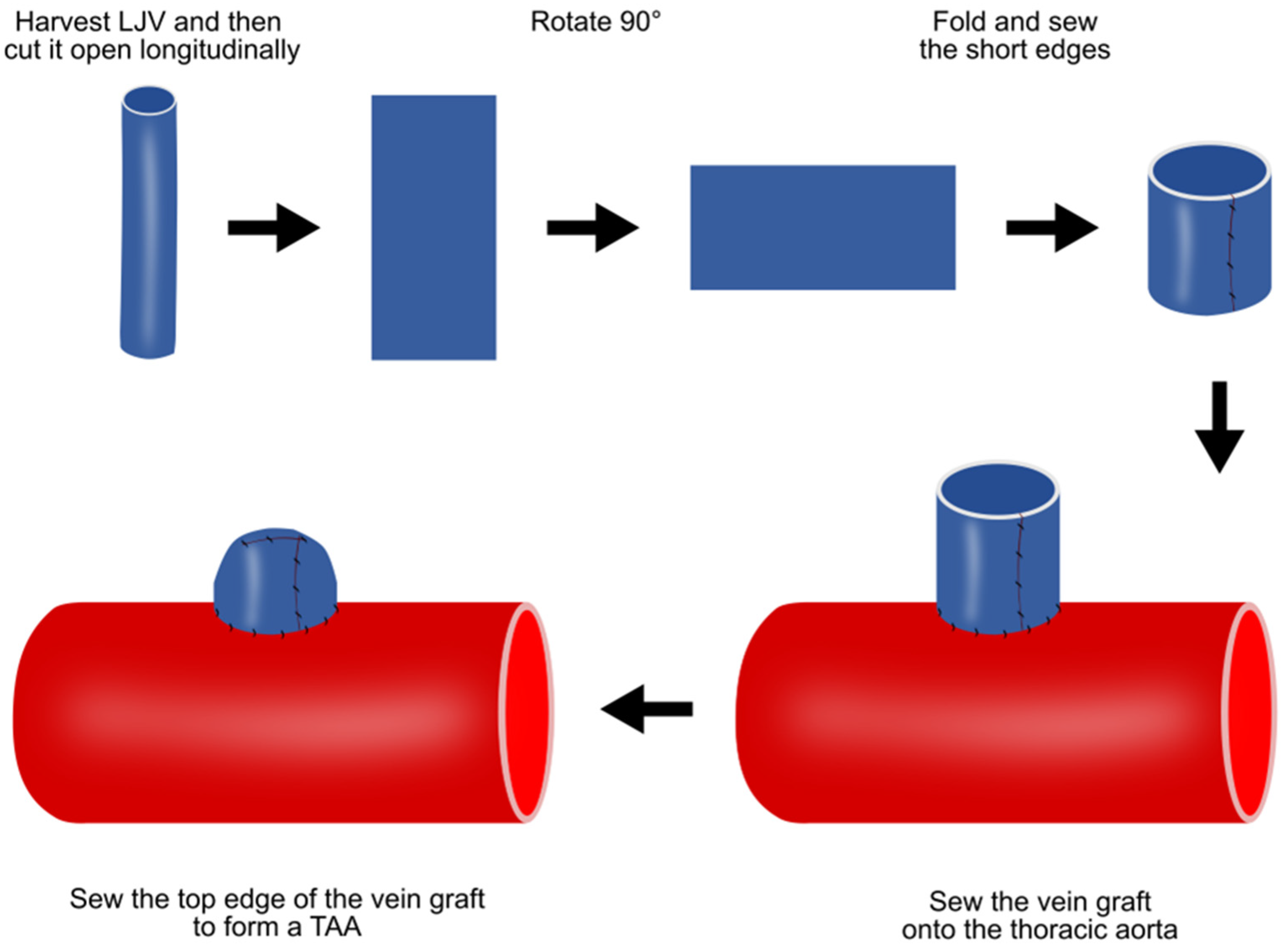
4.3. Vein Patch Method
4.4. Pericardium Pouch Method
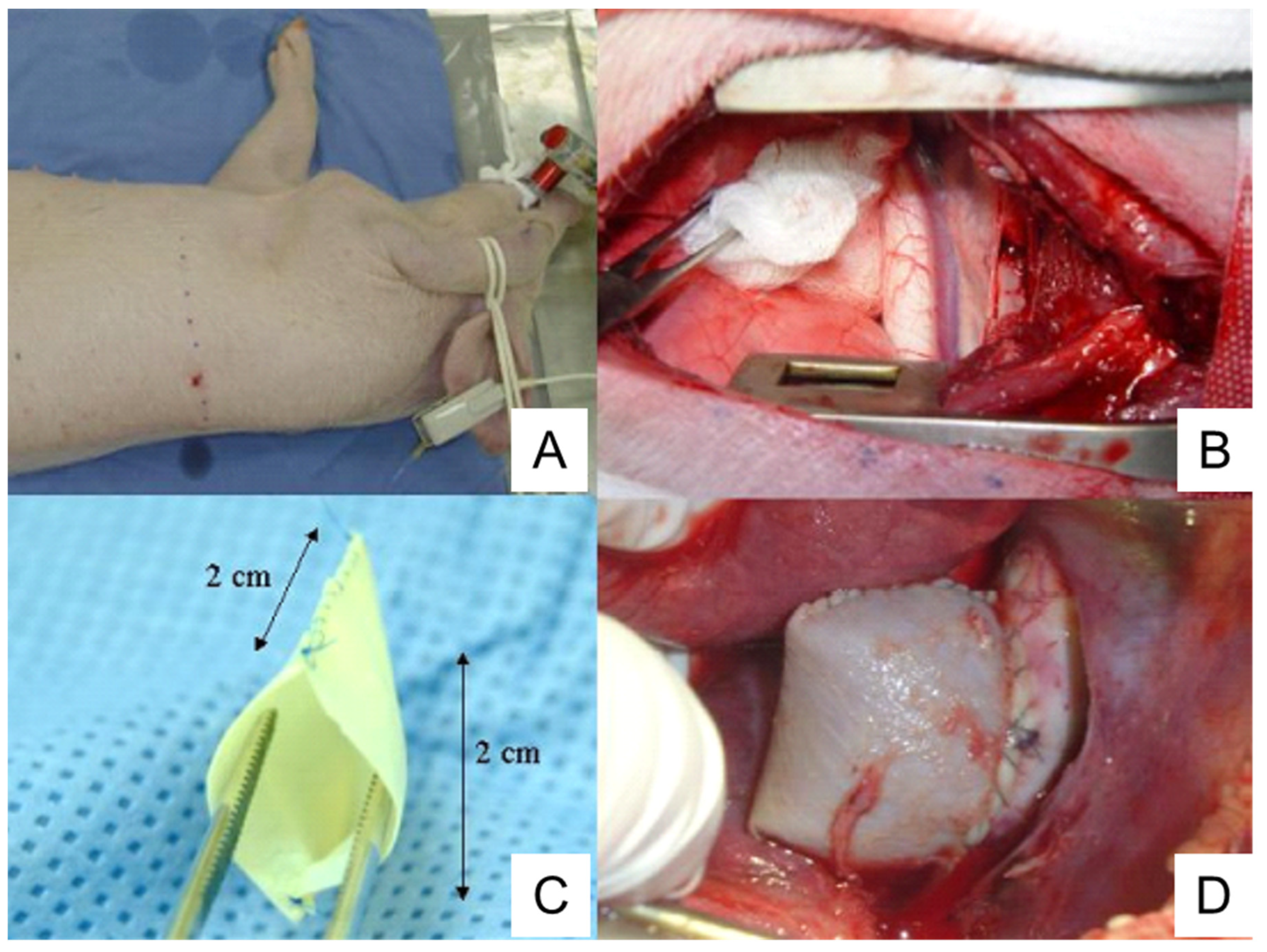
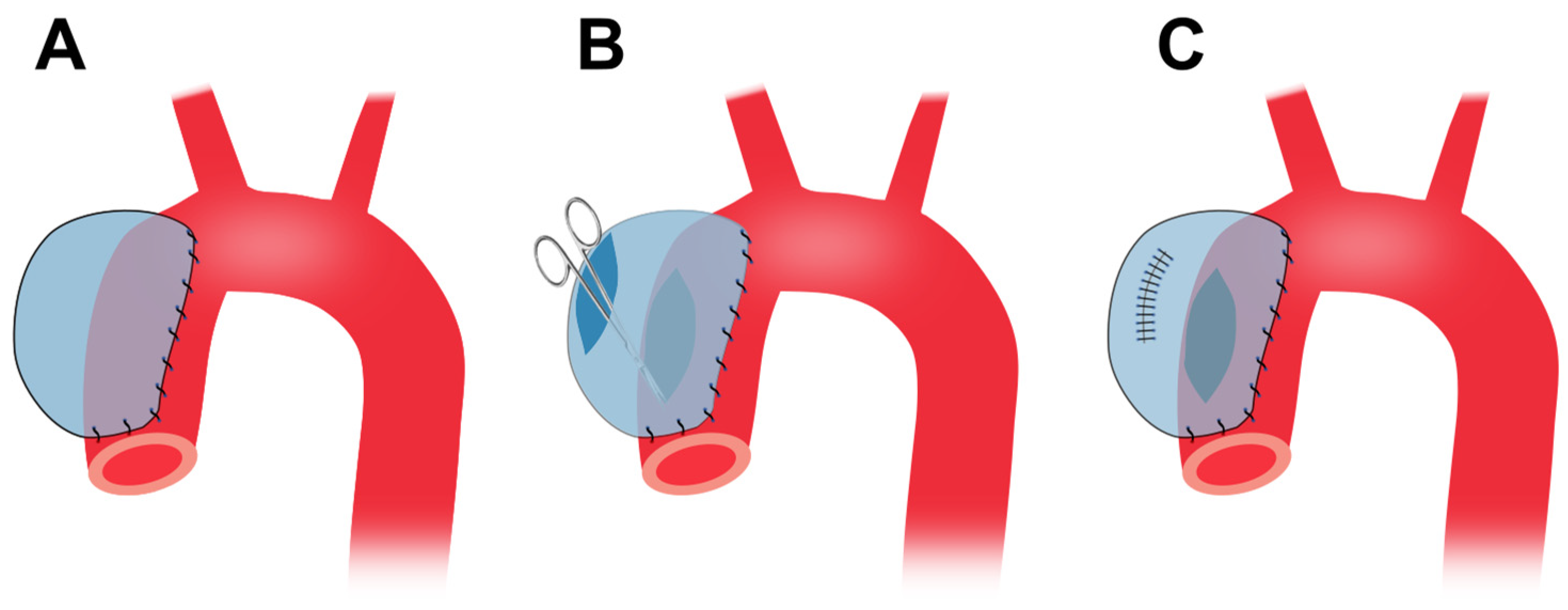
4.5. Cover-Then-Cut Method
4.6. Media and Intima Resection
5. Zebrafish TAA Models
6. Summary of the Animal Models of TAA
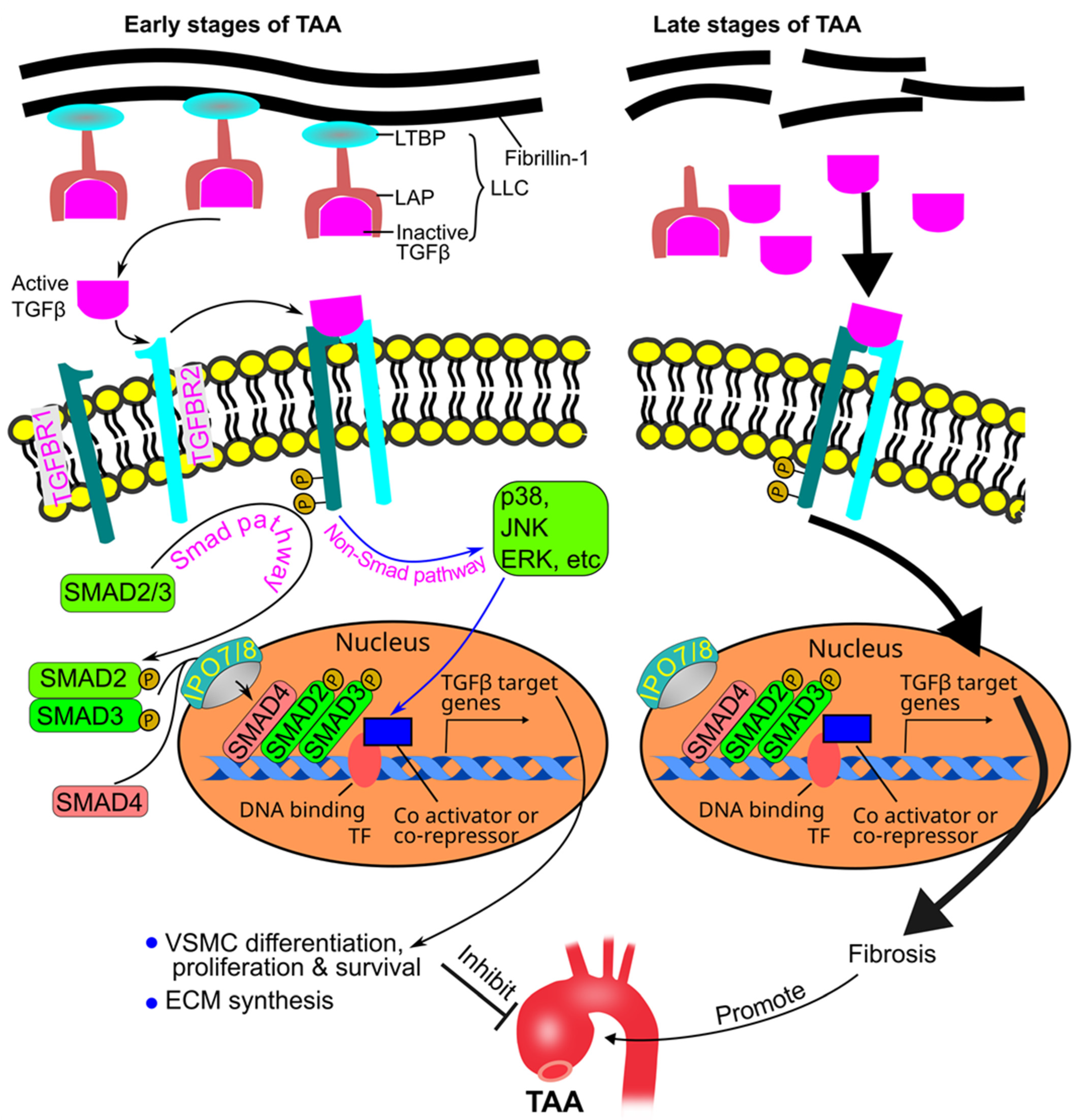
7. Role of TGFβ in TAA Pathogenesis
8. Role of the Tunica Intima in TAA Pathogenesis
9. Role of the Tunica Media in TAA Pathogenesis
9.1. VSMC-Elastin-Contractile Unit
9.2. Role of VSMC-Elastin-Contractile Unit Components in TAA Pathogenesis
9.3. Role of VSMC Apoptosis in TAA Pathogenesis
9.4. Role of Inflammation and Reactive Oxygen Species in TAA Pathogenesis
9.5. Role of Glycosaminoglycans and Proteoglycans in TAA Pathogenesis
10. Role of the Tunica Adventitia in TAA Pathogenesis
11. Potential TAA Treatments Derived from Recent Preclinical Studies
11.1. Inhibition of Inflammation
11.2. Inhibition of Apoptosis
11.3. Inhibition of Elastin Degradation
11.4. Other Recent Interventions
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.J.; Wu, W.W.; Schermerhorn, M.L. Open and Endovascular Management of Aortic Aneurysms. Circ. Res. 2019, 124, 647–661. [Google Scholar] [CrossRef]
- Quintana, R.A.; Taylor, W.R. Introduction to the Compendium on Aortic Aneurysms. Circ. Res. 2019, 124, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Renard, M.; Francis, C.; Ghosh, R.; Scott, A.F.; Witmer, P.D.; Adès, L.C.; Andelfinger, G.U.; Arnaud, P.; Boileau, C.; Callewaert, B.L.; et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018, 72, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Faiza, Z.; Sharma, T. Thoracic Aorta Aneurysm. [Updated 1 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554567/ (accessed on 11 October 2023).
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.; Reed, C.; Stemmermann, G.; Hayashi, T. Are aortic aneurysms caused by atherosclerosis? Circulation 1992, 85, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pauka, D.; Poór, V.S.; Maróti, P.; Told, R.; Tóth, D.; Tornóczky, T.; Molnár, T.F.; Simon, G. Biomechanical study on the effect of atherosclerosis on the vulnerability of thoracic aorta, and it’s role in the development of traumatic aorta injury. PLoS ONE 2023, 18, e0287652. [Google Scholar] [CrossRef]
- Dolmaci, O.B.; El Mathari, S.; Driessen, A.H.G.; Klautz, R.J.M.; Poelmann, R.E.; Lindeman, J.H.N.; Grewal, N. Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? J. Clin. Med. 2022, 12, 272. [Google Scholar] [CrossRef]
- Diehm, N.; Becker, G.; Katzen, B.; Benenati, J.; Kovacs, M.; Dick, F. Statins are associated with decreased mortality in abdominal, but not in thoracic aortic aneurysm patients undergoing endovascular repair: Propensity score-adjusted analysis. Vasa 2008, 37, 241–249. [Google Scholar] [CrossRef]
- Walsh, S.R.; Tang, T.Y.; Sadat, U.; Naik, J.; Gaunt, M.E.; Boyle, J.R.; Hayes, P.D.; Varty, K. Endovascular stenting versus open surgery for thoracic aortic disease: Systematic review and meta-analysis of perioperative results. J. Vasc. Surg. 2008, 47, 1094–1098. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Appoo, J.J.; Makaroun, M.S.; Verter, J.; Yu, Z.F.; Mitchell, R.S. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: A multicenter comparative trial. J. Thorac. Cardiovasc. Surg. 2007, 133, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Goodney, P.P.; Travis, L.; Lucas, F.L.; Fillinger, M.F.; Goodman, D.C.; Cronenwett, J.L.; Stone, D.H. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation 2011, 124, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Martin, J.; Shennib, H.; Dunning, J.; Muneretto, C.; Schueler, S.; Von Segesser, L.; Sergeant, P.; Turina, M. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J. Am. Coll. Cardiol. 2010, 55, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Makaroun, M.S.; Dillavou, E.D.; Wheatley, G.H.; Cambria, R.P. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J. Vasc. Surg. 2008, 47, 912–918. [Google Scholar] [CrossRef]
- Takano, T.; Katada, Y.; Komaki, N.; Onozawa, S.; Yokoyama, H. A technique for creating an experimental type Ia endoleak model in the thoracic aorta of swine. Jpn. J. Radiol. 2021, 39, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, W.; Sun, J.; Zhai, H.; Yu, Y.; Qi, X.; Jones, J.A.; Zhong, H. A reproducible swine model of proximal descending thoracic aortic aneurysm created with intra-adventitial application of elastase. J. Vasc. Surg. 2018, 67, 300–308.e302. [Google Scholar] [CrossRef]
- Muetterties, C.E.; Menon, R.; Wheatley, G.H., 3rd. A systematic review of primary endovascular repair of the ascending aorta. J. Vasc. Surg. 2018, 67, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Mokashi, S.A.; Svensson, L.G. Guidelines for the management of thoracic aortic disease in 2017. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 59–65. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Ramirez, F. Therapies for Thoracic Aortic Aneurysms and Acute Aortic Dissections. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 126–136. [Google Scholar] [CrossRef]
- Gillis, E.; Van Laer, L.; Loeys, B.L. Genetics of thoracic aortic aneurysm: At the crossroad of transforming growth factor-β signaling and vascular smooth muscle cell contractility. Circ. Res. 2013, 113, 327–340. [Google Scholar] [CrossRef]
