Heat Shock Proteins and Breast Cancer
Abstract
1. Introduction
2. The Biological Function of Hsps in Breast Cancer
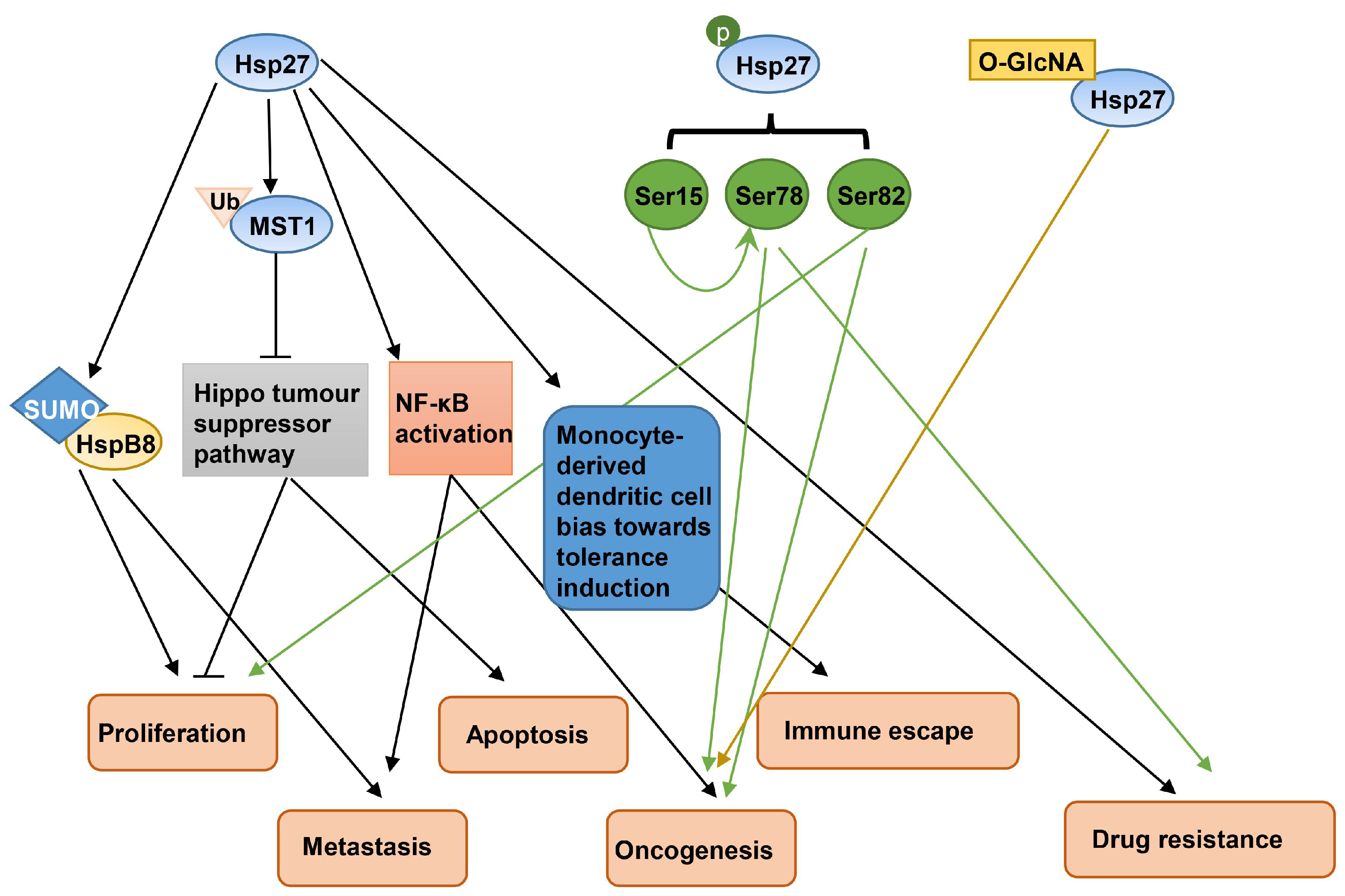
2.1. Hsp27
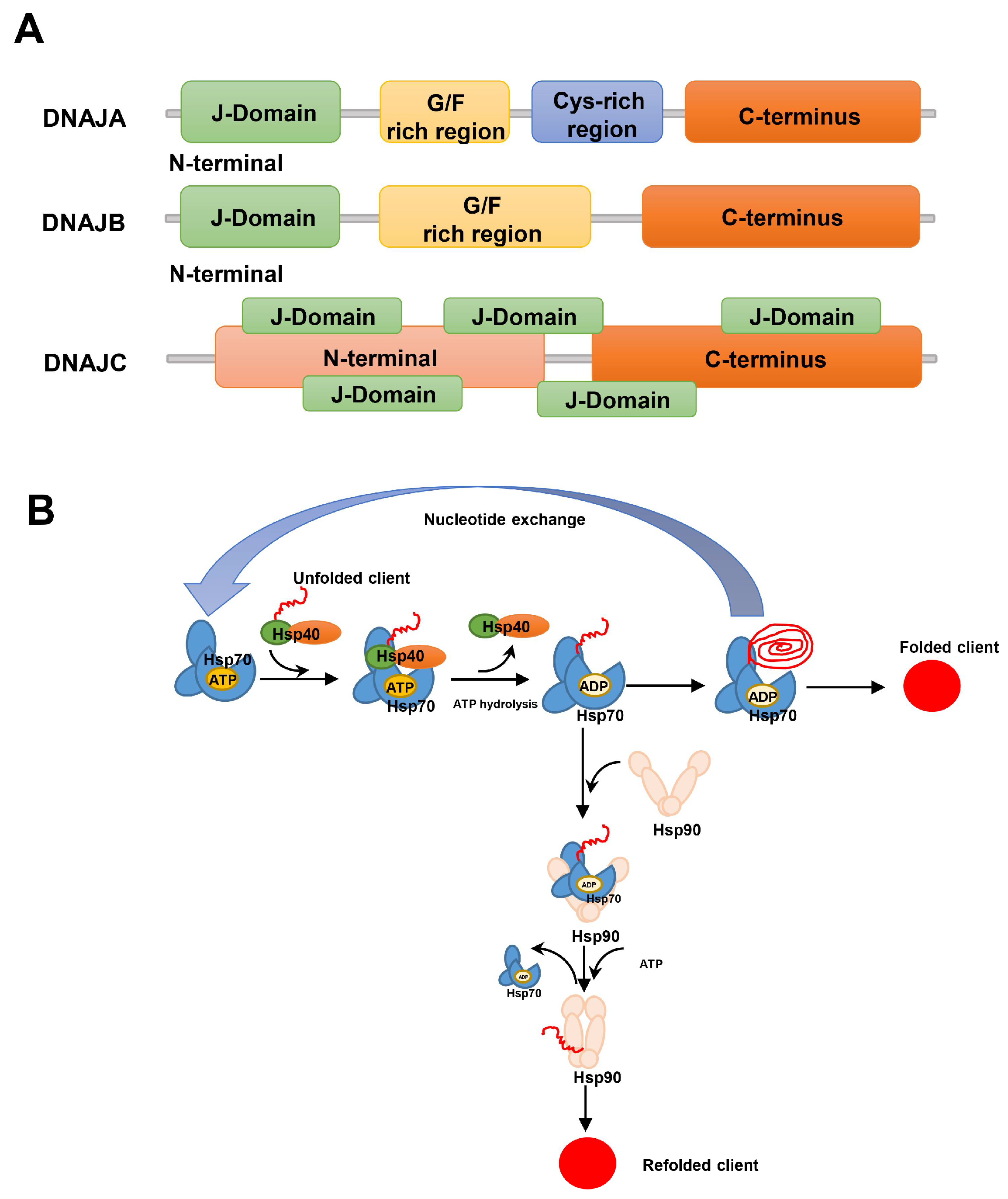
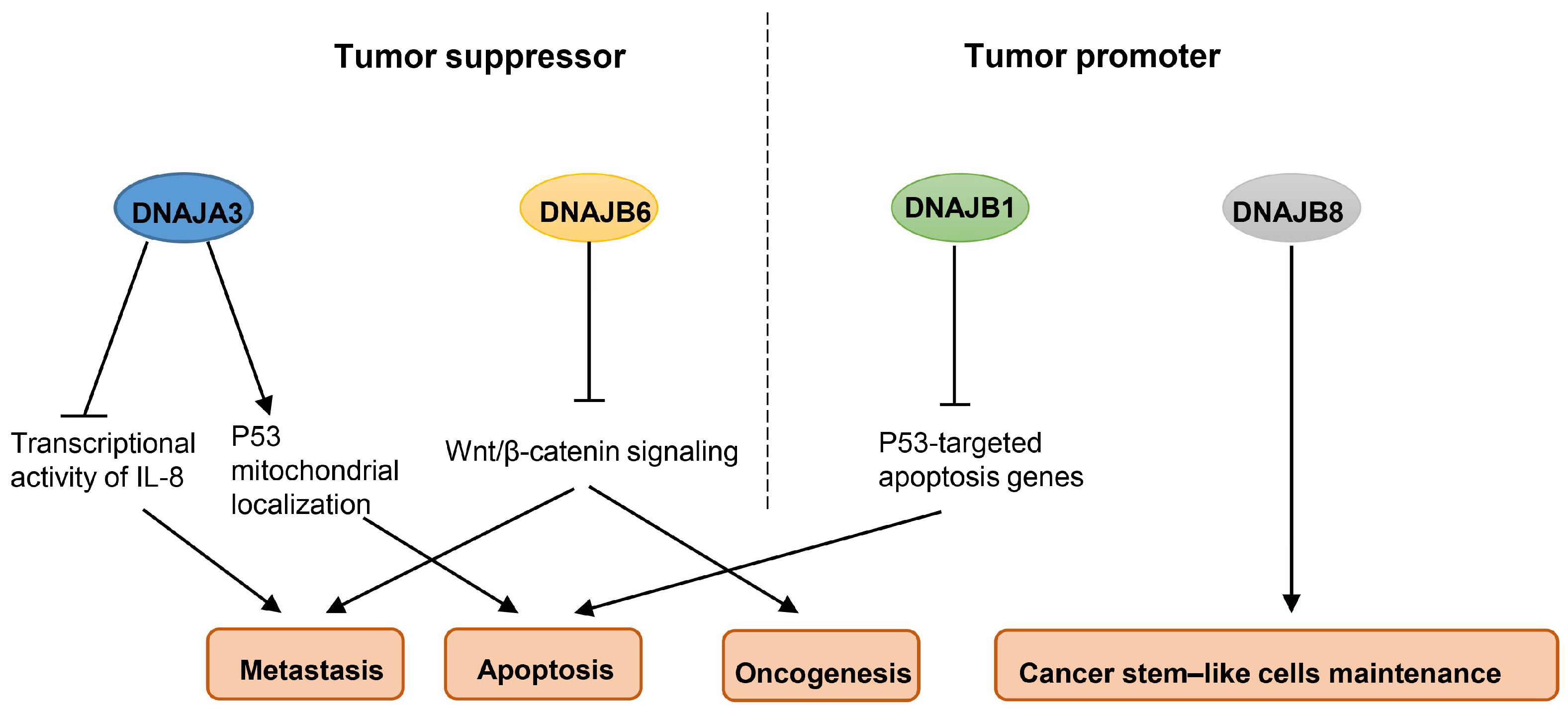
2.2. Hsp40
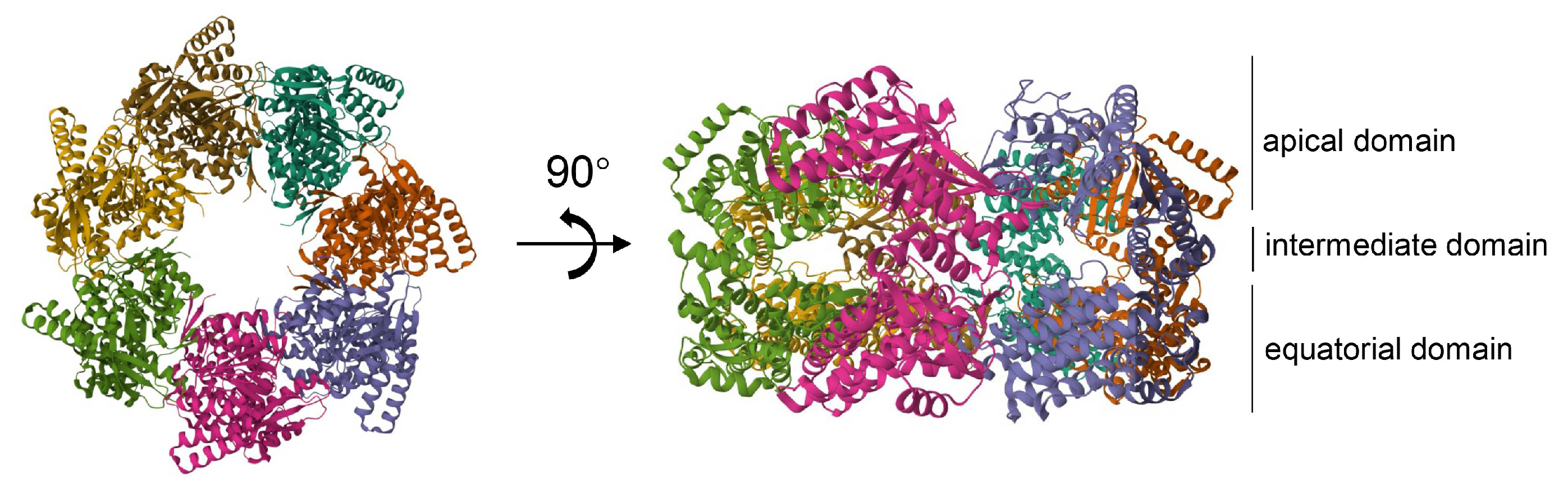
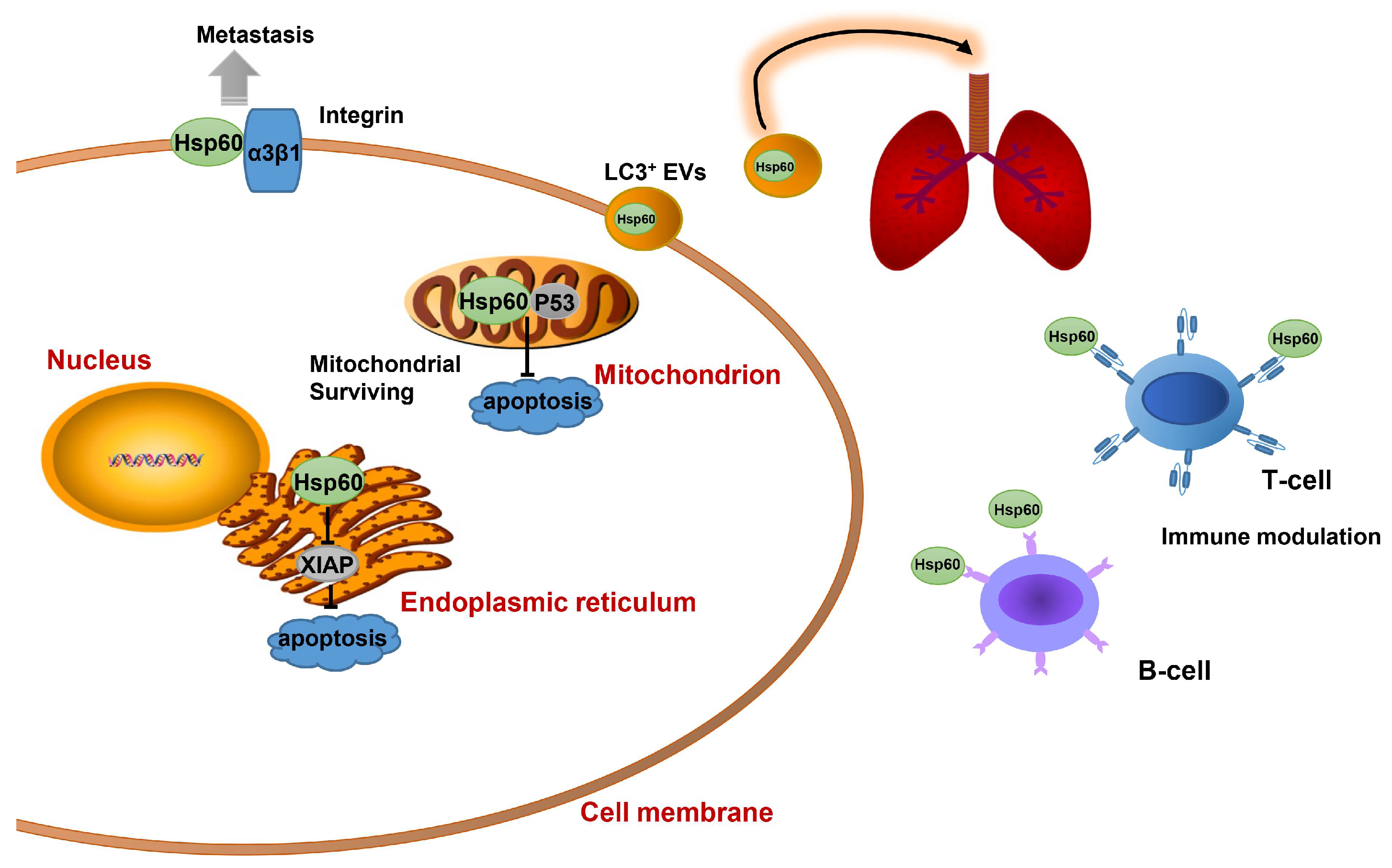
2.3. Hsp60
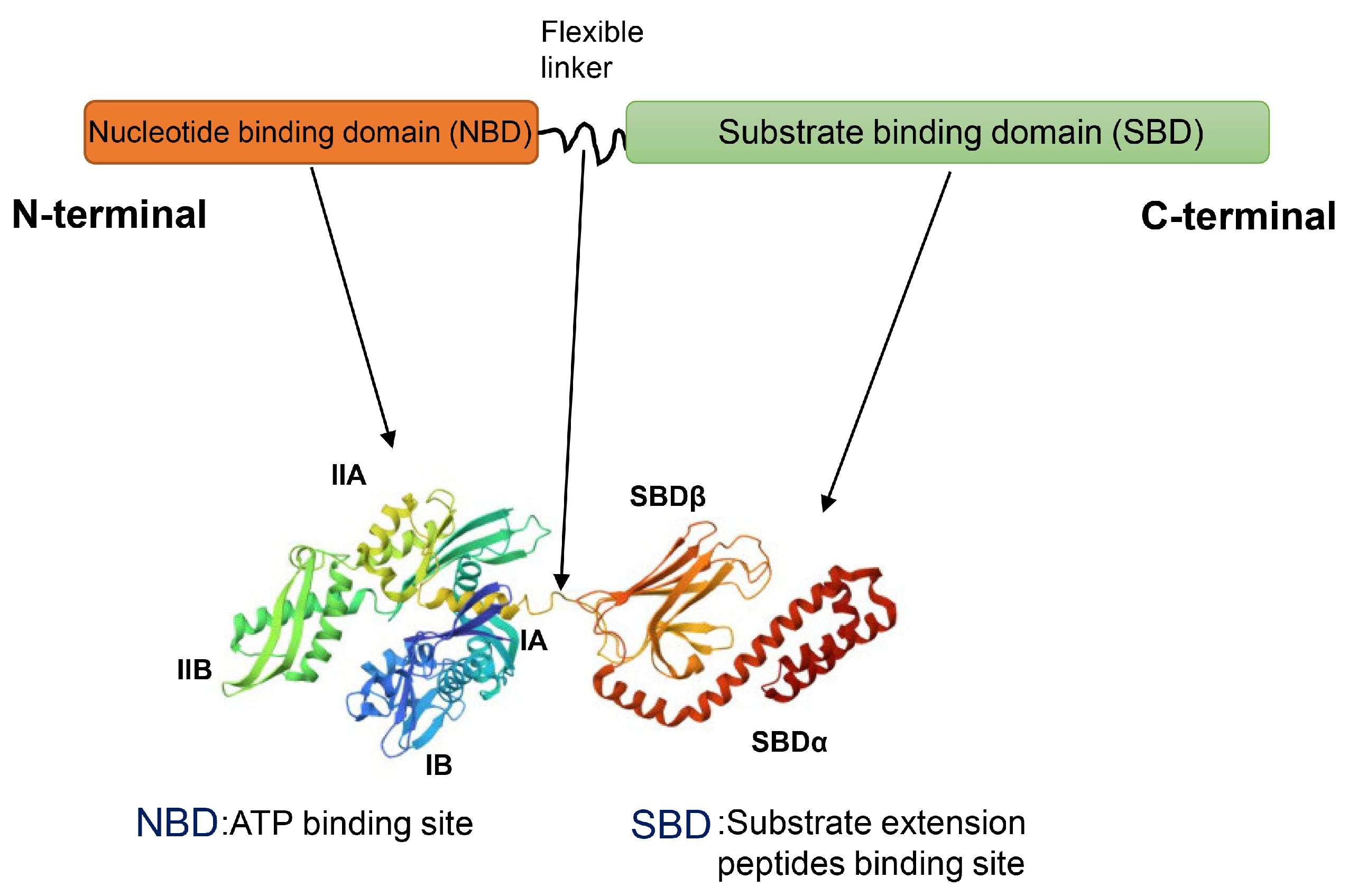
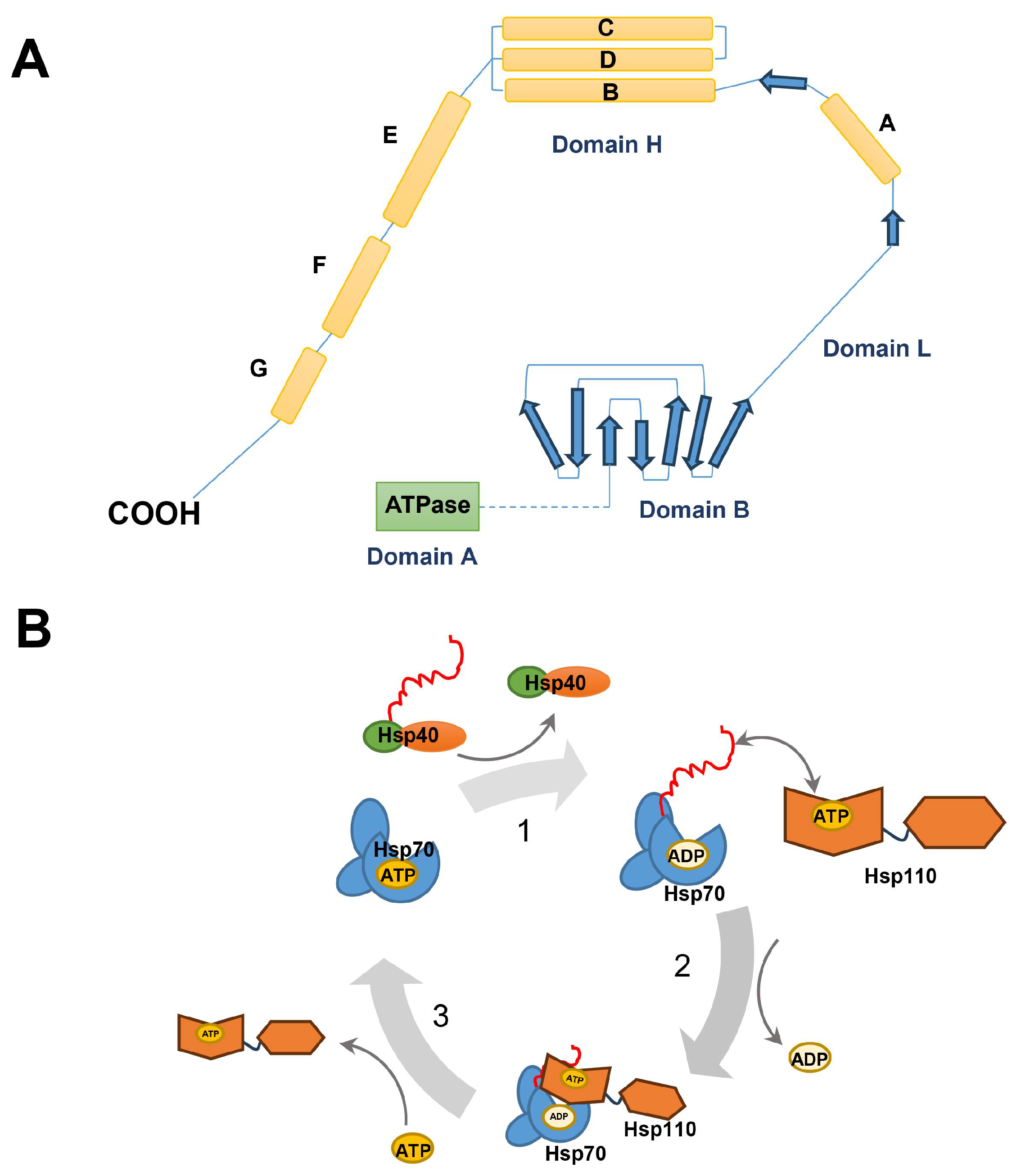
2.4. Hsp70
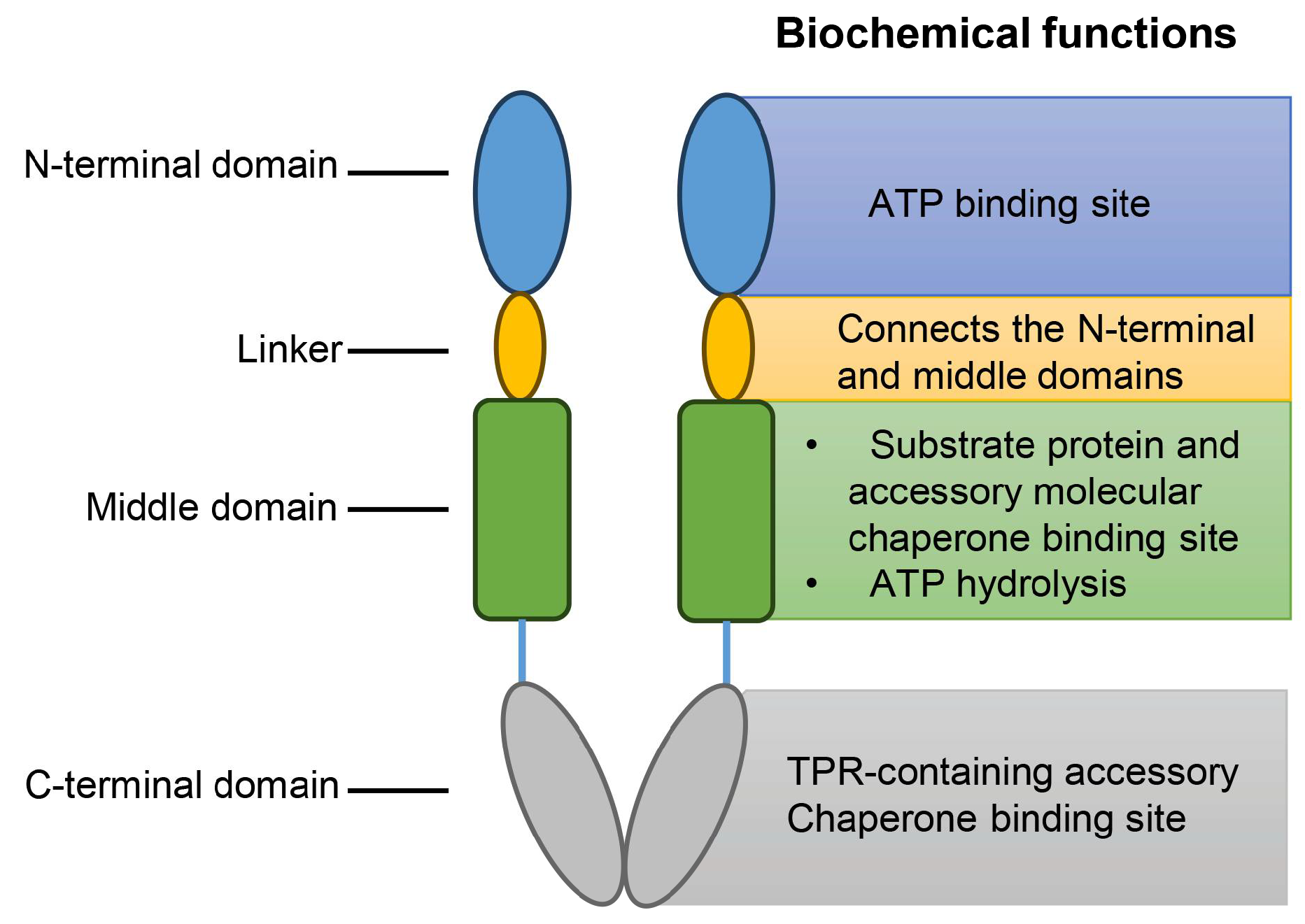
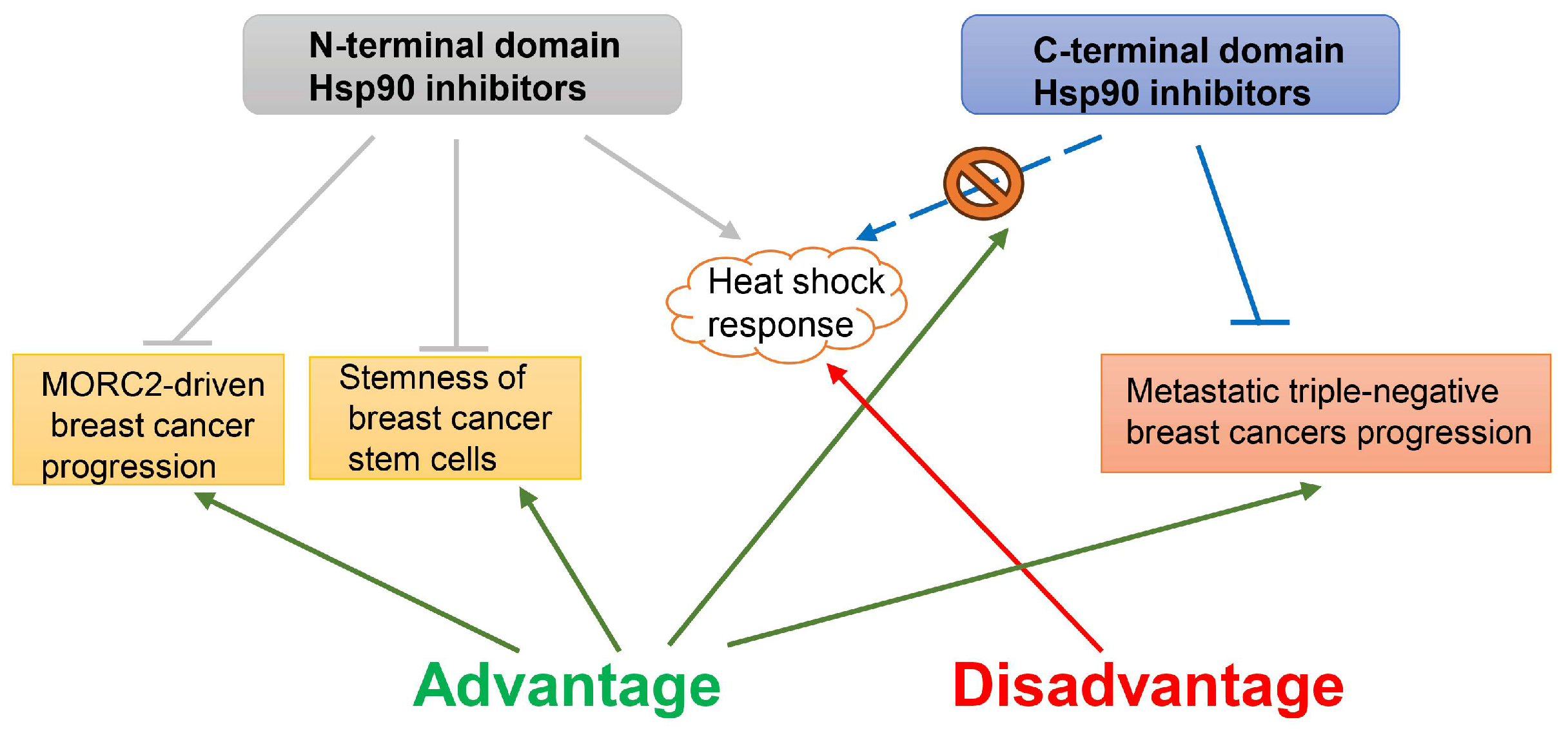
2.5. Hsp90
2.6. Hsp110
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldstein, M.G.; Li, Z. Heat-shock proteins in infection-mediated inflammation-induced tumorigenesis. J. Hematol. Oncol. 2009, 2, 5. [Google Scholar] [PubMed]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar]
- Tsan, M.F.; Gao, B. Heat shock protein and innate immunity. Cell Mol. Immunol. 2004, 1, 274–279. [Google Scholar] [PubMed]
- Janowska, M.K.; Baughman, H.E.R.; Woods, C.N.; Klevit, R.E. Mechanisms of Small Heat Shock Proteins. Cold Spring Harb. Perspect. Biol. 2019, 11, a034025. [Google Scholar]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress. Chaperones 2009, 14, 105–111. [Google Scholar] [PubMed]
- Li, J.; Song, P.; Jiang, T.; Dai, D.; Wang, H.; Sun, J.; Zhu, L.; Xu, W.; Feng, L.; Shin, V.Y.; et al. Heat Shock Factor 1 Epigenetically Stimulates Glutaminase-1-Dependent mTOR Activation to Promote Colorectal Carcinogenesis. Mol. Ther. 2018, 26, 1828–1839. [Google Scholar]
- Bascos, N.A.D.; Landry, S.J. A History of Molecular Chaperone Structures in the Protein Data Bank. Int. J. Mol. Sci. 2019, 20, 6195. [Google Scholar]
- Klimczak, M.; Biecek, P.; Zylicz, A.; Zylicz, M. Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 2019, 9, 7507. [Google Scholar]
- Das, J.K.; Xiong, X.; Ren, X.; Yang, J.M.; Song, J. Heat Shock Proteins in Cancer Immunotherapy. J. Oncol. 2019, 2019, 3267207. [Google Scholar]
- Singh, M.K.; Sharma, B.; Tiwari, P.K. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017, 69, 149–154. [Google Scholar]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair. 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, S.; Moens, U. Heat shock protein 27 phosphorylation: Kinases, phosphatases, functions and pathology. Cell Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [PubMed]
- Bi, X.; Xu, M.; Li, J.; Huang, T.; Jiang, B.; Shen, L.; Luo, L.; Liu, S.; Yin, Z. Heat shock protein 27 inhibits HMGB1 translocation by regulating CBP acetyltransferase activity and ubiquitination. Mol. Immunol. 2019, 108, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Gan, L.; Zhang, S.; Cui, F.J.; Cun, W.; Li, Y.; Kang, N.X.; Gao, M.D.; Liu, K.Y. Translocation of HSP27 into liver cancer cell nucleus may be associated with phosphorylation and O-GlcNAc glycosylation. Oncol. Rep. 2012, 28, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Wang, H.; Wang, Y.; Chen, P.; Wang, L. Heat Shock Protein 27 Enhances SUMOylation of Heat Shock Protein B8 to Accelerate the Progression of Breast Cancer. Am. J. Pathol. 2020, 190, 2464–2477. [Google Scholar] [PubMed]
- Vahid, S.; Thaper, D.; Gibson, K.F.; Bishop, J.L.; Zoubeidi, A. Molecular chaperone Hsp27 regulates the Hippo tumor suppressor pathway in cancer. Sci. Rep. 2016, 6, 31842. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011, 13, R101. [Google Scholar]
- de Azevedo-Santos, A.P.S.; Rocha, M.C.B.; Guimaraes, S.J.A.; Vale, A.A.M.; Laginha, F.M.; Nascimento, F.R.F.; Nagai, M.A.; Bergami-Santos, P.C.; Barbuto, J.A.M. Could Increased Expression of Hsp27, an “Anti-Inflammatory” Chaperone, Contribute to the Monocyte-Derived Dendritic Cell Bias towards Tolerance Induction in Breast Cancer Patients? Mediat. Inflamm. 2019, 2019, 8346930. [Google Scholar]
