Effect of Fillers Modification with ILs on Fillers Textural Properties: Thermal Properties of SBR Composites
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Properties of Pristine GnPs and GnPs Modified from Solution with 1-butylpyridinium Bromide (BPyBr) and 4-methyl-1-butylpyridinium Bromide (BmPyBr)
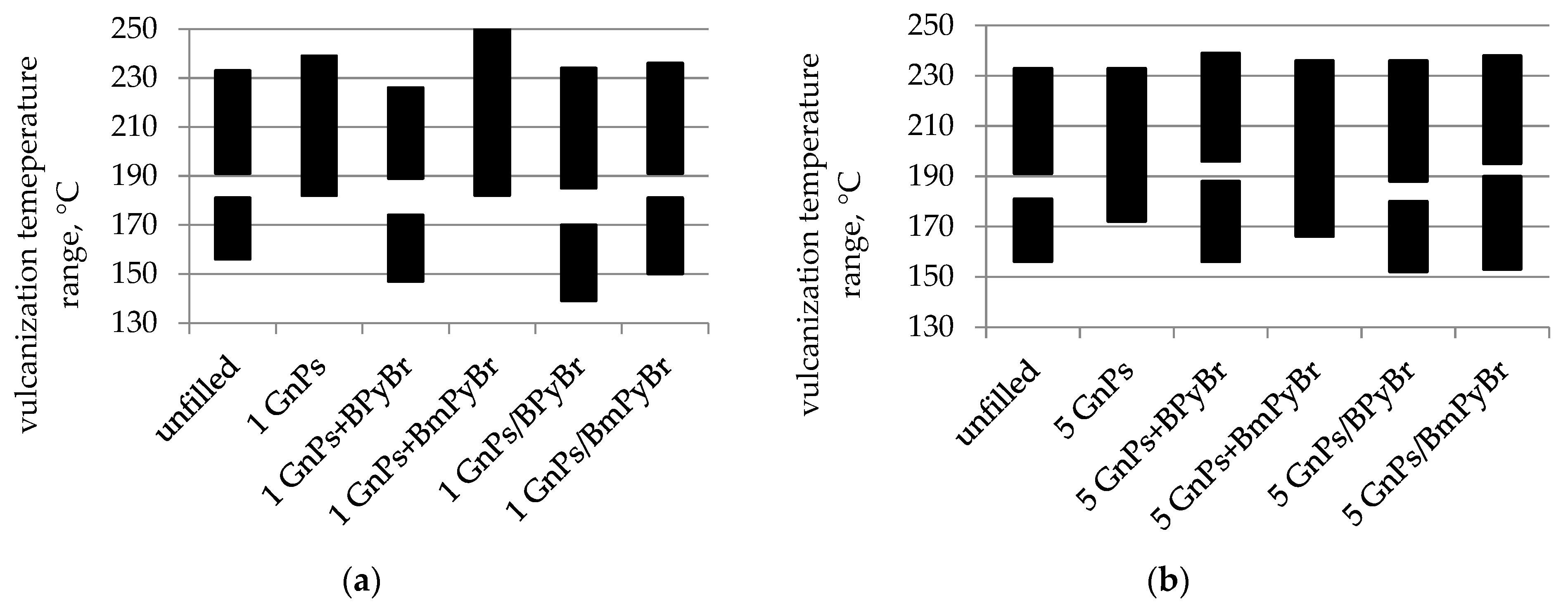
2.2. Curing Kinetics of SBR Compounds by DSC Measurements
2.3. Thermal Stability of SBR Composites and ILs Alone Used to Prepare Composites
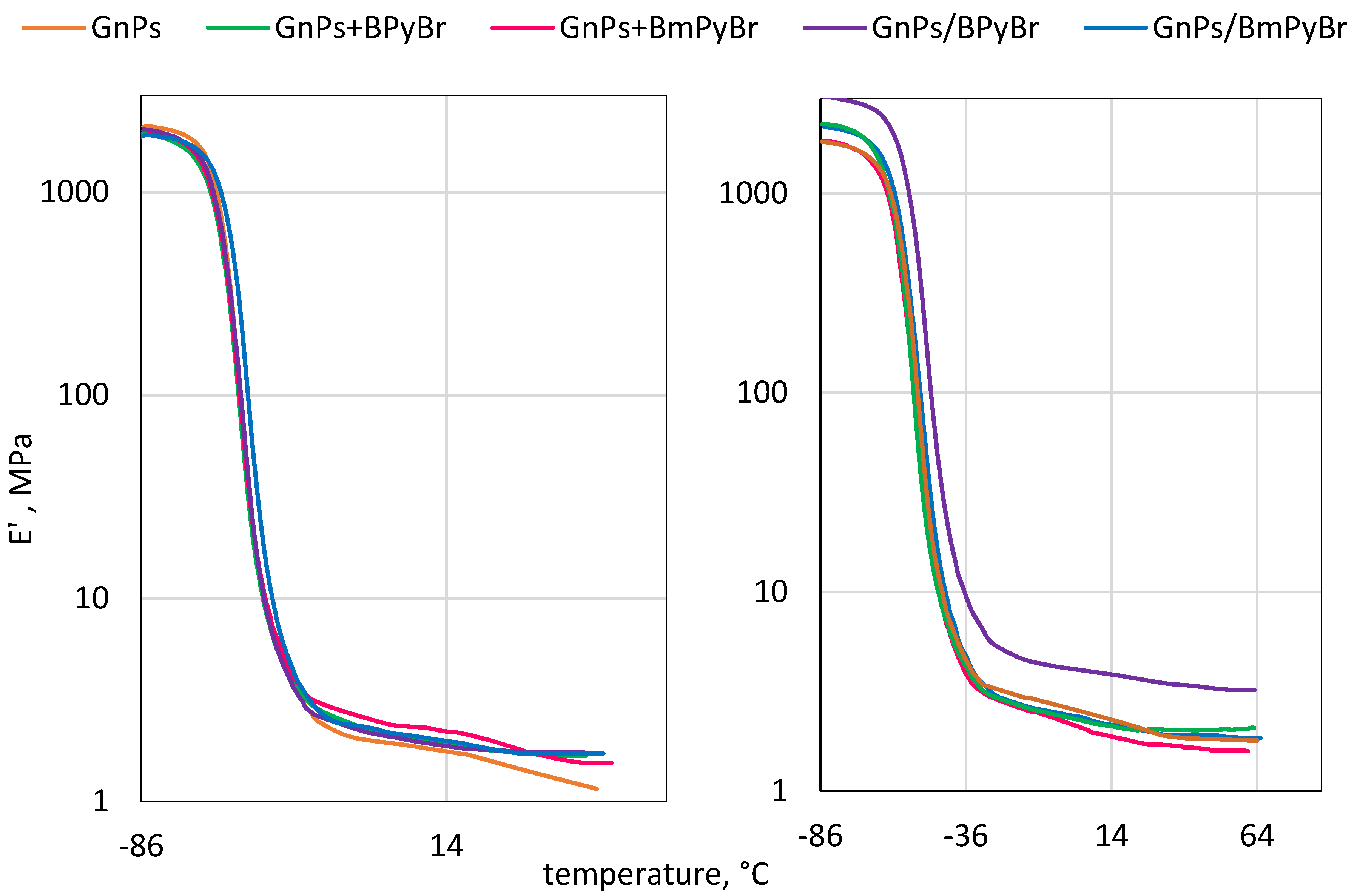
2.4. Dynamic Mechanical Analysis of SBR Composites
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Graphene Nanoplatelets Modified with BPyBr and BmPyBr from Solution (GnPs/BPyBr and GnPs/BmPyBr, Respectively)
3.3. Preparation of Rubber Mixes and Vulcanizates
3.4. Methods of Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, B.; Kim, H.G.; Lee, J.-H.; Arepalli, S.; Nah, C. Carbon nanotube-reinforced elastomeric nanocomposites: A review. Int. J. Smart Nano Mater. 2015, 6, 211–238. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Gabor, R.A.; Nicolae, C.A.; Parau, A.C.; Vitelaru, C.; Raditoiu, V.; Chipara, M. Block copolymer elastomer with graphite filler: Effect of processing conditions and silane coupling agent on the composite properties. Polymers 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Kumar, V.; Potiyaraj, P.; Lee, D.-J.; Choi, J. Mutual dispersion of graphite—Silica binary fillers and its effects on curing, mechanical, and aging properties of natural rubber composites. Polym. Bull. 2022, 79, 2707–2724. [Google Scholar] [CrossRef]
- Somaweera, D.; Abeygunawardane, G.A.; Weragoda, S.; Ranatunga, S. Effect of vein graphite powder on mechanical, curing, and thermal properties of solid tire vulcanizate. Mater. Today Proc. 2022, 59, 316–323. [Google Scholar] [CrossRef]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Gao, X.; Song, W. Effect of various carbon nanofillers and different filler aspect ratios on the thermal conductivity of epoxy matrix nanocomposites. Results Phys. 2019, 15, 102771. [Google Scholar] [CrossRef]