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Jiao, X.L.; Yu, H.H.; Li, L.Y.; Li, J.; Zhang, X.P.; Qin, Y.W. Angiopoietin-like protein 8 deficiency attenuates thoracic aortic aneurysm/dissection development in β-aminopropionitrile monofumarate-induced model mice. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166619. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Abudupataer, M.; Liu, G.; Zhou, X.; Liu, D.; Zhu, S.; Ming, Y.; Yin, X.; Yan, S.; Sun, Y.; et al. Ciprofloxacin exacerbates dysfunction of smooth muscle cells in a microphysiological model of thoracic aortic aneurysm. JCI Insight 2023, 8, e161729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Qiu, T.; Yang, Y.; Li, Q.; Zhang, X. Dexamethasone reduces the formation of thoracic aortic aneurysm and dissection in a murine model. Exp. Cell Res. 2021, 405, 112703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, W.; Zhao, G.; Yu, B.; Ma, B.; Liu, Z.; Xie, N.; Fu, Y.; Gong, Z.; Dai, R.; et al. Rapamycin prevents thoracic aortic aneurysm and dissection in mice. J. Vasc. Surg. 2019, 69, 921–932.e923. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Liu, Y.; Zhang, K.; Zhang, Y.; Xu, K.; Zhou, M.; Zhao, H.; Zhu, S.; Chen, J.; Li, P.; et al. Artificial Intelligence Uncovers Natural MMP Inhibitor Crocin as a Potential Treatment of Thoracic Aortic Aneurysm and Dissection. Front. Cardiovasc. Med. 2022, 9, 871486. [Google Scholar] [CrossRef]
- Wang, F.; Tu, Y.; Gao, Y.; Chen, H.; Liu, J.; Zheng, J. Smooth Muscle Sirtuin 1 Blocks Thoracic Aortic Aneurysm/Dissection Development in Mice. Cardiovasc. Drugs Ther. 2020, 34, 641–650. [Google Scholar] [CrossRef]
- Xia, L.; Sun, C.; Zhu, H.; Zhai, M.; Zhang, L.; Jiang, L.; Hou, P.; Li, J.; Li, K.; Liu, Z.; et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020, 69, e12661. [Google Scholar] [CrossRef]
- Le, S.; Zhang, H.; Huang, X.; Chen, S.; Wu, J.; Chen, S.; Ding, X.; Chen, S.; Zhao, J.; Xu, H.; et al. PKM2 Activator TEPP-46 Attenuates Thoracic Aortic Aneurysm and Dissection by Inhibiting NLRP3 Inflammasome-Mediated IL-1β Secretion. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, J.; Le, S.; Wang, H.; Luo, J.; Li, R.; Chen, X.; Song, Y.; Wu, L.; Ye, P.; et al. Oltipraz, the activator of nuclear factor erythroid 2-related factor 2 (Nrf2), protects against the formation of BAPN-induced aneurysms and dissection of the thoracic aorta in mice by inhibiting activation of the ROS-mediated NLRP3 inflammasome. Eur. J. Pharmacol. 2022, 936, 175361. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Zhu, G.; Yang, H.; Li, W.; Luo, M.; Shu, C.; Zhou, Z. Single-cell RNA sequencing identifies an Il1rn(+)/Trem1(+) macrophage subpopulation as a cellular target for mitigating the progression of thoracic aortic aneurysm and dissection. Cell Discov. 2022, 8, 11. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Xie, N.; Huang, C.; Li, Z.; Yu, F.; Fu, Y.; Cui, Q.; Kong, W. Pan-HDAC (Histone Deacetylase) Inhibitors Increase Susceptibility of Thoracic Aortic Aneurysm and Dissection in Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2848–2850. [Google Scholar] [CrossRef]
- Yang, H.; Yang, F.; Luo, M.; Chen, Q.; Liu, X.; Zhang, Y.; Zhu, G.; Chen, W.; Li, T.; Shu, C.; et al. Metabolomic Profile Reveals That Ceramide Metabolic Disturbance Plays an Important Role in Thoracic Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 826861. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Wang, D.; Yang, S.; Ma, Z.; Fu, Y.; Ma, Q.; Kong, W. ADAMTS-7 deficiency attenuates thoracic aortic aneurysm and dissection in mice. J. Mol. Med. 2023, 101, 237–248. [Google Scholar] [CrossRef]
- Yang, X.; Xu, C.; Yao, F.; Ding, Q.; Liu, H.; Luo, C.; Wang, D.; Huang, J.; Li, Z.; Shen, Y.; et al. Targeting endothelial tight junctions to predict and protect thoracic aortic aneurysm and dissection. Eur. Heart J. 2023, 44, 1248–1261. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Liu, S.; Yan, S.; Xu, K.; Zhang, Y.S.; Abudupataer, M.; Ming, Y.; Zhu, S.; Xiang, B.; et al. Patient-derived microphysiological model identifies the therapeutic potential of metformin for thoracic aortic aneurysm. EBioMedicine 2022, 81, 104080. [Google Scholar] [CrossRef]
- Da, X.; Li, Z.; Huang, X.; He, Z.; Yu, Y.; Tian, T.; Xu, C.; Yao, Y.; Wang, Q.K. AGGF1 therapy inhibits thoracic aortic aneurysms by enhancing integrin α7-mediated inhibition of TGF-β1 maturation and ERK1/2 signaling. Nat. Commun. 2023, 14, 2265. [Google Scholar] [CrossRef]
- Aicher, B.O.; Zhang, J.; Muratoglu, S.C.; Galisteo, R.; Arai, A.L.; Gray, V.L.; Lal, B.K.; Strickland, D.K.; Ucuzian, A.A. Moderate aerobic exercise prevents matrix degradation and death in a mouse model of aortic dissection and aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1786–H1801. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, M.; Zhao, L.; Qi, F.; Zou, H.; He, P.; Zhou, H.; Shi, K.; Du, J. 68Ga-labeled WVP peptide as a novel PET probe for molecular biological diagnosis of unstable thoracic aortic aneurysm and early dissection: An animal study. Front. Cardiovasc. Med. 2023, 10, 1048927. [Google Scholar] [CrossRef]
- Luo, B.Y.; Zhou, J.; Guo, D.; Yang, Q.; Tian, Q.; Cai, D.P.; Zhou, R.M.; Xu, Z.Z.; Wang, H.J.; Chen, S.Y.; et al. Methamphetamine induces thoracic aortic aneurysm/dissection through C/EBPβ. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166447. [Google Scholar] [CrossRef]
- Wang, Y.; Emeto, T.I.; Lee, J.; Marshman, L.; Moran, C.; Seto, S.W.; Golledge, J. Mouse models of intracranial aneurysm. Brain Pathol. 2015, 25, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Krishna, S.M.; Wang, Y. Mouse models for abdominal aortic aneurysm. Br. J. Pharmacol. 2022, 179, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, Y.; Wang, X.; Jia, L.; Piao, C.; Lan, F.; Du, J. β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci. Rep. 2016, 6, 28149. [Google Scholar] [CrossRef]
- Wu, H.; Xie, C.; Wang, R.; Cheng, J.; Xu, Q.; Zhao, H. Comparative analysis of thoracic and abdominal aortic aneurysms across the segment and species at the single-cell level. Front. Pharmacol. 2022, 13, 1095757. [Google Scholar] [CrossRef] [PubMed]
- Jadli, A.S.; Ballasy, N.N.; Gomes, K.P.; Mackay, C.D.A.; Meechem, M.; Wijesuriya, T.M.; Belke, D.; Thompson, J.; Fedak, P.W.M.; Patel, V.B. Attenuation of Smooth Muscle Cell Phenotypic Switching by Angiotensin 1-7 Protects against Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 15566. [Google Scholar] [CrossRef]
- Bersi, M.R.; Bellini, C.; Humphrey, J.D.; Avril, S. Local variations in material and structural properties characterize murine thoracic aortic aneurysm mechanics. Biomech. Model. Mechanobiol. 2019, 18, 203–218. [Google Scholar] [CrossRef]