- Lee, C.H.; Wu, Y.T.; Hsieh, H.C.; Yu, Y.; Yu, A.L.; Chang, W.W. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie 2014, 104, 117–126. [Google Scholar]
- Zhang, D.; Wong, L.L.; Koay, E.S. Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status and lymph node positivity in breast cancer. Mol. Cancer 2007, 6, 52. [Google Scholar] [CrossRef]
- Liu, X.; Feng, C.; Liu, J.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Jiao, J.; Niu, Y. Androgen receptor and heat shock protein 27 co-regulate the malignant potential of molecular apocrine breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 90. [Google Scholar] [CrossRef]
- Netsirisawan, P.; Chaiyawat, P.; Chokchaichamnankit, D.; Lirdprapamongkol, K.; Srisomsap, C.; Svasti, J.; Champattanachai, V. Decreasing O-GlcNAcylation affects the malignant transformation of MCF-7 cells via Hsp27 expression and its O-GlcNAc modification. Oncol. Rep. 2018, 40, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.K.; Parra, I.; Lemieux, P.; Oesterreich, S.; Hilsenbeck, S.G.; Fuqua, S.A. Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 1999, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kang, K.W.; Kim, K.H.; Kwon, B.; Kim, S.K.; Lee, H.Y.; Kong, S.Y.; Lee, E.S.; Jang, S.G.; Yoo, B.C. Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer 2008, 8, 286. [Google Scholar] [CrossRef]
- Zhang, D.; Putti, T.C. Over-expression of ERp29 attenuates doxorubicin-induced cell apoptosis through up-regulation of Hsp27 in breast cancer cells. Exp. Cell Res. 2010, 316, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Wang, B.Y.; Chi, W.Y.; Chang-Chien, J.; Yang, J.J.; Lee, H.T.; Tzeng, Y.M.; Chang, W.W. Ovatodiolide Inhibits Breast Cancer Stem/Progenitor Cells through SMURF2-Mediated Downregulation of Hsp27. Toxins 2016, 8, 127. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Choi, S.K.; Seo, S.H.; Jo, H.; Shin, J.H.; Na, Y.; Lee, Y.S.; Kwon, Y. Specific Roles of HSP27 S15 Phosphorylation Augmenting the Nuclear Function of HER2 to Promote Trastuzumab Resistance. Cancers 2020, 12, 1540. [Google Scholar] [CrossRef]
- Tong, X.B.; Kita, K.; Chen, S.P.; Jiang, X.; Sugaya, S.; Jing, W.L.; Zhang, S.F.; Suzuki, N. Involvement of heat shock protein 27 in the susceptibility of KT human breast cancer cells to UVC and interferon lethality. Exp. Ther. Med. 2012, 4, 913–917. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, M.; Zhou, J.; Yan, X.; Cheng, L.; Luo, L.; Huang, C.; Yin, Z. Phosphorylated Hsp27 promotes adriamycin resistance in breast cancer cells through regulating dual phosphorylation of c-Myc. Cell Signal 2023, 112, 110913. [Google Scholar] [CrossRef]
- Cayado-Gutierrez, N.; Moncalero, V.L.; Rosales, E.M.; Beron, W.; Salvatierra, E.E.; Alvarez-Olmedo, D.; Radrizzani, M.; Ciocca, D.R. Downregulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress. Chaperones 2013, 18, 243–249. [Google Scholar] [CrossRef]
- Ma, W.; Teng, Y.; Hua, H.; Hou, J.; Luo, T.; Jiang, Y. Upregulation of heat shock protein 27 confers resistance to actinomycin D-induced apoptosis in cancer cells. FEBS J. 2013, 280, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Fong, P.C.; Yu, C.C.; Tsai, W.C.; Tzeng, Y.M.; Chang, W.W. Methyl Antcinate A suppresses the population of cancer stem-like cells in MCF7 human breast cancer cell line. Molecules 2013, 18, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef] [PubMed]