- Sreenath, P.R.; Singh, S.; Satyanarayana, M.S.; Das, P.; Kumar, K.D. Carbon dot—Unique reinforcing filler for polymer with special reference to physico—Mechanical properties. Polymer 2017, 112, 189–200. [Google Scholar] [CrossRef]
- Aldroubi, S.; Brun, N.; Malham, I.B.; Mehdi, A. When graphene meets ionic liquids: A good match for the design of functional materials. Nanoscale 2021, 13, 2750–2779. [Google Scholar] [CrossRef]
- Parviz, D.; Das, S.; Ahmed, H.S.T.; Irin, F.; Bhattacharia, S.; Green, M.J. Dispersions of non—Covalently functionalized graphene with minimal stabilizer. ACS Nano 2012, 6, 8857–8867. [Google Scholar] [CrossRef]
- Gaca, M.; Ilcikova, M.; Mrlik, M.; Cvek, M.; Vaulot, C.; Urbanek, P.; Pietrasik, R.; Krupa, I.; Pietrasik, J. Impact of ionic liquids on the processing and photo—Actuations behavior of SBR composites containing graphene nanoplatelets. Sens. Actuators B Chem. 2021, 329, 129195. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Simg, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.H. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A.; Radushkevich, L.V. Physical Adsorption of Gases and Vapors in Micropores. In Progress and Membrane Science, 2nd ed.; Cadenhead, D.A., Danielli, J.F., Rosenberg, M.D., Eds.; Academic Press: New York, NY, USA, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Ravikovitch, P.I.; Haller, G.L.; Neimark, A.V. Density functional theory model for calculating pore size distributions: Pore structure of nanoporous catalysts. Adv. Colloid Interface Sci. 1998, 76–77, 203–226. [Google Scholar] [CrossRef]