- Atchison, D.K.; O’Connor, C.L.; Converso-Baran, K.; Bergin, I.L.; Zhang, H.; Wang, Y.; Hartman, J.R.; Ju, W.; Smrcka, A.V.; Ganesh, S.K.; et al. Phospholipase Cε insufficiency causes ascending aortic aneurysm and dissection. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1376–H1387. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Jia, Y.; Kong, W.; Li, W. LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes 2021, 12, 513. [Google Scholar] [CrossRef]
- Oller, J.; Gabandé-Rodríguez, E.; Ruiz-Rodríguez, M.J.; Desdín-Micó, G.; Aranda, J.F.; Rodrigues-Diez, R.; Ballesteros-Martínez, C.; Blanco, E.M.; Roldan-Montero, R.; Acuña, P.; et al. Extracellular Tuning of Mitochondrial Respiration Leads to Aortic Aneurysm. Circulation 2021, 143, 2091–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Jin, L.; Liu, Y.; Hong, X. Changes in expressions of miR-22-3p and MMP-9 in rats with thoracic aortic aneurysm and their significance. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6949–6954. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, D.T.; Anesi, J.; Alramahi, A.; Witting, P.K.; Chai, Z.; Khan, A.W.; Kelly, J.; Denton, K.M.; Golledge, J. Moxonidine Increases Uptake of Oxidised Low-Density Lipoprotein in Cultured Vascular Smooth Muscle Cells and Inhibits Atherosclerosis in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2023, 24, 3857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dinh, T.N.; Nield, A.; Krishna, S.M.; Denton, K.; Golledge, J. Renal Denervation Promotes Atherosclerosis in Hypertensive Apolipoprotein E-Deficient Mice Infused with Angiotensin II. Front. Physiol. 2017, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sargisson, O.; Nguyen, D.T.; Parker, K.; Pyke, S.J.R.; Alramahi, A.; Thihlum, L.; Fang, Y.; Wallace, M.E.; Berzins, S.P.; et al. Effect of Hydralazine on Angiotensin II-Induced Abdominal Aortic Aneurysm in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2023, 24, 15955. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Biros, E.; Moran, C.S.; Wang, Y.; Clancy, P.; Golledge, J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 389–398. [Google Scholar] [CrossRef]
- Deng, J.; Li, D.; Zhang, X.; Lu, W.; Rong, D.; Wang, X.; Sun, G.; Jia, S.; Zhang, H.; Jia, X.; et al. Murine model of elastase-induced proximal thoracic aortic aneurysm through a midline incision in the anterior neck. Front. Cardiovasc. Med. 2023, 10, 953514. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Salmon, M.; Su, G.; Lu, G.; Leroy, V.; Bontha, S.V.; Mas, V.R.; Upchurch, G.R., Jr.; Ailawadi, G.; Sharma, A.K. Mesenchymal Stem Cells Alter MicroRNA Expression and Attenuate Thoracic Aortic Aneurysm Formation. J. Surg. Res. 2021, 268, 221–231. [Google Scholar] [CrossRef]
- Tyerman, Z.; Dahl, J.; Shannon, A.; Johnston, W.F.; Pope, N.H.; Lu, G.; Upchurch, G.R., Jr.; Ailawadi, G.; Salmon, M. Murine Surgical Model of Topical Elastase Induced Descending Thoracic Aortic Aneurysm. J. Vis. Exp. 2019, 150, e60105. [Google Scholar] [CrossRef]
- Li, K.; Cui, M.Z.; Zhang, K.W.; Wang, G.Q.; Zhai, S.T. Effect of miR-21 on rat thoracic aortic aneurysm model by regulating the expressions of MMP-2 and MMP-9. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 878–884. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Gibson, W.C.; Gardner, J.; Sweterlitsch, S.; Thompson, R.P.; Mukherjee, R.; Spinale, F.G. A murine model of thoracic aortic aneurysms. J. Surg. Res. 2003, 115, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.W.; Collins, E.N.; Peterson, A.R.; Collins, L.B.; Harrison, J.K.; DeVaughn, A.; Townsend, J.M.; Vanbuskirk, R.L.; Riopedre-Maqueira, J.; Reyes, A.; et al. miR-133a Replacement Attenuates Thoracic Aortic Aneurysm in Mice. J. Am. Heart. Assoc. 2021, 10, e019862. [Google Scholar] [CrossRef]
- Fan, J.; Li, X.; Yan, Y.W.; Tian, X.H.; Hou, W.J.; Tong, H.; Bai, S.L. Curcumin attenuates rat thoracic aortic aneurysm formation by inhibition of the c-Jun N-terminal kinase pathway and apoptosis. Nutrition 2012, 28, 1068–1074. [Google Scholar] [CrossRef]
- Zhou, M.; Zha, Z.; Zheng, Z.; Pan, Y. Cordycepin suppresses vascular inflammation, apoptosis and oxidative stress of arterial smooth muscle cell in thoracic aortic aneurysm with VEGF inhibition. Int. Immunopharmacol. 2023, 116, 109759. [Google Scholar] [CrossRef]
- Wang, Y.; Krishna, S.M.; Moxon, J.; Dinh, T.N.; Jose, R.J.; Yu, H.; Golledge, J. Influence of apolipoprotein E, age and aortic site on calcium phosphate induced abdominal aortic aneurysm in mice. Atherosclerosis 2014, 235, 204–212. [Google Scholar] [CrossRef]
- Wang, Y.; Krishna, S.; Golledge, J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013, 226, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Zalghout, S.; Vo, S.; Arocas, V.; Jadoui, S.; Hamade, E.; Badran, B.; Oudar, O.; Charnaux, N.; Longrois, D.; Boulaftali, Y.; et al. Syndecan-1 Is Overexpressed in Human Thoracic Aneurysm but Is Dispensable for the Disease Progression in a Mouse Model. Front. Cardiovasc. Med. 2022, 9, 839743. [Google Scholar] [CrossRef]
- Qi, X.; Wang, F.; Chun, C.; Saldarriaga, L.; Jiang, Z.; Pruitt, E.Y.; Arnaoutakis, G.J.; Upchurch, G.R., Jr.; Jiang, Z. A validated mouse model capable of recapitulating the protective effects of female sex hormones on ascending aortic aneurysms and dissections (AADs). Physiol. Rep. 2020, 8, e14631. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Qu, Y.; Jiang, L.; Liang, R.; Li, B.; Li, L.; Jiang, Y. Label-free quantitative proteomic analysis of serum exosomes in mice with thoracic aortic aneurysm. Proteome Sci. 2023, 21, 19. [Google Scholar] [CrossRef]
- Ageedi, W.; Zhang, C.; Frankel, W.C.; Dawson, A.; Li, Y.; Coselli, J.S.; Shen, H.Y.; LeMaire, S.A. AIM2 Inflammasome Activation Contributes to Aortic Dissection in a Sporadic Aortic Disease Mouse Model. J. Surg. Res. 2022, 272, 105–116. [Google Scholar] [CrossRef]
