Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds
Abstract
1. Introduction
2. Results
2.1. Generation and Characterization of NF
2.2. Repeated In Vivo Microscopy of Healing Wounds
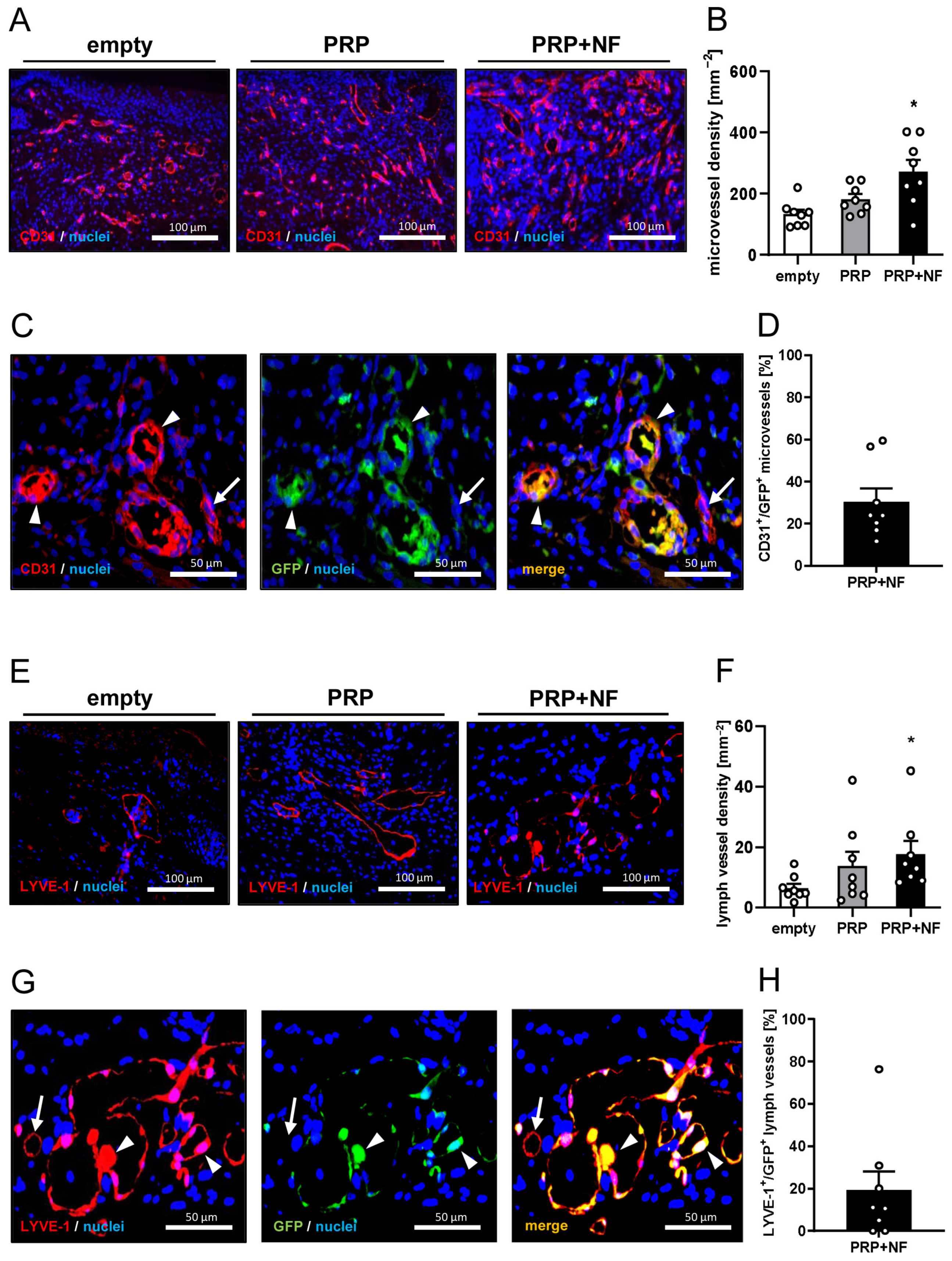
2.3. Histological and Immunohistochemical Analyses of Wounds
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Anesthesia
4.3. Generation of PRP
4.4. Generation of NF
4.5. Dorsal Skinfold Chamber Model
4.6. Stereomicroscopy
4.7. Intravital Fluorescence Microscopy
4.8. Histology and Immunohistochemistry
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 1170, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar]
- Mieczkowski, M.; Mrozikiewicz-Rakowska, B.; Kowara, M.; Kleibert, M.; Czupryniak, L. The Problem of Wound Healing in Diabetes—From Molecular Pathways to the Design of an Animal Model. Int. J. Mol. Sci. 2022, 23, 7930. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef]
- Nepal, S.; Venkataram, A.; Mysore, V. The Role of Adipose Tissue in Hair Regeneration: A Potential Tool for Management? J. Cutan. Aesthet. Surg. 2021, 14, 295–304. [Google Scholar]
- Abouzaid, A.M.; El Mokadem, M.E.; Aboubakr, A.K.; Kassem, M.A.; Al Shora, A.K.; Solaiman, A. Effect of autologous fat transfer in acute burn wound management: A randomized controlled study. Burns 2022, 48, 1368–1385. [Google Scholar] [CrossRef]
- La Padula, S.; Ponzo, M.; Lombardi, M.; Iazzetta, V.; Errico, C.; Polverino, G.; Russo, F.; D’Andrea, L.; Hersant, B.; Meningaud, J.P.; et al. Nanofat in Plastic Reconstructive, Regenerative, and Aesthetic Surgery: A Review of Advancements in Face-Focused Applications. J. Clin. Med. 2023, 12, 4351. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Osinga, R.; Menzi, N.R.; Tchang, L.A.H.; Caviezel, D.; Kalbermatten, D.F.; Martin, I.; Schaefer, D.J.; Scherberich, A.; Largo, R.D. Effects of intersyringe processing on adipose tissue and its cellular components: Implications in autologous fat grafting. Plast. Reconstr. Surg. 2015, 135, 1618–1628. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Huang, H.; Wang, Z.; Xu, P.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice. Aesthet. Surg. J. 2018, 38, 667–675. [Google Scholar] [CrossRef]
