Knockdown of the ABCG23 Gene Disrupts the Development and Lipid Accumulation of Panonychus citri (Acari/Tetranychidae)
Abstract
1. Introduction
2. Results
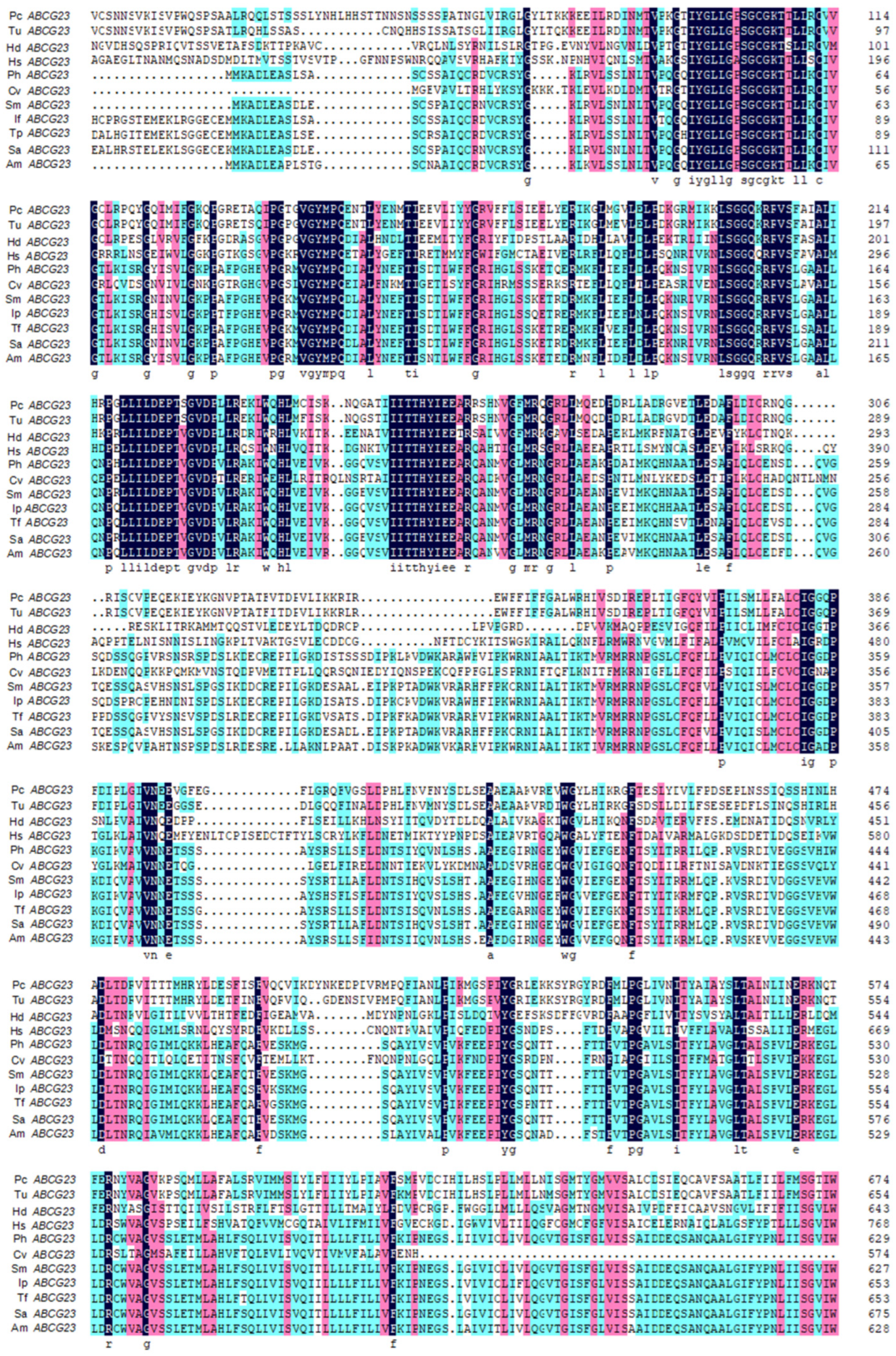
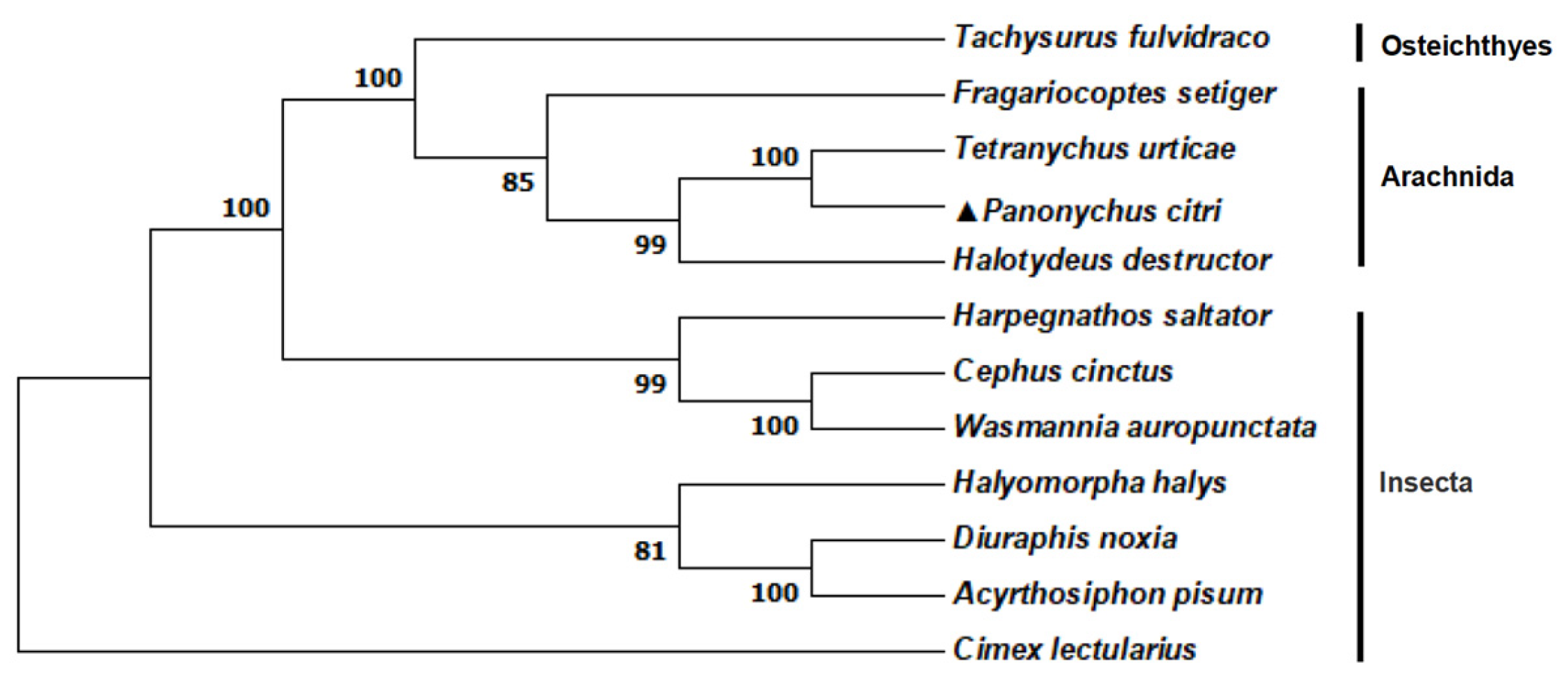
2.1. Cloning and Sequence Analysis
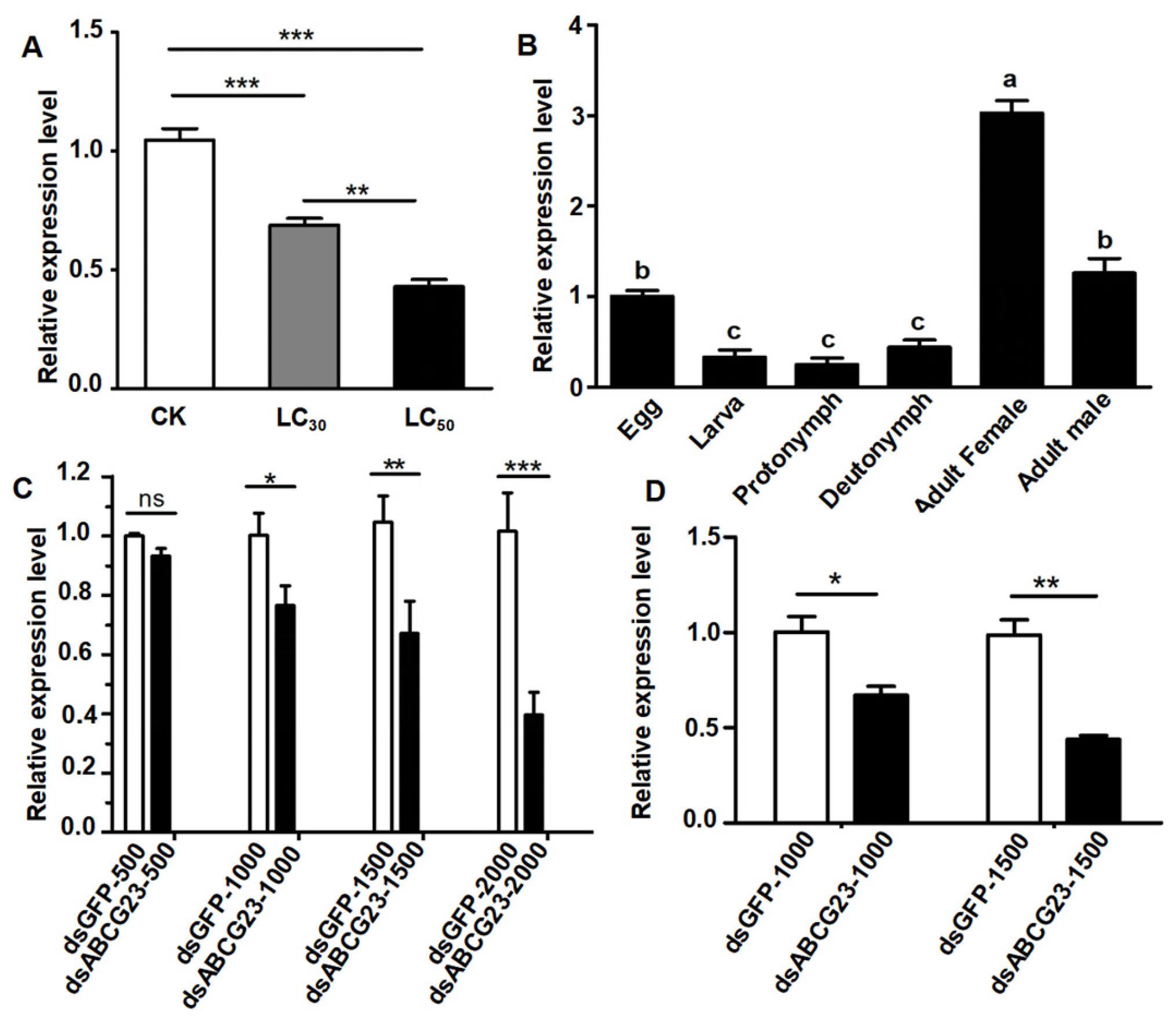
2.2. Expression of the PcABCG23
2.3. Effect of PcABCG23 Knockdown in P. citri
3. Materials and Methods
3.1. Mite Rearing and Acaricide
3.2. Cloning and Sequencing the ABCG23 Gene
3.2.1. RNA Extraction, cDNA Synthesis
3.2.2. Target Gene Fragment Cloning
3.3. PcABCG23 Expression Analysis
3.4. Synthesis of dsRNA and RNAi Efficiency Evaluation
3.4.1. dsRNA Synthesis and Delivery
3.4.2. RNAi Efficiency Evaluation
3.5. Evaluation of Biological Changes in P. citri after RNAi
3.5.1. Biological Observation after RNAi
3.5.2. Evaluation of Fecundity after RNAi
3.5.3. Determination of Triacylglycerol (TG) after RNAi
3.6. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higgins, C. ABC Transporters: From Microorganisms to Man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Sara, E.; Daniele, P.; Valentina, M.; Francesco, C.; Davide, S.; Paolo, R.; Claudia, C.; Domenico, O.; Guido, F.; Claudio, G.; et al. ABC transporters are involved in defense against permethrin insecticide in the malaria vector Anopheles stephensi. Parasites Vectors 2014, 7, 349. [Google Scholar]
- Rees, D.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Charles, R.; Rishil, J.; Pranav, G. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates 2015, 18, 1–17. [Google Scholar]