- Kuang, S.Q.; Geng, L.; Prakash, S.K.; Cao, J.M.; Guo, S.; Villamizar, C.; Kwartler, C.S.; Peters, A.M.; Brasier, A.R.; Milewicz, D.M. Aortic Remodeling After Transverse Aortic Constriction in Mice Is Attenuated With AT1 Receptor Blockade. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bedja, D.; Koitabashi, N.; Xing, D.; Chen, J.; Fox-Talbot, K.; Rouf, R.; Chen, S.; Steenbergen, C.; Harmon, J.W.; et al. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-β signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E841–E850. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.M.; Niaz, T.; Bowen, J.M. What Is Marfan Syndrome? JAMA 2023, 329, 1618. [Google Scholar] [CrossRef] [PubMed]
- Gharraee, N.; Sun, Y.; Swisher, J.A.; Lessner, S.M. Age and sex dependency of thoracic aortopathy in a mouse model of Marfan syndrome. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H44–H56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Sawada, H.; Ye, D.; Katsumata, Y.; Kukida, M.; Ohno-Urabe, S.; Moorleghen, J.J.; Franklin, M.K.; Howatt, D.A.; Sheppard, M.B.; et al. Deletion of AT1a (Angiotensin II Type 1a) Receptor or Inhibition of Angiotensinogen Synthesis Attenuates Thoracic Aortopathies in Fibrillin1(C1041G/+) Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; Lemties, J.; Braumann, S.; Geißen, S.; Bokredenghel, S.; Nies, R.; Hof, A.; Winkels, H.; Freeman, B.A.; Klinke, A.; et al. Nitro-oleic acid reduces thoracic aortic aneurysm progression in a mouse model of Marfan syndrome. Cardiovasc. Res. 2022, 118, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Boileau, A.; Lino Cardenas, C.L.; Courtois, A.; Zhang, L.; Rodosthenous, R.S.; Das, S.; Sakalihasan, N.; Michel, J.B.; Lindsay, M.E.; Devaux, Y. MiR-574-5p: A Circulating Marker of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2019, 20, 3924. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, Y.; Zhou, Z.; Qi, F.; Zheng, S.; Gao, S.; Li, Y.; Liu, Y.; Du, J. Fibroblast-Secreted Phosphoprotein 1 Mediates Extracellular Matrix Deposition and Inhibits Smooth Muscle Cell Contractility in Marfan Syndrome Aortic Aneurysm. J. Cardiovasc. Transl. Res. 2022, 15, 959–970. [Google Scholar] [CrossRef]
- de la Fuente-Alonso, A.; Toral, M.; Alfayate, A.; Ruiz-Rodríguez, M.J.; Bonzón-Kulichenko, E.; Teixido-Tura, G.; Martínez-Martínez, S.; Méndez-Olivares, M.J.; López-Maderuelo, D.; González-Valdés, I.; et al. Aortic disease in Marfan syndrome is caused by overactivation of sGC-PRKG signaling by NO. Nat. Commun. 2021, 12, 2628. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Siu, K.L.; Zhang, Y.; Cai, H. Targeting feed-forward signaling of TGFβ/NOX4/DHFR/eNOS uncoupling/TGFβ axis with anti-TGFβ and folic acid attenuates formation of aortic aneurysms: Novel mechanisms and therapeutics. Redox Biol. 2021, 38, 101757. [Google Scholar] [CrossRef]
- Tashima, Y.; He, H.; Cui, J.Z.; Pedroza, A.J.; Nakamura, K.; Yokoyama, N.; Iosef, C.; Burdon, G.; Koyano, T.; Yamaguchi, A.; et al. Androgens Accentuate TGF-β Dependent Erk/Smad Activation During Thoracic Aortic Aneurysm Formation in Marfan Syndrome Male Mice. J. Am. Heart. Assoc. 2020, 9, e015773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, X.; Ji, C.; Shi, J.; Pan, Y. LncRNA Sox2ot modulates the progression of thoracic aortic aneurysm by regulating miR-330-5p/Myh11. Biosci. Rep. 2020, 40, BSR20194040. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, M.; Caulk, A.W.; Cilfone, N.A.; Gujja, S.; Qin, L.; Chen, P.Y.; Chen, Z.; Yousef, S.; Jiao, Y.; et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J. Clin. Investig. 2020, 130, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, M.S.; Ham, S.; Park, E.S.; Kim, K.L.; Suh, W. Transforming Growth Factor β Receptor Type I Inhibitor, Galunisertib, Has No Beneficial Effects on Aneurysmal Pathological Changes in Marfan Mice. Biomol. Ther. 2020, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, L.; Zhu, J.; He, C.; Xu, Q.; Sun, A.; Kong, W.; Li, W.; Zhang, X. Rapamycin attenuates a murine model of thoracic aortic aneurysm by downregulating the miR-126-3p mediated activation of MAPK/ERK signalling pathway. Biochem. Biophys. Res. Commun. 2019, 512, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chang, H.H.; Guo, Y.R.; Chang, W.C.; Chen, Y.F. Vitamin B Mitigates Thoracic Aortic Dilation in Marfan Syndrome Mice by Restoring the Canonical TGF-β Pathway. Int. J. Mol. Sci. 2021, 22, 11737. [Google Scholar] [CrossRef]
- Judge, D.P.; Biery, N.J.; Keene, D.R.; Geubtner, J.; Myers, L.; Huso, D.L.; Sakai, L.Y.; Dietz, H.C. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Investig. 2004, 114, 172–181. [Google Scholar] [CrossRef]
- Chen, M.; Cavinato, C.; Hansen, J.; Tanaka, K.; Ren, P.; Hassab, A.; Li, D.S.; Youshao, E.; Tellides, G.; Iyengar, R.; et al. FN (Fibronectin)-Integrin α5 Signaling Promotes Thoracic Aortic Aneurysm in a Mouse Model of Marfan Syndrome. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e132–e150. [Google Scholar] [CrossRef]
- Zhang, R.M.; Tiedemann, K.; Muthu, M.L.; Dinesh, N.E.H.; Komarova, S.; Ramkhelawon, B.; Reinhardt, D.P. Fibrillin-1-regulated miR-122 has a critical role in thoracic aortic aneurysm formation. Cell. Mol. Life Sci. 2022, 79, 314. [Google Scholar] [CrossRef]
- Jespersen, K.; Li, C.; Batra, R.; Stephenson, C.A.; Harding, P.; Sestak, K.; Foley, R.T.; Greene, H.; Meisinger, T.; Cook, J.R.; et al. Impact of Notch3 Activation on Aortic Aneurysm Development in Marfan Syndrome. J. Immunol. Res. 2022, 2022, 7538649. [Google Scholar] [CrossRef]