- Lin, J.; Hu, D.; Luo, Y.; Zhong, B.; Jia, Z.; Xu, T.; Jia, D. Enhanced mechanical performance and antioxidative efficiency of styrene—Butadiene rubber via 4—Aminodiphenylamine functionalized mesoporous silica. Ind. Eng. Chem. Res. 2018, 57, 4935–4940. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D. A method to improve the mechanical performance of styrene—Butadiene rubber via vulcanization accelerator modified silica. Compos. Sci. Technol. 2015, 117, 46–53. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Luo, Y.; Zhang, D.; Jia, Z.; Jia, D.; Liu, F. Simultaneous reduction and functionalization of graphene oxide via antioxidant for highly aging resistant and thermal conductive elastomer composites. Compos. Sci. Technol. 2017, 151, 156–163. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Bokobza, L.; Pissis, P. Dynamics near the filler surface in natural rubber—Silica nanocomposites. Polymer 2011, 52, 3175–3182. [Google Scholar] [CrossRef]
- Simon, P.; Kućma, S. DSC analysis of the induction period in the vulcanisation of rubber compounds. J. Therm. Anal. Calorim. 1999, 56, 1107–1113. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.-H.; Liu, Z.-Y.; Qing, M.-L.; Wang, G. DSC and curing kinetics of epoxy resin using cyclohexanediol diglycidyl ether as active dilutents. J. Therm. Anal. Calorim. 2014, 116, 411–416. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowińska-Baranowska, A. The synergistic effect of dibenzyldithiocarbamate based accelerator on the vulcanization and performance of the silica filled styrene—Butadiene rubber. Materials 2022, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Sowińska-Baranowska, A.; Maciejewska, M. Influence of the silica specific surface area and ionic liquids on the curing characteristics and performance of styrene–butadiene rubber composites. Materials 2021, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Razzaghi-Kashani, M. Catalytic and networking effects of carbon black on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber. Soft Matter. 2018, 14, 9191–9208. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Gaca, M.; Vaulot, C.; Maciejewska, M.; Lipińska, M. Preparation and properties of SBR composites containing graphene nanoplatelets modified with pyridinium derivative. Materials 2020, 13, 5407. [Google Scholar] [CrossRef]