- Shang, F.; Niu, J.; Ding, B.; Zhang, W.; Wei, D.D.; Wei, D.; Jiang, H.; Wang, J. The miR-9b microRNA mediates dimorphism and development of wing in aphids. Proc. Natl. Acad. Sci. USA 2020, 117, 8404–8409. [Google Scholar] [CrossRef] [PubMed]
- Broehan, G.; Kroeger, T.; Lorenzen, M.; Merzendorfer, H. Functional analysis of the ATP-binding cassette(ABC) transporter gene family of Tribolium castaneum. BMC Genom. 2013, 14, 6. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, M.; Wang, K.; Qiao, X.; Chen, M. Molecular Cloning, Expression Pattern of Multidrug Resistance Associated Protein 1 (Mrp1, Abcc1) Gene, and the Synergistic Effects of Verapamil on Toxicity of Two Insecticides in the Bird Cherry-Oat Aphid. Arch. Insect Biochem. Physiol. 2016, 92, 65–84. [Google Scholar] [CrossRef]
- Rösner, J.; Merzendorfer, H. Transcriptional plasticity of different ABC transporter genes from Tribolium castaneum contributes to diflubenzuron resistance. Insect Biochem Mol. Biol. 2020, 116, 103282. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Zhao, X.; Liu, X.; Ma, E.; Moussian, B.; Zhang, J. The ABC transporter ABCH-9C is needed for cuticle barrier construction in Locusta migratoria. Insect Biochem. Mol. Biol. 2017, 87, 90–99. [Google Scholar] [CrossRef]
- Michael, D.; Yannick, H.; Giovanna, C. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar]
- Pignatelli, P.; Ingham, V.A.; Balabanidou, V.; Vontas, J.; Lycett, G.; Ranson, H. The Anopheles gambiae ATP-binding cassette transporter family: Phylogenetic analysis and tissue localization provide clues on function and role in insecticide resistance. Insect Mol. Biol. 2017, 27, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. ABC Transporters and Their Role in Protecting Insects from Pesticides and Their Metabolites. Adv. Insect Physiol. 