- Pulido, P.; Leister, D. Novel DNAJ-related proteins in Arabidopsis thaliana. New Phytol. 2018, 217, 480–490. [Google Scholar] [CrossRef]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.B.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 proteins use class-specific regulation to drive HSP70 functional diversity. Nature 2020, 587, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Fauvet, B.; Finka, A.; Castanie-Cornet, M.P.; Cirinesi, A.M.; Genevaux, P.; Quadroni, M.; Goloubinoff, P. Bacterial Hsp90 Facilitates the Degradation of Aggregation-Prone Hsp70-Hsp40 Substrates. Front. Mol. Biosci. 2021, 8, 653073. [Google Scholar] [CrossRef]
- Doyle, S.M.; Hoskins, J.R.; Kravats, A.N.; Heffner, A.L.; Garikapati, S.; Wickner, S. Intermolecular Interactions between Hsp90 and Hsp70. J. Mol. Biol. 2019, 431, 2729–2746. [Google Scholar] [CrossRef]
- De Bessa, S.A.; Salaorni, S.; Patrao, D.F.; Neto, M.M.; Brentani, M.M.; Nagai, M.A. JDP1 (DNAJC12/Hsp40) expression in breast cancer and its association with estrogen receptor status. Int. J. Mol. Med. 2006, 17, 363–367. [Google Scholar] [CrossRef]
- Kok, M.; Koornstra, R.H.; Margarido, T.C.; Fles, R.; Armstrong, N.J.; Linn, S.C.; Van’t Veer, L.J.; Weigelt, B. Mammosphere-derived gene set predicts outcome in patients with ER-positive breast cancer. J. Pathol. 2009, 218, 316–326. [Google Scholar] [CrossRef]
- Incekara, O.; Acun, T. DNAJC9 expression in basal-like and luminal A breast cancer subtypes predicts worse survival. Mol. Biol. Rep. 2023, 50, 7275–7282. [Google Scholar] [CrossRef]
- Furkan Celebi Ileri, T.A. High expression of DNAJA1 (HDJ2) predicts unfavorable survival outcomes in breast cancer. Biomark. Med. 2021, 15, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Mo, L.; Pan, X.; Yang, Z.; Huang, H.; Zhu, L.; Wang, Y.; Jiang, G. DNAJB4 promotes triple-negative breast cancer cell apoptosis via activation of the Hippo signaling pathway. Discov. Oncol. 2023, 14, 40. [Google Scholar] [CrossRef]
- Mitra, A.; Fillmore, R.A.; Metge, B.J.; Rajesh, M.; Xi, Y.; King, J.; Ju, J.; Pannell, L.; Shevde, L.A.; Samant, R.S. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. 2008, 10, R22. [Google Scholar] [CrossRef] [PubMed]
- Acun, T.; Senses, K.M. Downregulation of DNAJC10 (ERDJ5) is associated with poor survival in breast cancer. Breast Cancer 2020, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Hayashi, M.; Lo, J.F.; Fearns, C.; Xiang, R.; Lazennec, G.; Yang, Y.; Lee, J.D. Tid1 negatively regulates the migratory potential of cancer cells by inhibiting the production of interleukin-8. Cancer Res. 2005, 65, 8784–8791. [Google Scholar] [CrossRef]
- Trinh, D.L.; Elwi, A.N.; Kim, S.W. Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis. Oncotarget 2010, 1, 396–404. [Google Scholar] [CrossRef]
- Menezes, M.E.; Mitra, A.; Shevde, L.A.; Samant, R.S. DNAJB6 governs a novel regulatory loop determining Wnt/beta-catenin signalling activity. Biochem. J. 2012, 444, 573–580. [Google Scholar] [CrossRef]
- Cui, X.; Choi, H.K.; Choi, Y.S.; Park, S.Y.; Sung, G.J.; Lee, Y.H.; Lee, J.; Jun, W.J.; Kim, K.; Choi, K.C.; et al. DNAJB1 destabilizes PDCD5 to suppress p53-mediated apoptosis. Cancer Lett. 2015, 357, 307–315. [Google Scholar] [CrossRef]
- Nishizawa, S.; Hirohashi, Y.; Torigoe, T.; Takahashi, A.; Tamura, Y.; Mori, T.; Kanaseki, T.; Kamiguchi, K.; Asanuma, H.; Morita, R.; et al. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012, 72, 2844–2854. [Google Scholar] [CrossRef]
- Izbicka, E.; Campos, D.; Carrizales, G.; Patnaik, A. Biomarkers of anticancer activity of R115777 (Tipifarnib, Zarnestra) in human breast cancer models in vitro. Anticancer Res. 2005, 25, 3215–3223. [Google Scholar]