- Mondal, S.; Khastgir, D. Elastomer reinforcement by graphene nanoplatelets and synergistic improvements of electrical and mechanical properties of composites by hybrid nano fillers of graphene—Carbon black & graphene—MWCNT. Compos. Part A Appl. Sci. Manuf. 2017, 102, 154–165. [Google Scholar]
- Hamdani, S.; Longuet, C.; Lopez-Cuesta, J.-M.; Ganachaud, F. Calcium and aluminium—Based fillers as flame—Retardant additives in silicone matrices. I. Blend preparation and thermal properties. Polym. Degrad. Stab. 2010, 95, 1911–1919. [Google Scholar] [CrossRef]
- Wen, Y.; Yin, Q.; Jia, H.; Yin, B.; Zhang, X.; Liu, P.; Wang, J.; Ji, Q.; Xu, Z. Tailoring rubber—Filler interfacial interactions and multifunctional rubber nanocomposites by usage of graphene oxide with different oxidation degree. Compos. Part B Eng. 2017, 124, 250–259. [Google Scholar] [CrossRef]
- Hou, G.; Tao, W.; Liu, J.; Zhang, X.; Dong, M.; Zhang, L. Effect of the structural characteristics of solution styrene—Butadiene rubber on the properties of rubber composites. J. Appl. Polym. Sci. 2018, 135, 45749. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zhang, K.; Liu, L.; Ji, L.; Liu, Q. Vulcanization, interfacial interaction, and dynamic mechanical properties of in-situ organic amino modified kaolinite/SBR nanocomposites based on latex compounding method. Appl. Clay Sci. 2020, 185, 105366. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Z.; Yang, Z.; Guo, B. Bioinspired interface engineering in elastomer/graphene composites by constructing sacrificial metal—Ligand bonds. Macromol. Rapid Commun. 2016, 37, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- ISO 11357-1:2016; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2016.