2014, 46, 1–72. [Google Scholar]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Song, T.; He, R.; Zeng, Y.; Xie, W.; Wu, Q.; Wang, S.; Zhou, X.; Zhang, Y. Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweetpotato whitefly, Bemisia tabaci. BMC Genom. 2017, 18, 330. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Michaela, N.; Yiwen, W.; Kathrin, O.; Nicole, G.; Davide, A.; Slawomir, B.; Jurgen, B.; Matthias, F.; Bernard, M. The ABC transporter Snu and the extracellular protein Snsl cooperate in the formation of the lipid-based inward and outward barrier in the skin of Drosophila. Eur. J. Cell Biol. 2018, 97, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Gary, D.; Anthony, J. ABC Transporters Involved in Transport of Eye Pigment Precursors in Drosophila melanogaster. Method Enaymol. 1998, 292, 213–224. [Google Scholar]
- Grubbs, N.; Haas, S.; Beeman, R.; Lorenzen, M. The ABCs of eye color in Tribolium castaneum: Orthologs of the Drosophila white, scarlet, and brown Genes. Genetics 2015, 199, 749–759. [Google Scholar] [CrossRef]
- Yamanaka, N.; Marques, G.; O’Connor, M. Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell 2015, 163, 907–919. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Niu, N.; Zhao, Y.; Liu, W.; Ma, E.; Moussian, B.; Zhang, J. The fatty acid elongase gene LmELO7 is required for hydrocarbon biosynthesis and cuticle permeability in the migratory locust, Locusta migratoria. J. Insect Physiol. 2020, 123, 104052. [Google Scholar] [CrossRef] [PubMed]