- Tang, Y.; Zhou, Y.; Fan, S.; Wen, Q. The multiple roles and therapeutic potential of HSP60 in cancer. Biochem. Pharmacol. 2022, 201, 115096. [Google Scholar] [CrossRef] [PubMed]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.K.; Singh, A.; Yadav, N.K.; Cheruvu, S.H.; Hossain, Z.; Meena, S.; Maheshwari, S.; Singh, A.K.; Shahab, U.; Sharma, C.; et al. Anti-breast tumor activity of Eclipta extract in-vitro and in-vivo: Novel evidence of endoplasmic reticulum specific localization of Hsp60 during apoptosis. Sci. Rep. 2015, 5, 18457. [Google Scholar] [CrossRef] [PubMed]
- Desmetz, C.; Bibeau, F.; Boissiere, F.; Bellet, V.; Rouanet, P.; Maudelonde, T.; Mange, A.; Solassol, J. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J. Proteome Res. 2008, 7, 3830–3837. [Google Scholar] [CrossRef]
- Ghosh, J.C.; Dohi, T.; Kang, B.H.; Altieri, D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008, 283, 5188–5194. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Marino Gammazza, A.; Palumbo Piccionello, A.; Campanella, C.; Pace, A.; Conway de Macario, E.; Macario, A.J. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert. Opin. Ther. Targets 2014, 18, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, X.; Yan, C.; Zheng, S.; Gao, R.; Huang, F.; Wei, Y.; Wen, Z.; Chen, Y.; Zhou, X.; et al. Tumor cell-released LC3-positive EVs promote lung metastasis of breast cancer through enhancing premetastatic niche formation. Cancer Sci. 2022, 113, 3405–3416. [Google Scholar] [CrossRef]
- Li, D.Q.; Wang, L.; Fei, F.; Hou, Y.F.; Luo, J.M.; Wei, C.; Zeng, R.; Wu, J.; Lu, J.S.; Di, G.H.; et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics 2006, 6, 3352–3368. [Google Scholar] [CrossRef]
- Chalmers, S.A.; Eidelman, A.S.; Ewer, J.C.; Ricca, J.M.; Serrano, A.; Tucker, K.C.; Vail, C.M.; Kurt, R.A. A role for HMGB1, HSP60 and Myd88 in growth of murine mammary carcinoma in vitro. Cell Immunol. 2013, 282, 136–145. [Google Scholar] [CrossRef]
- Ghosh, J.C.; Siegelin, M.D.; Dohi, T.; Altieri, D.C. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010, 70, 8988–8993. [Google Scholar] [CrossRef]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef]
- Kravats, A.N.; Doyle, S.M.; Hoskins, J.R.; Genest, O.; Doody, E.; Wickner, S. Interaction of E. coli Hsp90 with DnaK Involves the DnaJ Binding Region of DnaK. J. Mol. Biol. 2017, 429, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Sarbeng, E.B.; Liu, Q.; Le, K.Q.; Xu, X.; Xu, H.; Yang, J.; Wong, J.L.; Vorvis, C.; Hendrickson, W.A.; et al. Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nat. Struct. Mol. Biol. 2013, 20, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, N.; Agarwal, S.; Gupta, N.; Fatima, R.; Devi, S.; Kumar, V.; Suri, V.; Kumar, R.; Suri, V.; Sadasukhi, T.C.; et al. Heat shock protein 70-2 (HSP70-2) overexpression in breast cancer. J. Exp. Clin. Cancer Res. 2016, 35, 150. [Google Scholar] [CrossRef]
- Orfanelli, T.; Giannopoulos, S.; Zografos, E.; Athanasiou, A.; Bongiovanni, A.M.; Doulaveris, G.; Moo, T.A.; LaPolla, D.; Bakoyiannis, C.N.; Theodoropoulos, G.E.; et al. Alterations of the 70 kDa heat shock protein (HSP70) and sequestosome-1 (p62) in women with breast cancer. Sci. Rep. 2021, 11, 22220. [Google Scholar] [CrossRef] [PubMed]
- Nadin, S.B.; Sottile, M.L.; Montt-Guevara, M.M.; Gauna, G.V.; Daguerre, P.; Leuzzi, M.; Gago, F.E.; Ibarra, J.; Cuello-Carrion, F.D.; Ciocca, D.R.; et al. Prognostic implication of HSPA (HSP70) in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Cell Stress. Chaperones 2014, 19, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Z.; Zhu, S.; Sun, W.; Wang, X.; Tan, C.; Zhang, Y.; Zhang, G.; Xu, Y.; Tang, J. Small extracellular vesicle-mediated Hsp70 intercellular delivery enhances breast cancer adriamycin resistance. Free Radic. Biol. Med. 2021, 164, 85–95. [Google Scholar] [CrossRef]