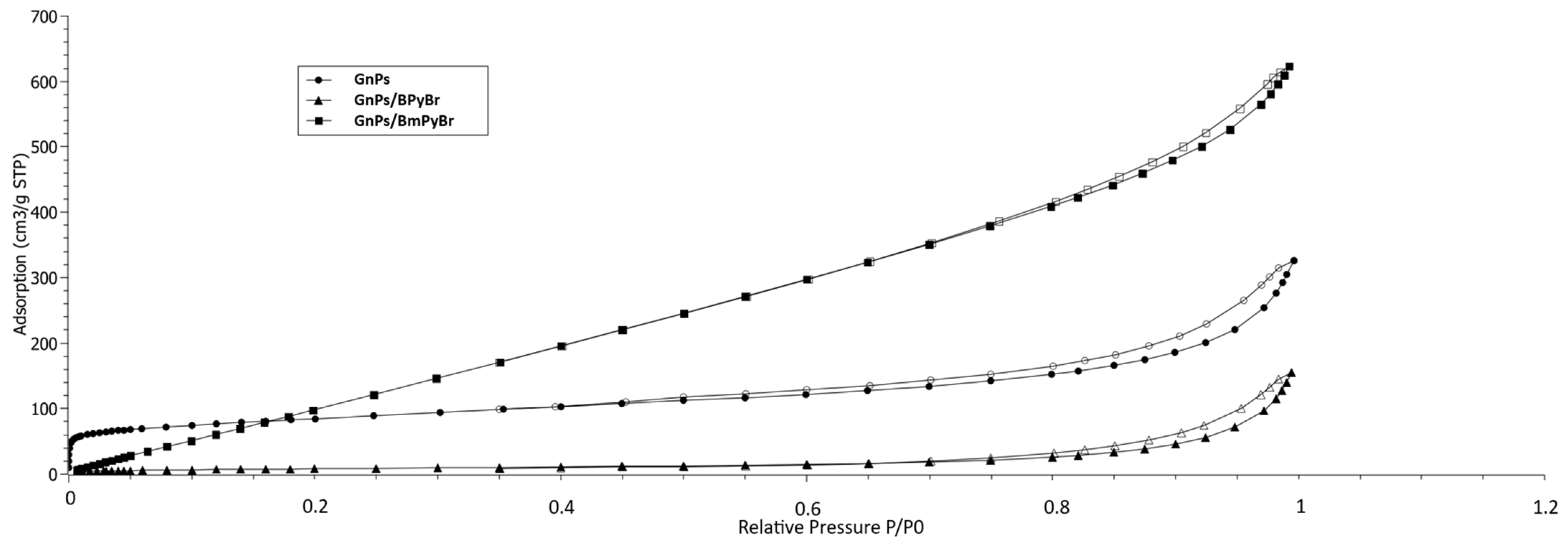
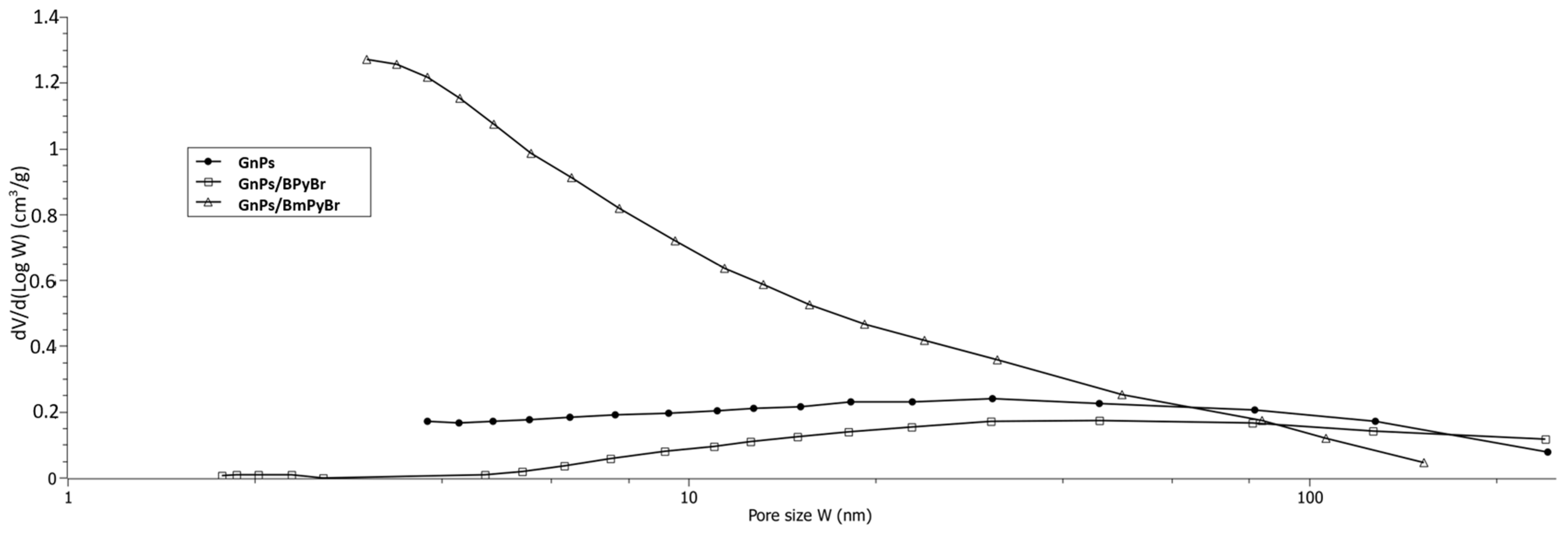




| BET Model | BJH Model | Dubinin–Astakhov Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Vmax (cm3 g−1 STP) | SBET (m2 g−1) | DBET (nm) | SBJH (m2 g−1) | VBJH (cm3 g−1) | DBJH (nm) | SDA (m2 g−1) | VDA (cm3 g−1) |
| pristine GnPs | 325 | 298 ± 1 | 5.5 | 118.6 | 0.40 | 13.5 | 263 | 0.11 |
| GnPs/BPyBr | 35 | 30 ± 0.3 | 31 | 45.0 | 0.24 | 21.4 | n.a. | n.a. |
| GnPs/BmPyBr | 623 | 767 ± 10 | 5.0 | 391.0 | 0.84 | 8.5 | 403 | 0.33 |
| SBR Composites | GnPs Content (phr) | Modifier/Method of Modification | χ (-) | Tg (°C) | Δcp (J g−1 K−1) | ΔH (J g−1) | |
|---|---|---|---|---|---|---|---|
| unfilled | 0 | – | – | 0.00 | −51.1 ± 0.9 | 0.42 ± 0.08 | 5.96 ± 1.50 |
| 1 GnPs | 1 | – | – | 0.01 | −50.3 ± 0.9 | 0.41 ± 0.08 | 12.7 ± 1.50 |
| 1 GnPs + BPyBr | BPyBr | mm | 0.01 | −51.2 ± 0.9 | 0.41 ± 0.08 | 7.28 ± 1.50 | |
| 1 GnPs/BPyBr | BPyBr | s | 0.16 | −51.4 ± 0.9 | 0.35 ± 0.08 | 6.56 ± 1.50 | |
| 1 GnPs + BmPyBr | BmPyBr | mm | 0.11 | −51.2 ± 0.9 | 0.37 ± 0.08 | 18.44 ± 1.50 | |
| 1 GnPs/BmPyBr | BmPyBr | s | 0.01 | −50.3 ± 0.9 | 0.41 ± 0.08 | 10.95 ± 1.50 | |
| 5 GnPs | 5 | – | – | 0.10 | −51.3 ± 0.9 | 0.36 ± 0.08 | 24.6 ± 1.50 |
| 5 GnPs + BPyBr | BPyBr | mm | 0.38 | −49.9 ± 0.9 | 0.25 ± 0.08 | 9.99 ± 1.50 | |
| 5 GnPs/BPyBr | BPyBr | s | 0.04 | −51.2 ± 0.9 | 0.38 ± 0.08 | 16.28 ± 1.50 | |