- Frueh, F.S.; Später, T.; Lindenblatt, N.; Calcagni, M.; Giovanoli, P.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Adipose Tissue-Derived Microvascular Fragments Improve Vascularization, Lymphangiogenesis, and Integration of Dermal Skin Substitutes. J. Investig. Dermatol. 2017, 137, 217–227. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Sharma, S.; Ganta, C.; Ranjan, R.; Jha, S.K. Nanofat: A therapeutic paradigm in regenerative medicine. World J. Stem Cells 2021, 13, 1733–1746. [Google Scholar] [CrossRef]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Boosting Tissue Vascularization: Nanofat as a Potential Source of Functional Microvessel Segments. Front. Bioeng. Biotechnol. 2022, 10, 820835. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef]
- Park, G.T.; Lim, J.K.; Choi, E.B.; Lim, M.J.; Yun, B.Y.; Kim, D.K.; Yoon, J.W.; Hong, Y.G.; Chang, J.H.; Bae, S.H.; et al. Transplantation of adipose tissue-derived microvascular fragments promotes therapy of critical limb ischemia. Biomater. Res. 2023, 27, 70. [Google Scholar] [CrossRef] [PubMed]
- Uyulmaz, S.; Macedo, N.S.; Rezaeian, F.; Giovanoli, P.; Lindenblatt, N. Nanofat grafting for scar treatment and skin quality improvement. Aesthet. Surg. J. 2018, 38, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Jiao, H.; Fan, J.; Liu, L.; Tian, J.; Gan, C.; Yang, Z.; Zhang, T.; Zeng, Y.; Su, Z. Nanofat Injection for the Treatment of Depressed Facial Scars. Aesthetic. Plast. Surg. 2021, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, Y.S.; Porwal, R.; Dhaker, H.O.; Arif, M.; Shekhawat, T. Nano fat injection for rejuvenation of scars: A prospective observational study. Eur. J. Mol. Clin. Med. 2022, 9, 2294–2301. [Google Scholar]
- Nalbach, L.; Roma, L.P.; Schmitt, B.M.; Becker, V.; Körbel, C.; Wrublewsky, S.; Pack, M.; Später, T.; Metzger, W.; Menger, M.M.; et al. Improvement of islet transplantation by the fusion of islet cells with functional blood vessels. EMBO Mol. Med. 2021, 13, e12616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.C.; Ma, J.J.; Sun, W.J.; Wang, S.W.; Gu, Z.C.; Yang, X. Autologous nanofat transplantation accelerates foot wound healing in diabetic rats. Regen. Med. 2019, 14, 231–241. [Google Scholar] [CrossRef]