- Bausero, M.A.; Gastpar, R.; Multhoff, G.; Asea, A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J. Immunol. 2005, 175, 2900–2912. [Google Scholar] [CrossRef]
- Peterko, A.C.; Rajković-Molek, K.; Gulić, T.; Vujaklija, D.V.; Lovasić, I.B.; Lovasić, F.; Mustać, E.; Avirović, M. HSP70 In triple negative breast cancer: Prognostic value and clinical significance. Pathol. Res. Pract. 2022, 238, 154127. [Google Scholar] [CrossRef]
- Yamaguchi-Tanaka, M.; Takagi, K.; Miki, Y.; Sato, A.; Iwabuchi, E.; Miyashita, M.; Suzuki, T. The Pro-Tumorigenic Role of Chemotherapy-Induced Extracellular HSP70 from Breast Cancer Cells via Intratumoral Macrophages. Cancers 2023, 15, 1903. [Google Scholar] [CrossRef]
- Rothammer, A.; Sage, E.K.; Werner, C.; Combs, S.E.; Multhoff, G. Increased heat shock protein 70 (Hsp70) serum levels and low NK cell counts after radiotherapy—Potential markers for predicting breast cancer recurrence? Radiat. Oncol. 2019, 14, 78. [Google Scholar] [CrossRef]
- Mawatari, T.; Ninomiya, I.; Inokuchi, M.; Harada, S.; Hayashi, H.; Oyama, K.; Makino, I.; Nakagawara, H.; Miyashita, T.; Tajima, H.; et al. Valproic acid inhibits proliferation of HER2-expressing breast cancer cells by inducing cell cycle arrest and apoptosis through Hsp70 acetylation. Int. J. Oncol. 2015, 47, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Nylandsted, J.; Rohde, M.; Brand, K.; Bastholm, L.; Elling, F.; Jaattela, M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA 2000, 97, 7871–7876. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Kim, S.A.; Choi, H.S.; Yoon, J.H.; Ahn, S.G. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer 2010, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Sulphoraphane, a naturally occurring isothiocyanate induces apoptosis in breast cancer cells by targeting heat shock proteins. Biochem. Biophys. Res. Commun. 2012, 427, 80–85. [Google Scholar] [CrossRef]
- Fani, S.; Dehghan, F.; Karimian, H.; Mun Lo, K.; Ebrahimi Nigjeh, S.; Swee Keong, Y.; Soori, R.; May Chow, K.; Kamalidehghan, B.; Mohd Ali, H.; et al. Monobenzyltin Complex C1 Induces Apoptosis in MCF-7 Breast Cancer Cells through the Intrinsic Signaling Pathway and through the Targeting of MCF-7-Derived Breast Cancer Stem Cells via the Wnt/beta-Catenin Signaling Pathway. PLoS ONE 2016, 11, e0160836. [Google Scholar] [CrossRef][Green Version]
- Mostafavinia, S.E.; Khorashadizadeh, M.; Hoshyar, R. Antiproliferative and Proapoptotic Effects of Crocin Combined with Hyperthermia on Human Breast Cancer Cells. DNA Cell Biol. 2016, 35, 340–347. [Google Scholar] [CrossRef]
- Kodiha, M.; Mahboubi, H.; Maysinger, D.; Stochaj, U. Gold Nanoparticles Impinge on Nucleoli and the Stress Response in MCF7 Breast Cancer Cells. Nanobiomedicine 2016, 3, 3. [Google Scholar] [CrossRef]
- Khan, G.N.; Kim, E.J.; Shin, T.S.; Lee, S.H. Azacytidine-induced Chemosensitivity to Doxorubicin in Human Breast Cancer MCF7 Cells. Anticancer. Res. 2017, 37, 2355–2364. [Google Scholar] [CrossRef]
- Sims, J.D.; McCready, J.; Jay, D.G. Extracellular heat shock protein (Hsp)70 and Hsp90alpha assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS ONE 2011, 6, e18848. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, H.; Zhang, X.; Li, H. Visualizing and Quantifying the Effect of the Inhibition of HSP70 on Breast Cancer Cells Based on Laser Scanning Microscopy. Technol. Cancer Res. Treat. 