| 5 GnPs + BmPyBr | BmPyBr | mm | 0.43 | −50.2 ± 0.9 | 0.23 ± 0.08 | 12.71 ± 1.50 | |
| 5 GnPs/BmPyBr | BmPyBr | s | 0.07 | −51.8 ± 0.9 | 0.37 ± 0.08 | 6.29 ± 1.50 | |
| SBR Composites | GnPs Content (phr) | Modifier/Method of Modification | T02 (°C) | T50 (°C) | |
|---|---|---|---|---|---|
| unfilled | 0 | – | – | 245 ± 1 | 441 ± 1 |
| 1 GnPs | 1 | – | – | 259 ± 1 | 445 ± 1 |
| 1 GnPs + BPyBr | BPyBr | mm | 251 ± 1 | 441 ± 1 | |
| 1 GnPs/BPyBr | BPyBr | s | 245 ± 1 | 441 ± 1 | |
| 1 GnPs + BmPyBr | BmPyBr | mm | 235 ± 1 | 443 ± 1 | |
| 1 GnPs/BmPyBr | BmPyBr | s | 231 ± 1 | 439 ± 1 | |
| 5 GnPs | 5 | – | – | 257 ± 1 | 445 ± 1 |
| 5 GnPs + BPyBr | BPyBr | mm | 233 ± 1 | 439 ± 1 | |
| 5 GnPs/BPyBr | BPyBr | s | 229 ± 1 | 443 ± 1 | |
| 5 GnPs + BmPyBr | BmPyBr | mm | 253 ± 1 | 443 ± 1 | |
| 5 GnPs/BmPyBr | BmPyBr | s | 237 ± 1 | 443 ± 1 | |
| Ionic Liquid | T02 (°C) | TMAX (°C) |
|---|---|---|
| BPyBr | 213 ± 1 | 268 ± 1 |
| BmPyBr | 153 ± 1 | 278 ± 1 |
| SBR Composites | GnPs Content (phr) | Modifier/Method of Modification | Tg (°C) | Tan δTg (-) | Tan δ0 (-) | Tan δ60 (-) | |
|---|---|---|---|---|---|---|---|
| unfilled | 0 | – | – | −48.4 | 2.04 | 0.115 | 0.099 |
| 1 GnPs | 1 | – | – | −49.4 | 2.03 | 0.118 | 0.118 |
| 1 GnPs + BPyBr | BPyBr | mm | −50.3 | 1.94 | 0.116 | 0.065 | |
| 1 GnPs/BPyBr | BPyBr | s | −49.3 | 2.03 | 0.086 | 0.059 | |
| 1 GnPs + BmPyBr | BmPyBr | mm | −49.3 | 1.92 | 0.093 | 0.087 | |