- Caescu, C.I.; Hansen, J.; Crockett, B.; Xiao, W.; Arnaud, P.; Spronck, B.; Weinberg, A.; Hashimoto, T.; Murtada, S.I.; Borkar, R.; et al. Inhibition of HIPK2 Alleviates Thoracic Aortic Disease in Mice With Progressively Severe Marfan Syndrome. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Galatioto, J.; Caescu, C.I.; Arnaud, P.; Calizo, R.C.; Spronck, B.; Murtada, S.I.; Borkar, R.; Weinberg, A.; Azeloglu, E.U.; et al. Systems pharmacology-based integration of human and mouse data for drug repurposing to treat thoracic aneurysms. JCI Insight 2019, 4, e127652. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Asano, K.; Sedes, L.; Cantalupo, A.; Hansen, J.; Iyengar, R.; Walsh, M.J.; Ramirez, F. Dissecting aortic aneurysm in Marfan syndrome is associated with losartan-sensitive transcriptomic modulation of aortic cells. JCI Insight 2023, 8, e168793. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Sawada, H.; Moorleghen, J.J.; Weiland, M.; Daugherty, A.; Sheppard, M.B. Aortic Strain Correlates with Elastin Fragmentation in Fibrillin-1 Hypomorphic Mice. Circ. Rep. 2019, 1, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Sulser Ponce De Leon, S.; Tsukahara, Y.; Liu, H.; Huangfu, Y.; Wang, Y.; Seabra, P.M.; Yang, X.; Goodman, J.; Wan, X.; et al. Redox Dysregulation of Vascular Smooth Muscle Sirtuin-1 in Thoracic Aortic Aneurysm in Marfan Syndrome. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e339–e357. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Schwill, S.; Seppelt, P.; Grünhagen, J.; Ott, C.E.; Jugold, M.; Ruhparwar, A.; Robinson, P.N.; Karck, M.; Kallenbach, K. The fibrillin-1 hypomorphic mgR/mgR murine model of Marfan syndrome shows severe elastolysis in all segments of the aorta. J. Vasc. Surg. 2013, 57, 1628–1636.e3. [Google Scholar] [CrossRef]
- Bazzi, M.S.; Balouchzadeh, R.; Pavey, S.N.; Quirk, J.D.; Yanagisawa, H.; Vedula, V.; Wagenseil, J.E.; Barocas, V.H. Experimental and Mouse-Specific Computational Models of the Fbln4(SMKO) Mouse to Identify Potential Biomarkers for Ascending Thoracic Aortic Aneurysm. Cardiovasc. Eng. Technol. 2022, 13, 558–572. [Google Scholar] [CrossRef]
- Nguyen, T.A.V.; Lino, C.A.; Hang, H.T.; Alves, J.V.; Thang, B.Q.; Shin, S.J.; Sugiyama, K.; Matsunaga, H.; Takeyama, H.; Yamashiro, Y.; et al. Protective Role of Endothelial Fibulin-4 in Valvulo-Arterial Integrity. J. Am. Heart. Assoc. 2023, 12, e026942. [Google Scholar] [CrossRef]
- Sugiyama, K.; Marzi, J.; Alber, J.; Brauchle, E.M.; Ando, M.; Yamashiro, Y.; Ramkhelawon, B.; Schenke-Layland, K.; Yanagisawa, H. Raman microspectroscopy and Raman imaging reveal biomarkers specific for thoracic aortic aneurysms. Cell Rep. Med. 2021, 2, 100261. [Google Scholar] [CrossRef]
- Correction to: Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans. Circ. Res. 2020, 127, e142. [CrossRef]
- Shin, S.J.; Hang, H.T.; Thang, B.Q.; Shimoda, T.; Sakamoto, H.; Osaka, M.; Hiramatsu, Y.; Yamashiro, Y.; Yanagisawa, H. Role of PAR1-Egr1 in the Initiation of Thoracic Aortic Aneurysm in Fbln4-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Davis, E.C.; Chapman, S.L.; Budatha, M.; Marmorstein, L.Y.; Word, R.A.; Yanagisawa, H. Fibulin-4 deficiency results in ascending aortic aneurysms: A potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2010, 106, 583–592. [Google Scholar] [CrossRef]
- Hanada, K.; Vermeij, M.; Garinis, G.A.; De Waard, M.C.; Kunen, M.G.S.; Myers, L.; Maas, A.; Duncker, D.J.; Meijers, C.; Dietz, H.C.; et al. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ. Res. 2007, 100, 738–746. [Google Scholar] [CrossRef]
- Igoucheva, O.; Alexeev, V.; Halabi, C.M.; Adams, S.M.; Stoilov, I.; Sasaki, T.; Arita, M.; Donahue, A.; Mecham, R.P.; Birk, D.E.; et al. Fibulin-4 E57K Knock-in Mice Recapitulate Cutaneous, Vascular and Skeletal Defects of Recessive Cutis Laxa 1B with both Elastic Fiber and Collagen Fibril Abnormalities. J. Biol. Chem. 2015, 290, 21443–21459. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.L.; Wu, Y.; Kailash, K.A.; Bersi, M.R.; Halabi, C.M.; Wagenseil, J.E. Changes in transmural mass transport correlate with ascending thoracic aortic aneurysm diameter in a fibulin-4 E57K knockin mouse model. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H113–H124. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Li, W.; Zhang, T.; Li, X.; Jiao, Y.; Zhang, X.; Jiang, J.; Zhang, X.; Zhang, X. Dexamethasone attenuated thoracic aortic aneurysm and dissection in vascular smooth muscle cell Tgfbr2-disrupted mice with CCL8 suppression. Exp. Physiol. 2022, 107, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Jiao, Y.; Qin, L.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Investig. 2014, 124, 755–767. [Google Scholar] [CrossRef]
- Ghadie, N.M.; St-Pierre, J.P.; Labrosse, M.R. The Contribution of Glycosaminoglycans/Proteoglycans to Aortic Mechanics in Health and Disease: A Critical Review. IEEE Trans. Biomed. Eng. 2021, 68, 3491–3500. [Google Scholar] [CrossRef]
- Tolar, J.; Braunlin, E.; Riddle, M.; Peacock, B.; McElmurry, R.T.; Orchard, P.J.; Blazar, B.R. Gender-related dimorphism in aortic insufficiency in murine mucopolysaccharidosis type I. J. Heart Valve Dis. 2009, 18, 524–529. [Google Scholar]
- Shen, Y.H.; Lu, H.S.; LeMaire, S.A.; Daugherty, A. Unfolding the Story of Proteoglycan Accumulation in Thoracic Aortic Aneurysm and Dissection. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.; Abrahante, J.E.; McElmurry, R.; Evans, M.; Smith, M.; Seelig, D.; O’Sullivan, M.G.; Tolar, J.; Whitley, C.B.; McIvor, R.S. Contribution of the innate and adaptive immune systems to aortic dilation in murine mucopolysaccharidosis type I. Mol. Genet. Metab. 2022, 135, 193–205. [Google Scholar] [CrossRef]
- Deleeuw, V.; Carlson, E.; Renard, M.; Zientek, K.D.; Wilmarth, P.A.; Reddy, A.P.; Manalo, E.C.; Tufa, S.F.; Keene, D.R.; Olbinado, M.; et al. Unraveling the role of TGFβ signaling in thoracic aortic aneurysm and dissection using Fbn1 mutant mouse models. Matrix Biol. 2023, 123, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Perlman, R.L. Mouse models of human disease: An evolutionary perspective. Evol. Med. Public Health 2016, 2016, 170–176. [Google Scholar] [CrossRef]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienke, A.A.; Sides, G.D.; Winters, K.J.; Kovacs, R.J.; Rebhun, D.M.; Bloom, J.C.; Groh, W.J.; Eisenberg, P.R. Electrocardiogram Reference Ranges Derived from a Standardized Clinical Trial Population. Drug Inf. J. 2005, 39, 395–405. [Google Scholar] [CrossRef]
- Patel, N.; Durland, J.; Makaryus, A. Physiology, Cardiac Index. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539905/ (accessed on 20 November 2023).