2018, 17, 1533033818785274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, R.; Zhang, T.; Li, S.; Zhong, W. Design and synthesis of piperidine derivatives as novel human heat shock protein 70 inhibitors for the treatment of drug-resistant tumors. Eur. J. Med. Chem. 2015, 97, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.Q.; Cao, R.Y.; Yang, J.L.; Li, X.Z.; Li, S.; Zhong, W. Design, synthesis and biological evaluation of novel HSP70 inhibitors: N, N’-disubstituted thiourea derivatives. Eur. J. Med. Chem. 2016, 119, 83–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sannino, S.; Yates, M.E.; Schurdak, M.E.; Oesterreich, S.; Lee, A.V.; Wipf, P.; Brodsky, J.L. Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised. eLife 2021, 10, e64977. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an allosteric small-molecule inhibitor selective for the inducible form of heat shock protein 70. Chem. Biol. 2014, 21, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Cutress, R.I.; Johnson, P.W.; Packham, G.; Townsend, P.A. Short peptides derived from the BAG-1 C-terminus inhibit the interaction between BAG-1 and HSC70 and decrease breast cancer cell growth. FEBS Lett. 2009, 583, 3405–3411. [Google Scholar] [CrossRef]
- Yaglom, J.A.; Wang, Y.; Li, A.; Li, Z.; Monti, S.; Alexandrov, I.; Lu, X.; Sherman, M.Y. Cancer cell responses to Hsp70 inhibitor JG-98: Comparison with Hsp90 inhibitors and finding synergistic drug combinations. Sci. Rep. 2018, 8, 3010. [Google Scholar] [CrossRef]
- Shao, H.; Li, X.; Moses, M.A.; Gilbert, L.A.; Kalyanaraman, C.; Young, Z.T.; Chernova, M.; Journey, S.N.; Weissman, J.S.; Hann, B.; et al. Exploration of Benzothiazole Rhodacyanines as Allosteric Inhibitors of Protein-Protein Interactions with Heat Shock Protein 70 (Hsp70). J. Med. Chem. 2018, 61, 6163–6177. [Google Scholar] [CrossRef]
- Elnatan, D.; Betegon, M.; Liu, Y.; Ramelot, T.; Kennedy, M.A.; Agard, D.A. Symmetry broken and rebroken during the ATP hydrolysis cycle of the mitochondrial Hsp90 TRAP1. eLife 2017, 6, e25235. [Google Scholar] [CrossRef]
- Lopez, A.; Dahiya, V.; Delhommel, F.; Freiburger, L.; Stehle, R.; Asami, S.; Rutz, D.; Blair, L.; Buchner, J.; Sattler, M. Client binding shifts the populations of dynamic Hsp90 conformations through an allosteric network. Sci. Adv. 2021, 7, eabl7295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, C.; Zhu, H.; Liu, Z.; Su, H.; Zhang, X.; Chen, T.; Zhong, Y.; Hu, H.; Xiong, M.; et al. Allosteric Regulation of Hsp90alpha’s Activity by Small Molecules Targeting the Middle Domain of the Chaperone. iScience 2020, 23, 100857. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.A.; Woodford, M.R.; Backe, S.J.; Makedon, A.M.; Baker-Williams, A.J.; DiGregorio, B.T.; Loiselle, D.R.; Haystead, T.A.; Zachara, N.E.; Prodromou, C.; et al. Post-translational Regulation of FNIP1 Creates a Rheostat for the Molecular Chaperone Hsp90. Cell Rep. 2019, 26, 1344–1356.e5. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Lagadari, M.; Toneatto, J.; Piwien-Pilipuk, G.; Galigniana, M.D. Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim. Biophys. Acta 2014, 1839, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Kluger, Y.; Giltnane, J.M.; Moeder, C.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007, 67, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, S.A.; Toss, M.; Alsaleem, M.; Aleskandarany, M.; Joseph, C.; Kurozumi, S.; Ball, G.; Mongan, N.; Green, A.; Rakha, E. Prognostic significance of heat shock protein 90AA1 (HSP90α) in invasive breast cancer. J. Clin. Pathol. 2022, 75, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.Y.; Lin, H.Y.; Jan, H.M.; Chen, Y.J.; Ong, L.L.; Yu, A.L.; Lin, C.H. S-glutathionylation of Hsp90 enhances its degradation and correlates with favorable prognosis of breast cancer. Redox Biol. 2022, 57, 102501. [Google Scholar] [CrossRef]