- Perez, A.G.; Rodrigues, A.A.; Luzo, A.C.; Lana, J.F.; Belangero, W.D.; Santana, M.H. Fibrin network architectures in pure platelet-rich plasma as characterized by fiber radius and correlated with clotting time. J. Mater. Sci. Mater. Med. 2014, 25, 1967–1977. [Google Scholar] [CrossRef]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. 2018, 59, 265–275. [Google Scholar] [CrossRef]
- Limido, E.; Weinzierl, A.; Harder, Y.; Menger, M.D.; Laschke, M.W. Fatter Is Better: Boosting the Vascularization of Adipose Tissue Grafts. Tissue Eng. Part. B Rev. 2023, 29, 605–622. [Google Scholar] [CrossRef]
- Laschke, M.W.; Später, T.; Menger, M.D. Microvascular Fragments: More Than Just Natural Vascularization Units. Trends Biotechnol. 2021, 39, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Corral, I.; Olmeda, D.; Diéguez-Hurtado, R.; Tammela, T.; Alitalo, K.; Ortega, S. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 6223–6228. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Q.; Yu, Z.; Karvar, M.; Aoki, S.; Hamaguchi, R.; Ma, C.; Orgill, D.P.; Panayi, A.C. Negative-Pressure Wound Therapy Induces Lymphangiogenesis in Murine Diabetic Wound Healing. Plast. Reconstr. Surg. 2023, 151, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Guerret, S.; Govignon, E.; Hartmann, D.J.; Ronfard, V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf) grafted onto nude mice: Immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen. 2003, 11, 35–45. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.O. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019, 2019, 7921760. [Google Scholar] [CrossRef]
- Sorg, H.; Grambow, E.; Eckl, E.; Vollmar, B. Oxytocin effects on experimental skin wound healing. Innov. Surg. Sci. 2017, 2, 219–232. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. The dorsal skinfold chamber: A versatile tool for preclinical research in tissue engineering and regenerative medicine. Eur. Cells Mater. 2016, 32, 202–215. [Google Scholar] [CrossRef]