- Hannon, J.P.; Bossone, C.A.; Wade, C.E. Normal Physiological Values for Conscious Pigs Used in Biomedical Research; LAIR, Military Trauma Research: San Francisco, CA, USA, 1989. [Google Scholar]
- World Health Organization. Hypertension Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 20 November 2023).
- Fukushima, S.; Ohki, T.; Koizumi, M.; Ohta, H.; TakahasHi, T.; Okano, H.J. A reproducible swine model of a surgically created saccular thoracic aortic aneurysm. Exp. Anim. 2021, 70, 257–263. [Google Scholar] [CrossRef]
- Debus, E.S.; Kölbel, T.; Duprée, A.; Daum, G.; Sandhu, H.K.; Manzoni, D.; Wipper, S.H. Feasibility Study of a Novel Thoraco-abdominal Aortic Hybrid Device (SPIDER-graft) in a Translational Pig Model. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 196–205. [Google Scholar] [CrossRef]
- Meylaerts, S.A.; De Haan, P.; Kalkman, C.J.; Jaspers, J.; Vanicky, I.; Jacobs, M.J. Prevention of paraplegia in pigs by selective segmental artery perfusion during aortic cross-clamping. J. Vasc. Surg. 2000, 32, 160–170. [Google Scholar] [CrossRef][Green Version]
- Mirra, A.; Gamez Maidanskaia, E.; Carmo, L.P.; Levionnois, O.; Spadavecchia, C. How is depth of anaesthesia assessed in experimental pigs? A scoping review. PLoS ONE 2023, 18, e0283511. [Google Scholar] [CrossRef]
- Eckhouse, S.R.; Logdon, C.B.; Oelsen, J.M.; Patel, R.K.; Rice, A.D.; Stroud, R.E.; Wince, W.B.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S.; et al. Reproducible Porcine Model of Thoracic Aortic Aneurysm. Circulation 2013, 128, S186–S193. [Google Scholar] [CrossRef][Green Version]
- Argenta, R.; Perini, S.C.; Pereira, A.H. Thoracic aortic aneurysm. An experimental model in pigs. Acta Cir. Bras. 2021, 36, e360602. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Feng, R.; Feng, J.; Li, Y.; Bao, X.; Qin, F.; Li, T.; Zhou, J.; Jing, Z. A new porcine model of ascending aortic aneurysm established using a cover-then-cut method. Surg. Today 2021, 51, 906–915. [Google Scholar] [CrossRef]
- Hoareau, M.; El Kholti, N.; Debret, R.; Lambert, E. Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. Int. J. Mol. Sci. 2022, 23, 2102. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Abrial, M.; Basu, S.; Huang, M.; Butty, V.; Schwertner, A.; Jeffrey, S.; Jordan, D.; Burns, C.E.; Burns, C.G. Latent TGFβ-binding proteins 1 and 3 protect the larval zebrafish outflow tract from aneurysmal dilatation. Dis. Model. Mech. 2022, 15, dmm046979. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.; Ziganshin, B.A.; Papanikolaou, D.; Zafar, M.A.; Nicoli, S.; Mukherjee, S.; Elefteriades, J.A. Phenotyping Zebrafish Mutant Models to Assess Candidate Genes Associated with Aortic Aneurysm. Genes 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Boezio, G.L.M.; Bensimon-Brito, A.; Piesker, J.; Guenther, S.; Helker, C.S.M.; Stainier, D.Y.R. Endothelial TGF-β signaling instructs smooth muscle cell development in the cardiac outflow tract. eLife 2020, 9, e57603. [Google Scholar] [CrossRef]
- Folkesson, M.; Sadowska, N.; Vikingsson, S.; Karlsson, M.; Carlhäll, C.J.; Länne, T.; Wågsäter, D.; Jensen, L. Differences in cardiovascular toxicities associated with cigarette smoking and snuff use revealed using novel zebrafish models. Biol. Open 2016, 5, 970–978. [Google Scholar] [CrossRef]
- Takeda, N.; Yagi, H.; Hara, H.; Fujiwara, T.; Fujita, D.; Nawata, K.; Inuzuka, R.; Taniguchi, Y.; Harada, M.; Toko, H.; et al. Pathophysiology and Management of Cardiovascular Manifestations in Marfan and Loeys–Dietz Syndromes. Int. Heart J. 2016, 57, 271–277. [Google Scholar] [CrossRef]
- ten Dijke, P.; Arthur, H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, X.; Cottonham, C.; Xu, L. Preferential utilization of Imp7/8 in nuclear import of Smads. J. Biol. Chem. 2008, 283, 22867–22874. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Asano, K.; Cantalupo, A.; Sedes, L.; Ramirez, F. Pathophysiology and Therapeutics of Thoracic Aortic Aneurysm in Marfan Syndrome. Biomolecules 2022, 12, 128. [Google Scholar] [CrossRef]
- Wang, Y.; Krishna, S.; Walker, P.J.; Norman, P.; Golledge, J. Transforming growth factor-beta and abdominal aortic aneurysms. Cardiovasc. Pathol. 2013, 22, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Renard, M.; Callewaert, B.; Baetens, M.; Campens, L.; MacDermot, K.; Fryns, J.P.; Bonduelle, M.; Dietz, H.C.; Gaspar, I.M.; Cavaco, D.; et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ signaling in FTAAD. Int. J. Cardiol. 2013, 165, 314–321. [Google Scholar] [CrossRef]
- Hu, J.H.; Wei, H.; Jaffe, M.; Airhart, N.; Du, L.; Angelov, S.N.; Yan, J.; Allen, J.K.; Kang, I.; Wight, T.N.; et al. Postnatal Deletion of the Type II Transforming Growth Factor-β Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2647–2656. [Google Scholar] [CrossRef]
- Wei, H.; Hu, J.H.; Angelov, S.N.; Fox, K.; Yan, J.; Enstrom, R.; Smith, A.; Dichek, D.A. Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor β Signaling. J. Am. Heart. Assoc. 2017, 6, e004968. [Google Scholar] [CrossRef]
- Bramel, E.E.; Creamer, T.J.; Saqib, M.; Camejo Nunez, W.A.; Bagirzadeh, R.; Roker, L.A.; Goff, L.A.; MacFarlane, E.G. Postnatal Smad3 Inactivation in Murine Smooth Muscle Cells Elicits a Temporally and Regionally Distinct Transcriptional Response. Front. Cardiovasc. Med. 2022, 9, 826495. [Google Scholar] [CrossRef]
- Van Gucht, I.; Meester, J.A.N.; Bento, J.R.; Bastiaansen, M.; Bastianen, J.; Luyckx, I.; Van Den Heuvel, L.; Neutel, C.H.G.; Guns, P.J.; Vermont, M.; et al. A human importin-β-related disorder: Syndromic thoracic aortic aneurysm caused by bi-allelic loss-of-function variants in IPO8. Am. J. Hum. Genet. 2021, 108, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Boileau, C.; Guo, D.C.; Hanna, N.; Regalado, E.S.; Detaint, D.; Gong, L.; Varret, M.; Prakash, S.K.; Li, A.H.; d’Indy, H.; et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet. 2012, 44, 916–921. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Schepers, D.; Bolar, N.A.; Doyle, J.J.; Gallo, E.; Fert-Bober, J.; Kempers, M.J.; Fishman, E.K.; Chen, Y.; Myers, L.; et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 2012, 44, 922–927. [Google Scholar] [CrossRef]
- Cook, J.R.; Clayton, N.P.; Carta, L.; Galatioto, J.; Chiu, E.; Smaldone, S.; Nelson, C.A.; Cheng, S.H.; Wentworth, B.M.; Ramirez, F. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.Y.; Au Yeung, K.; Cortes, S.F.; Sandor, G.G.S.; Judge, D.P.; Dietz, H.C.; van Breemen, C. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. Br. J. Pharmacol. 2007, 150, 1075–1083. [Google Scholar] [CrossRef]
- Wilson, D.G.; Bellamy, M.F.; Ramsey, M.W.; Goodfellow, J.; Brownlee, M.; Davies, S.; Wilson, J.F.; Lewis, M.J.; Stuart, A.G. Endothelial function in Marfan syndrome: Selective impairment of flow-mediated vasodilation. Circulation 1999, 99, 909–915. [Google Scholar] [CrossRef]
- Sellers, S.L.; Milad, N.; Chan, R.; Mielnik, M.; Jermilova, U.; Huang, P.L.; de Crom, R.; Hirota, J.A.; Hogg, J.C.; Sandor, G.G.; et al. Inhibition of Marfan Syndrome Aortic Root Dilation by Losartan: Role of Angiotensin II Receptor Type 1-Independent Activation of Endothelial Function. Am. J. Pathol. 2018, 188, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; LeMaire, S.A. Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections. Curr. Probl. Surg. 2017, 54, 95–155. [Google Scholar] [CrossRef]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Trybus, K.M.; Guo, D.C.; Sweeney, H.L.; Regalado, E.; Kamm, K.; Stull, J.T. Altered Smooth Muscle Cell Force Generation as a Driver of Thoracic Aortic Aneurysms and Dissections. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 26–34. [Google Scholar] [CrossRef]
- Karimi, A.; Milewicz, D.M. Structure of the Elastin-Contractile Units in the Thoracic Aorta and How Genes That Cause Thoracic Aortic Aneurysms and Dissections Disrupt This Structure. Can. J. Cardiol. 2016, 32, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Anesi, J.; Maier, M.C.; Myers, M.A.; Oqueli, E.; Sobey, C.G.; Drummond, G.R.; Denton, K.M. Sympathetic Nervous System and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13132. [Google Scholar] [CrossRef] [PubMed]