- Tang, W.; Wu, Y.; Qi, X.; Yu, R.; Lu, Z.; Chen, A.; Fan, X.; Li, J. PGK1-coupled HSP90 stabilizes GSK3beta expression to regulate the stemness of breast cancer stem cells. Cancer Biol. Med. 2021, 19, 486–503. [Google Scholar] [CrossRef]
- Kotwal, A.; Suran, S.; Amere Subbarao, S. Hsp90 chaperone facilitates E2F1/2-dependent gene transcription in human breast cancer cells. Eur. J. Cell Biol. 2021, 100, 151148. [Google Scholar] [CrossRef]
- Alagundagi, D.B.; Ghate, S.D.; Rajendra, V.K.J.; Gollapalli, P.; Shetty, V.V.; D’Souza, C.; Shetty, P.; Patil, P. Exploring breast cancer exosomes for novel biomarkers of potential diagnostic and prognostic importance. 3 Biotech. 2023, 13, 7. [Google Scholar] [CrossRef]
- Caldas-Lopes, E.; Cerchietti, L.; Ahn, J.H.; Clement, C.C.; Robles, A.I.; Rodina, A.; Moulick, K.; Taldone, T.; Gozman, A.; Guo, Y.; et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl. Acad. Sci. USA 2009, 106, 8368–8373. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Wang, Q.; Li, Y.; Fu, L.; Fu, H.; Zhu, J.; Chen, Z.; Zhu, W.; Xie, C.; et al. DCZ3112, a novel Hsp90 inhibitor, exerts potent antitumor activity against HER2-positive breast cancer through disruption of Hsp90-Cdc37 interaction. Cancer Lett. 2018, 434, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cai, X.; Wu, C.; Liu, Y.; Zhang, J.; Gong, X.; Wang, X.; Wu, X.; Zhu, T.; Mo, L.; et al. Targeting HSP90-HDAC6 Regulating Network Implicates Precision Treatment of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sun, R.; Hou, Z.; Zhang, F.L.; Xiao, Y.; Yang, Y.S.; Yang, S.Y.; Xie, Y.F.; Liu, Y.Y.; Luo, C.; et al. HSP90 N-terminal inhibitors target oncoprotein MORC2 for autophagic degradation and suppress MORC2-driven breast cancer progression. Clin. Transl. Med. 2022, 12, e825. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Carnero, A.; Paz-Ares, L. Inhibition of HSP90 molecular chaperones: Moving into the clinic. Lancet Oncol. 2013, 14, e358–e369. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, T.M.; Park, J.M.; Park, S.; Park, M.; Nam, K.D.; Ko, D.; Seo, J.; Kim, S.; Jung, E.; et al. A novel HSP90 inhibitor SL-145 suppresses metastatic triple-negative breast cancer without triggering the heat shock response. Oncogene 2022, 41, 3289–3297. [Google Scholar] [CrossRef]
- Oh, H.J.; Easton, D.; Murawski, M.; Kaneko, Y.; Subjeck, J.R. The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J. Biol. Chem. 1999, 274, 15712–15718. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, X.; Oh, H.J.; Repasky, E.; Kazim, L.; Subjeck, J. Characterization of native interaction of hsp110 with hsp25 and hsc70. FEBS Lett. 2000, 465, 98–102. [Google Scholar] [CrossRef]
- Shorter, J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 2011, 6, e26319. [Google Scholar] [CrossRef]
- Polier, S.; Dragovic, Z.; Hartl, F.U.; Bracher, A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 2008, 133, 1068–1079. [Google Scholar] [CrossRef]
- Ishihara, K.; Yamagishi, N.; Hatayama, T. Protein kinase CK2 phosphorylates Hsp105 alpha at Ser509 and modulates its function. Biochem. J. 2003, 371 Pt 3, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Kakunda, M.; Pham, V.; Lill, J.R.; Du, P.; Wongchenko, M.; Yan, Y.; Firestein, R.; Huang, X. HSP105 recruits protein phosphatase 2A to dephosphorylate beta-catenin. Mol. Cell Biol. 2015, 35, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Manjili, M.H.; Park, J.; Facciponte, J.G.; Subjeck, J.R. HSP110 induces “danger signals” upon interaction with antigen presenting cells and mouse mammary carcinoma. Immunobiology 2005, 210, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Pabla, S.; Seager, R.J.; Van Roey, E.; Gao, S.; Hoefer, C.; Nesline, M.K.; DePietro, P.; Burgher, B.; Andreas, J.; Giamo, V.; et al. Integration of tumor inflammation, cell proliferation, and traditional biomarkers improves prediction of immunotherapy resistance and response. Biomark. Res. 2021, 9, 56. [Google Scholar] [CrossRef]