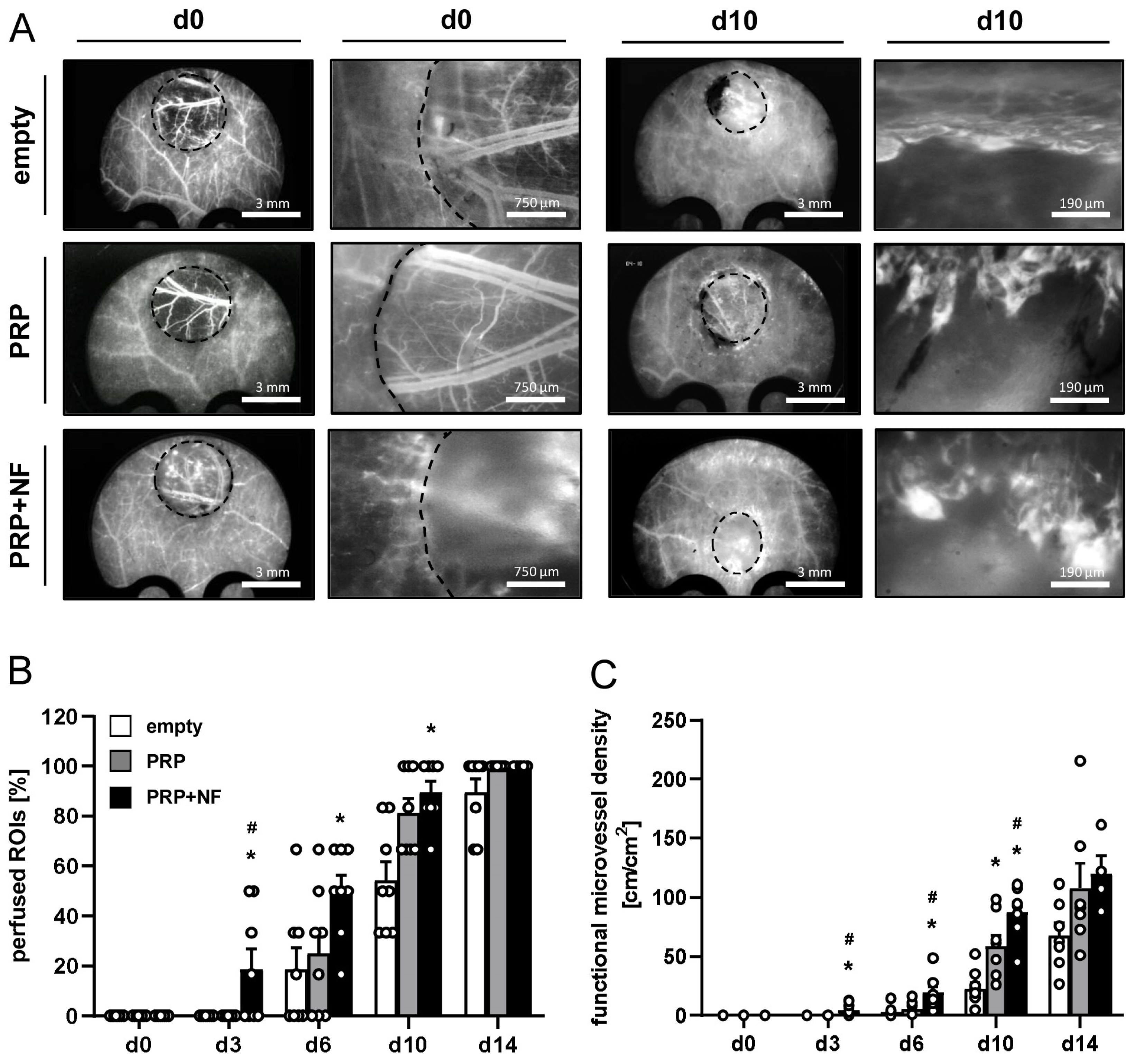


| d0 | d3 | d6 | d10 | d14 | |
|---|---|---|---|---|---|
| Diameter (µm): | |||||
| empty | - | - | 24.1 ± 2.2 | 17.2 ± 0.6 | 14.3 ± 0.4 |
| PRP | - | - | 23.2 ± 1.1 | 19.6 ± 1.1 | 15.4 ± 0.8 |
| PRP + NF | - | 17.6 ± 0.6 | 17.9 ± 0.5 * | 17.6 ± 0.6 | 15.6 ± 0.6 |
| Centerline RBC velocity (µm/s): | |||||
| empty | - | - | 201.0 ± 18.1 | 189.5 ± 20.5 | 207.7 ± 22.8 |
| PRP | - | - | 104.8 ± 17.9 * | 213.4 ± 27.7 | 227.7 ± 34.0 |
| PRP + NF | - | 203.6 ± 4.9 | 226.9 ± 13.2 # | 216.5 ± 12.4 | 223.6 ± 13.6 |
| Shear rate (s−1): | |||||
| empty | - | - | 74.5 ± 8.1 | 92.3 ± 11.4 | 121.9 ± 15.0 |
| PRP | - | - | 51.7 ± 16.3 | 95.3 ± 13.4 | 125.4 ± 19.4 |
| PRP + NF | - | 101.5 ± 7.7 | 110.7 ± 9.2 # | 101.4 ± 6.8 | 102.0 ± 9.9 |
| Volumetric blood flow (pL/s): | |||||
| empty | - | - | 58.9 ± 11.8 | 28.1 ± 3.3 | 20.7 ± 1.9 |
| PRP | - | - | 36.6 ± 7.1 | 39.3 ± 5.1 | 27.6 ± 5.3 |
| PRP + NF | - | 29.8 ± 3.4 | 38.9 ± 3.2 | 35.9 ± 4.1 | 29.5 ± 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limido, E.; Weinzierl, A.; Ampofo, E.; Harder, Y.; Menger, M.D.; Laschke, M.W. Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds. Int. J. Mol. Sci. 2024, 25, 851. https://doi.org/10.3390/ijms25020851
Limido E, Weinzierl A, Ampofo E, Harder Y, Menger MD, Laschke MW. Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds. International Journal of Molecular Sciences. 2024; 25(2):851. https://doi.org/10.3390/ijms25020851
Chicago/Turabian StyleLimido, Ettore, Andrea Weinzierl, Emmanuel Ampofo, Yves Harder, Michael D. Menger, and Matthias W. Laschke. 2024. "Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds" International Journal of Molecular Sciences 25, no. 2: 851. https://doi.org/10.3390/ijms25020851
APA StyleLimido, E., Weinzierl, A., Ampofo, E., Harder, Y., Menger, M. D., & Laschke, M. W. (2024). Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds. International Journal of Molecular Sciences, 25(2), 851. https://doi.org/10.3390/ijms25020851