- Renard, M.; Holm, T.; Veith, R.; Callewaert, B.L.; Adès, L.C.; Baspinar, O.; Pickart, A.; Dasouki, M.; Hoyer, J.; Rauch, A.; et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 2010, 18, 895–901. [Google Scholar] [CrossRef]
- Barbier, M.; Gross, M.S.; Aubart, M.; Hanna, N.; Kessler, K.; Guo, D.C.; Tosolini, L.; Ho-Tin-Noe, B.; Regalado, E.; Varret, M.; et al. MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections. Am. J. Hum. Genet. 2014, 95, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef]
- Li, Y.; Gao, S.; Han, Y.; Song, L.; Kong, Y.; Jiao, Y.; Huang, S.; Du, J.; Li, Y. Variants of Focal Adhesion Scaffold Genes Cause Thoracic Aortic Aneurysm. Circ. Res. 2021, 128, 8–23. [Google Scholar] [CrossRef]
- Elbitar, S.; Renard, M.; Arnaud, P.; Hanna, N.; Jacob, M.P.; Guo, D.C.; Tsutsui, K.; Gross, M.S.; Kessler, K.; Tosolini, L.; et al. Pathogenic variants in THSD4, encoding the ADAMTS-like 6 protein, predispose to inherited thoracic aortic aneurysm. Genet. Med. 2021, 23, 111–122. [Google Scholar] [CrossRef]
- Martin, E.; Golunski, E.; Laing, S.T.; Estrera, A.L.; Sharina, I.G. Alternative splicing impairs soluble guanylyl cyclase function in aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1565–H1575. [Google Scholar] [CrossRef]
- Guo, R.; Du, P.; Pei, Y.; Yang, J.; Li, S.; Chang, S.; Sun, H.; He, X.; Dong, J.; Zhou, J.; et al. Whole-Exome Sequencing Identified Genes Responsible for Thoracic Aortic Aneurysms and Dissections in three Chinese Families. Front. Genet. 2022, 13, 910932. [Google Scholar] [CrossRef]
- Guo, D.C.; Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Yu, R.K.; Avidan, N.; Bourgeois, S.; Estrera, A.L.; Safi, H.J.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007, 39, 1488–1493. [Google Scholar] [CrossRef]
- Wang, L.; Guo, D.-C.; Cao, J.; Gong, L.; Kamm, K.E.; Regalado, E.; Li, L.; Shete, S.; He, W.-Q.; Zhu, M.-S.; et al. Mutations in Myosin Light Chain Kinase Cause Familial Aortic Dissections. Am. J. Hum. Genet. 2010, 87, 701–707. [Google Scholar] [CrossRef]
- Guo, D.C.; Regalado, E.; Casteel, D.E.; Santos-Cortez, R.L.; Gong, L.; Kim, J.J.; Dyack, S.; Horne, S.G.; Chang, G.; Jondeau, G.; et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am. J. Hum. Genet. 2013, 93, 398–404. [Google Scholar] [CrossRef]
- Capuano, A.; Bucciotti, F.; Farwell, K.D.; Tippin Davis, B.; Mroske, C.; Hulick, P.J.; Weissman, S.M.; Gao, Q.; Spessotto, P.; Colombatti, A.; et al. Diagnostic Exome Sequencing Identifies a Novel Gene, EMILIN1, Associated with Autosomal-Dominant Hereditary Connective Tissue Disease. Hum. Mutat. 2016, 37, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Crepeau, M.W.; Mitchell, A.L.; Stephan, M.J.; Puntel, R.A.; Yin Loke, K.; Kirk, R.C.; Urban, Z. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J. Med. Genet. 2006, 43, 255–258. [Google Scholar] [CrossRef]
- Adamo, C.S.; Beyens, A.; Schiavinato, A.; Keene, D.R.; Tufa, S.F.; Mörgelin, M.; Brinckmann, J.; Sasaki, T.; Niehoff, A.; Dreiner, M.; et al. EMILIN1 deficiency causes arterial tortuosity with osteopenia and connects impaired elastogenesis with defective collagen fibrillogenesis. Am. J. Hum. Genet. 2022, 109, 2230–2252. [Google Scholar] [CrossRef] [PubMed]
- Sheen, V.L.; Jansen, A.; Chen, M.H.; Parrini, E.; Morgan, T.; Ravenscroft, R.; Ganesh, V.; Underwood, T.; Wiley, J.; Leventer, R.; et al. Filamin A mutations cause periventricular heterotopia with Ehlers-Danlos syndrome. Neurology 2005, 64, 254–262. [Google Scholar] [CrossRef]
- Craft, C.S.; Broekelmann, T.J.; Mecham, R.P. Microfibril-associated glycoproteins MAGP-1 and MAGP-2 in disease. Matrix Biol. 2018, 71–72, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.S.; Halabi, C.M.; Hoffman, E.P.; Carmichael, N.; Leshchiner, I.; Lian, C.G.; Bierhals, A.J.; Vuzman, D.; Medicine, B.G.; Mecham, R.P. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc. Natl. Acad. Sci. USA 2016, 113, 8759–8764. [Google Scholar] [CrossRef]
- Chen, J.; Kaw, K.; Lu, H.; Fagnant, P.M.; Chattopadhyay, A.; Duan, X.Y.; Zhou, Z.; Ma, S.; Liu, Z.; Huang, J.; et al. Resistance of Acta2(R149C/+) mice to aortic disease is associated with defective release of mutant smooth muscle α-actin from the chaperonin-containing TCP1 folding complex. J. Biol. Chem. 2021, 297, 101228. [Google Scholar] [CrossRef]
- Bellini, C.; Wang, S.; Milewicz, D.M.; Humphrey, J.D. Myh11(R247C/R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity. J. Biomech. 2015, 48, 113–121. [Google Scholar] [CrossRef]
- Zanetti, M.; Braghetta, P.; Sabatelli, P.; Mura, I.; Doliana, R.; Colombatti, A.; Volpin, D.; Bonaldo, P.; Bressan, G.M. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell Biol. 2004, 24, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Ciliberto, C.H.; Knutsen, R.H.; Levy, M.A.; Kovacs, A.; Mecham, R.P. The importance of elastin to aortic development in mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H257–H264. [Google Scholar] [CrossRef]
- Schmid, F.X.; Bielenberg, K.; Schneider, A.; Haussler, A.; Keyser, A.; Birnbaum, D. Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: Involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur. J. Cardiothorac. Surg. 2003, 23, 537–543. [Google Scholar] [CrossRef]
- Emrich, F.C.; Okamura, H.; Dalal, A.R.; Penov, K.; Merk, D.R.; Raaz, U.; Hennigs, J.K.; Chin, J.T.; Miller, M.O.; Pedroza, A.J.; et al. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2016, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Li, Y.; Zhang, C.; Li, Y.; LeMaire, S.A.; Shen, Y.H. Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target. J. Mol. Cell. Cardiol. 2022, 163, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.H.; Littlewood, T.D.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Bennett, M.R. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ. Res. 2008, 102, 1529–1538. [Google Scholar] [CrossRef]
- Oller, J.; Méndez-Barbero, N.; Ruiz, E.J.; Villahoz, S.; Renard, M.; Canelas, L.I.; Briones, A.M.; Alberca, R.; Lozano-Vidal, N.; Hurlé, M.A.; et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 2017, 23, 200–212. [Google Scholar] [CrossRef]
- Ju, X.; Ijaz, T.; Sun, H.; Lejeune, W.; Vargas, G.; Shilagard, T.; Recinos, A., 3rd; Milewicz, D.M.; Brasier, A.R.; Tilton, R.G. IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J. Am. Heart. Assoc. 2014, 3, e000476. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Yeager, M.E.; El Kasmi, K.C.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef]
- Schwarze, U.; Hata, R.-I.; McKusick, V.A.; Shinkai, H.; Hoyme, H.E.; Pyeritz, R.E.; Byers, P.H. Rare Autosomal Recessive Cardiac Valvular Form of Ehlers-Danlos Syndrome Results from Mutations in the COL1A2 Gene That Activate the Nonsense-Mediated RNA Decay Pathway. Am. J. Hum. Genet. 2004, 74, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Kontusaari, S.; Tromp, G.; Kuivaniemi, H.; Ladda, R.L.; Prockop, D.J. Inheritance of an RNA splicing mutation (G+I IVS20) in the type III procollagen gene (COL3AI) in a family having aortic aneurysms and easy bruisability: Phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am. J. Hum. Genet. 1990, 47, 112–120. [Google Scholar]
- Zhao, K.; Zhu, H.; He, X.; Du, P.; Liang, T.; Sun, Y.; Jing, Z.; Zhou, J. Senkyunolide I ameliorates thoracic aortic aneurysm and dissection in mice via inhibiting the oxidative stress and apoptosis of endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166819. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, H.; Luo, C.; Zhao, Y.; Dai, R.; Li, Z.; Zhang, X.; Gong, Z.; Cai, Z.; Shen, Y.; et al. Urate-Lowering Therapy Inhibits Thoracic Aortic Aneurysm and Dissection Formation in Mice. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e172–e189. [Google Scholar] [CrossRef]
- Ming, Y.; Zhou, X.; Liu, G.; Abudupataer, M.; Zhu, S.; Xiang, B.; Yin, X.; Lai, H.; Sun, Y.; Wang, C.; et al. PM2.5 exposure exacerbates mice thoracic aortic aneurysm and dissection by inducing smooth muscle cell apoptosis via the MAPK pathway. Chemosphere 2023, 313, 137500. [Google Scholar] [CrossRef]

| Species, Strain | Sex | Age (w) | BAPN Dose | Induction Time, w | Aortic Diameter Increase | TAA Rate, % | Rupt. Rate,% | Dissection | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | NR | Yes | [25] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | NR | Yes | [26] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | 45.7 | Yes | [27] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | 73.3 | 46.7 | Yes | [28] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | <80 | Yes | [29] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | NR | NR | 80 | Yes | [30] |