- Manjili, M.H.; Wang, X.Y.; Chen, X.; Martin, T.; Repasky, E.A.; Henderson, R.; Subjeck, J.R. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J. Immunol. 2003, 171, 4054–4061. [Google Scholar] [CrossRef]


| Hsp Family | Gene | Normal Type | BRCA Type | Hsp Family | Gene | Normal Type | BRCA Type |
|---|---|---|---|---|---|---|---|
| (FPKM) | (FPKM) | (FPKM) | (FPKM) | ||||
| Hsp27 | HSPB1 | 117.625 | 352.549 | Hsp40 | DNAJC17 | 2.77648 | 3.03727 |
| HSPB2 | 4.17935 | 0.795792 | DNAJC18 | 2.74528 | 1.37358 | ||
| Hsp40 | DNAJA1 | 60.6343 | 89.2385 | DNAJC19 | 7.09079 | 10.2867 | |
| DNAJA2 | 17.6651 | 16.4556 | DNAJC20 | 7.39097 | 7.49364 | ||
| DNAJA3 | 6.61321 | 11.5337 | DNAJC21 | 6.50134 | 7.68681 | ||
| DNAJA4 | 6.20316 | 12.74 | DNAJC22 | 1.48905 | 3.92159 | ||
| DNAJB1 | 51.4863 | 49.5621 | DNAJC23 | 17.9754 | 18.5166 | ||
| DNAJB2 | 14.7851 | 18.5249 | DNAJC24 | 1.57841 | 1.38519 | ||
| DNAJB3 | 0.00184269 | 0.00143597 | DNAJC25 | 4.83016 | 4.64115 | ||
| DNAJB4 | 13.8392 | 5.21584 | DNAJC26 | 4.61191 | 6.98525 | ||
| DNAJB5 | 1.98587 | 1.57866 | DNAJC27 | 2.26955 | 1.39313 | ||
| DNAJB6 | 7.20102 | 8.04553 | DNAJC28 | 0.858132 | 0.706425 | ||
| DNAJB7 | 0.0585917 | 0.056792 | DNAJC29 | 3.08858 | 1.35963 | ||
| DNAJB8 | 0.00133167 | 0.0017791 | DNAJC30 | 5.94151 | 6.78276 | ||
| DNAJB9 | 14.9374 | 14.5789 | Hsp60 | HSPD1 | 60.7563 | 93.6693 | |
| DNAJB11 | 7.70387 | 13.5074 | Hsp70 | HSPA1A | 36.4994 | 54.2845 | |
| DNAJB12 | 11.4622 | 12.1393 | HSPA1B | 39.2733 | 48.0456 | ||
| DNAJB13 | 0.0583365 | 0.116631 | HSPA1L | 1.29012 | 1.49757 | ||
| DNAJB14 | 3.77468 | 3.37966 | HSPA2 | 5.03015 | 9.5758 | ||
| DNAJC1 | 13.7391 | 39.0695 | HSPA4 | 25.4501 | 38.5318 | ||
| DNAJC2 | 4.11542 | 5.8259 | HSPA4L | 1.85338 | 1.29703 | ||
| DNAJC3 | 20.8626 | 21.6129 | HSPA5 | 123.308 | 210.117 | ||
| DNAJC4 | 10.4769 | 12.6855 | HSPA6 | 1.27087 | 3.92941 | ||
| DNAJC5 | 16.7853 | 23.3509 | HSPA7 | 2.43346 | 3.12447 | ||
| DNAJC5B | 0.186127 | 0.430458 | HSPA8 | 189.73 | 263.496 | ||
| DNAJC5G | 0.0200045 | 0.0112469 | HSPA9 | 53.5377 | 74.6974 | ||
| DNAJC6 | 0.5795 | 0.945219 | HSPA12A | 6.20892 | 1.81148 | ||
| DNAJC7 | 6.90192 | 8.76527 | HSPA12B | 8.11172 | 3.32468 | ||
| DNAJC8 | 32.1043 | 30.8205 | HSPA13 | 8.29694 | 11.8282 | ||
| DNAJC9 | 3.19184 | 5.79583 | HSPA14 | 4.70054 | 7.35153 | ||
| DNAJC10 | 5.90804 | 7.9543 | Hsp90 | HSP90AA1 | 222.658 | 304.964 | |
| DNAJC11 | 6.00964 | 6.45572 | HSP90AB1 | 416.171 | 611.813 | ||
| DNAJC12 | 5.79399 | 23.0184 | HSP90B1 | 151.788 | 202.219 | ||
| DNAJC13 | 8.89732 | 8.54604 | HSP90B2P | 0.120954 | 0.119044 | ||
| DNAJC14 | 5.97507 | 7.31624 | HSP90L | 10.2401 | 14.1013 | ||
| DNAJC15 | 5.41503 | 5.17456 | Hsp110 | HSPH1 | 12.0038 | 21.2046 | |
| DNAJC16 | 4.415 | 4.81457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Bi, X. Heat Shock Proteins and Breast Cancer. Int. J. Mol. Sci. 2024, 25, 876. https://doi.org/10.3390/ijms25020876
Zhang M, Bi X. Heat Shock Proteins and Breast Cancer. International Journal of Molecular Sciences. 2024; 25(2):876. https://doi.org/10.3390/ijms25020876
Chicago/Turabian StyleZhang, Miao, and Xiaowen Bi. 2024. "Heat Shock Proteins and Breast Cancer" International Journal of Molecular Sciences 25, no. 2: 876. https://doi.org/10.3390/ijms25020876
APA StyleZhang, M., & Bi, X. (2024). Heat Shock Proteins and Breast Cancer. International Journal of Molecular Sciences, 25(2), 876. https://doi.org/10.3390/ijms25020876