| 1 GnPs/BmPyBr | BmPyBr | s | −46.6 | 1.96 | 0.011 | 0.056 | |
| 5 GnPs | 5 | – | – | −48.6 | 1.88 | 0.124 | 0.088 |
| 5 GnPs + BPyBr | BPyBr | mm | −50.3 | 1.97 | 0.061 | 0.095 | |
| 5 GnPs/BPyBr | BPyBr | s | −45.1 | 1.78 | 0.112 | 0.096 | |
| 5 GnPs + BmPyBr | BmPyBr | mm | −49.2 | 1.80 | 0.071 | 0.059 | |
| 5 GnPs/BmPyBr | BmPyBr | s | −47.7 | 1.91 | 0.106 | 0.090 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaca, M.; Vaulot, C. Effect of Fillers Modification with ILs on Fillers Textural Properties: Thermal Properties of SBR Composites. Int. J. Mol. Sci. 2024, 25, 885. https://doi.org/10.3390/ijms25020885
Gaca M, Vaulot C. Effect of Fillers Modification with ILs on Fillers Textural Properties: Thermal Properties of SBR Composites. International Journal of Molecular Sciences. 2024; 25(2):885. https://doi.org/10.3390/ijms25020885
Chicago/Turabian StyleGaca, Magdalena, and Cyril Vaulot. 2024. "Effect of Fillers Modification with ILs on Fillers Textural Properties: Thermal Properties of SBR Composites" International Journal of Molecular Sciences 25, no. 2: 885. https://doi.org/10.3390/ijms25020885
APA StyleGaca, M., & Vaulot, C. (2024). Effect of Fillers Modification with ILs on Fillers Textural Properties: Thermal Properties of SBR Composites. International Journal of Molecular Sciences, 25(2), 885. https://doi.org/10.3390/ijms25020885