| Mice, C57BL/6 | M | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | 23.3 | Yes | [31] |
| Mice, C57BL/6 | NR | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | 14.3 | Yes | [32] |
| Mice, C57BL/6 | NR | 3 | 1 g/kg/d in DW | 4 w | Yes | NR | >50 | Yes | [33] |
| Mice, C57BL/10 | M | 3 | 0.5 g/kg/d in DW | 4 w | NR | NR | <60 | Yes | [34] |
| Mice, C57BL/6 | M | 3 | 0.5 g/kg/d in DW | 4 w | Yes | NR | 11 | Yes | [35] |
| Mice, C57BL/6 | M | 3 | 0.5 g/kg/d in DW | 4 w | NR | NR | 12.5 | Yes | [36] |
| Mice, C57BL/6 | M | 3 | 0.5 g/kg/d in DW | 1–4 w | Yes | NR | 86.7 | Yes | [37] |
| Mice, C57BL/6 | F | 3 | 0.5 g/kg/d in DW | 1–4 w | Yes | NR | 58.8 | Yes | [37] |
| Mice, C57BL/6 | M | 3 | 0.4 g/100 g diet | 18 d | Yes | NR | NR | Yes | [38] |
| Mice, C57BL/6 | M | 3 | 6 g/L in DW | 4 w | Yes | 83.3 | NR | Yes | [39] |
| Mice, C57BL/6 | NR | 3 | 2.5 g/L in DW | 4 w | Yes | 42.9 | 42.9 | Yes | [40] |
| Mice, C57BL/6 SJL | M | 3–4 | 3 g/L in DW | 26 w | Yes | 50 | 15.2 | NR | [41] |
| Mice, C57BL/6 | M | 3 | 1 g/L in DW | 6 w | Yes | NR | 66 | NR | [42] |
| Rats, SD | M | 3 | 1 g/kg/d, intragastric | 4 w | Yes | 16.7 | 0 | NR | [43] |
| Species | Strain | Sex | Age, w | Dose, μg/kg per min | Time, w | TAA Rate, % | Rupture Rate, % | Dissection Rate, % | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mice | ApoE−/− | M | 12 | 1 | 4 | NR | NR | NR | [47] |
| Mice | ApoE−/− | M | 8–10 | 1 | 4 | NR | NR | NR | [48] |
| Mice | ApoE−/− | M | adult | NR | NR | NR | NR | NR | [49] |
| Mice | WT & Plce1−/− | M&F | 10 to 12 | 1 | 4 | 80 | 43 | NR | [50] |
| Mice | WT & Loxl4−/− | M | 14 or 20 | 1 or 1.5 | 4 | 28.6 | NR | NR | [51] |
| Mice | WT & Tfam−/− | M | 4–5 | 1 | 4 | 100 | 70 | 70% | [52] |
| Rat | SD | F | 8 | 1.2 | 4 | 85 | NR | NR | [53] |
| Species, Strain | Sex | Age, w | PPE Site | PPE Dose | PPE Time, min | Experimental Period, w | TAA Rate, % | Rupture Rate, % | Dissection Rate, % | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Mice, C57BL/6 | M | 8–12 | ATA & arch | 15 µL | 5 or 10 | 1–4 | 43 or 71 | 0 or 18 | NR | [58] |
| Mice, C57BL/6 | M | 8–12 | DTA | NR | 4 | 2 | NR | NR | NR | [59] |
| Mice, C57BL/6 | M | 8–10 | DTA | 12 μL | 3 | 2 | 100 | NR | NR | [60] |
| Rats, SD | F | 12 | DTA | NR | 15–20 | NR | NR | NR | NR | [61] |
| Species, Strain | Sex | Age | CaCl2 Conc. | CaCl2 Time, min | Exp. Duration, Weeks | Aortic Diameter Increase | TAA Rate, % | Rupt. Rate,% | Dissection Rate, % (or Yes/No) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Mice, 129/SvE | M&F | NR | 0.5 M | 15 | 4 | 25% | NR | NR | NR | [62] |
| Mice, C57BL/6 | M&F | 10 w | 0.5 M | 15 | 4, 8, 16 | 59.5% 4 w 64.3% 8 w 62.9% 16 w | 90% | NR | NR | [63] |
| Rats, WS | M | NR | 0.5 M | 15 | 4 | 18% | NR | NR | NR | [64] |
| Rats, SD | NR | NR | 0.5 M | 15 | 4 | NR | NR | NR | NR | [65] |
| Mous Strain | Sex | Age, w | BAPN | Angiotensin II | TAA Rate, % | TAA Diam ↑ | Rup. | Dis. | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Time, Days | Route | Dose, μg/kg/min | Time, Day | ||||||||
| C57BL/6 | M | 3 | 0.15 μg/kg/d | 28 | i.p. | 1 | 3 | 40 | Yes | Yes | NR | [68] |
| C57BL/6 | M | 3 | 0.15 μg/kg/d | 28 | i.p. | 1 | 28 | 73 | Yes | Yes | NR | [68] |
| C57BL/6 | M & F | 10–15 | 0.2% | 28 | DW | 1 | 25 (3 days after start of BAPN) | NR | Yes | Yes | NR | [69] |
| C57BL/6, or FVB | M | 3 | 0.4% | 28 | Diet | 1 | 1 | NR | NR | Yes | Yes | [46] |
| C57BL/6 | M | 3 | 1 μg/kg/d | 28 | DW | 1 | 2 | 25 | Yes | Yes | Yes | [70] |
| Species | Models | Features/Advantages | Disadvantages |
|---|---|---|---|
| Mouse/rat | Overall | Low-cost compared with porcine models. Many GMO animals are available | Not suitable for angiographic imaging and testing TAA repair procedures/devices |
| BAPN | Showing thrombosis, elastic fiber degradation, VSMC apoptosis, and dissection | May not closely represent human TAA development which requires years/decades | |
| AngII | A model of thoracoabdominal aortic aneurysms with dissection | As above | |
| Elastase | TAA location can be controlled | TAAs decrease in size when they reach maximal dilatation at 2 weeks post-surgery | |
| CaCl2 | Showing aortic calcification, apoptosis, inflammation, and ECM degradation | This model is not suitable for investigating TAA progression and rupture | |
| BAPN + AngII | Showing sex phenotype, aortic dissection, intramural hematomas, elastic fiber degradation, and inflammation | May not closely represent human TAA development which requires years/decades | |
| HFD + AngII | Suitable to study TAA-associated dissection | As above | |
| TAC | It mimics pressure overload | The increase in aortic diameter is small | |
| Genetic | Fbn1C1041G/+ and Fbn1mgR/mgR mice are useful for studying Marfan syndrome | Genetic knockout may be lethal | |
| Porcine | Overall | Suitable for angiographic imaging, assessing new devices or interventions | Expensive; Lacking reliable methods to assess anesthetic depth during surgery; GMO pigs are not readily available |
| Elastase | Showing loss of VSMCs and degradation of elastic fibers | As above | |
| Collagenase + CaCl2 | Showing an increase in MMPs, and a decrease in VSMCs | Not showing rupture | |
| Vein patch | Valuable in developing novel treatments for endoleaks after TEVAR | TAAs are histologically different from real aneurysms; TAAs are saccular | |
| Pericardium pouch | Showing mural thrombi and increased inflammation | No elastic fibers and VSMCs on the patch; TAAs formed are saccular; etiology is different from human TAAs | |
| Cover-then-cut | Showing gradual TAA growth | As above | |
| MI resection | TAAs more resemble human TAAs than those derived from patch methods | TAAs formed are saccular; they do not involve inflammatory cell infiltration and calcification | |
| Zebrafish | Genetic and pharmacological | Low-cost; less infrastructure requirement; suitable for direct microscopic assessment and large-scale small-molecule suppressor screening in microwells | TAAs develop within 2–5 days and may not closely mimic human TAAs |
| Gene | Protein | Associated with TAA in Humans | Contributing to TAA in Animal Models |
|---|---|---|---|
| FBN1 | Fibrillin-1 | Yes [155] | Yes [88,97] |
| FBLN4 | Fibulin-4 | Yes [157] | Yes [104,105,106] |
| MFAP 2 & 5 | Microfibril-associated glycoprotein 1 & 2 | Yes [158] | Yes [171] |
| LOX | Lysyl oxidase | Yes [159] | Yes [172] |
| TES | Testin | Yes [160] | Yes [160] |
| THSD4 | Thrombospondin, type I, domain containing 4 a | Yes [161] | Yes [161] |
| GUCY | Soluble guanylate cyclase | Yes [162] | Yes [80] |
| PRKG1 | Type 1 cGMP-dependent protein kinase | Yes [166] | Yes [80] |
| ACTA2 | SMC-specific α actin | Yes [163] | No [173] |
| MYH11 | Smooth muscle myosin heavy chain | Yes [164] | No [174] |
| MYLK | Myosin light chain kinase | Yes [165] | No [165] |
| EMILIN1 | Elastin microfibril interface–located protein 1 | Yes [167] | No [175] |
| ELN | Elastin | Yes [168] | NR [176] |
| EMILIN1 | Elastin microfibril interfacer 1 | Yes [167,169] | NR [175] |
| FLNA | Filamin A | Yes [170] | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Panicker, I.S.; Anesi, J.; Sargisson, O.; Atchison, B.; Habenicht, A.J.R. Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2024, 25, 901. https://doi.org/10.3390/ijms25020901
Wang Y, Panicker IS, Anesi J, Sargisson O, Atchison B, Habenicht AJR. Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm. International Journal of Molecular Sciences. 2024; 25(2):901. https://doi.org/10.3390/ijms25020901
Chicago/Turabian StyleWang, Yutang, Indu S. Panicker, Jack Anesi, Owen Sargisson, Benjamin Atchison, and Andreas J. R. Habenicht. 2024. "Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm" International Journal of Molecular Sciences 25, no. 2: 901. https://doi.org/10.3390/ijms25020901
APA StyleWang, Y., Panicker, I. S., Anesi, J., Sargisson, O., Atchison, B., & Habenicht, A. J. R. (2024). Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm. International Journal of Molecular Sciences, 25(2), 901. https://doi.org/10.3390/ijms25020901