- Qayyoum, M.; Song, Z.; Zhang, B.; Li, D.; Blanco, C. Dispersal Mechanism Assessment for Panonychus citri (Acari: Tetranychidae) Secondary Outbreaks. Ann. Entomol. Soc. Am. 2021, 114, 501–510. [Google Scholar] [CrossRef]
- Cheng, L.; Hou, D.; Sun, Q.; Yu, S.; Li, S.; Liu, H.; Cong, L.; Ran, C. Biochemical and Molecular Analysis of Field Resistance to Spirodiclofen in Panonychus citri (McGregor). Insects 2022, 13, 1011. [Google Scholar] [CrossRef]
- Alavijeh, E.; Khajehali, J.; Snoeck, S.; Panteleri, R.; Ghadamyari, M.; Jonckheere, W.; Bajda, S.; Saalwaechter, C.; Geibel, S.; Douris, V.; et al. Molecular and genetic analysis of resistance to METI-I acaricides in Iranian populations of the citrus red mite Panon. citri. Pestic. Biochem. Physiol. 2020, 164, 73–84. [Google Scholar] [CrossRef]
- Wang, H.; Xin, T.; Wang, H.; Wen, K.; Liu, Y.; Wang, J.; Zou, Z.; Zhong, L.; Xia, B. Stress response and tolerance mechanisms of spirobudiclofen exposure based on multiomics in Panonychus citri (Acari: Tetranychidae). iScience 2023, 26, 107111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Ding, J.; Wang, Y.; Zhang, Z.; Liu, F.; Mu, W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2018, 148, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hughes, H. Structure of the ABC ATPase domain of humanTAP1, the transporter associated with antigen processing. EMBO J. 2001, 20, 4964–4972. [Google Scholar]
- Liu, S.; Zhou, S.; Tian, L.; Guo, E.; Luan, Y.; Zhang, J.; Li, S. Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm, Bombyx mori. BMC Genom. 2011, 12, 491. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 11. [Google Scholar] [CrossRef]
- Chen, N.; Fan, Y.; Bai, Y.; Li, X.D.; Zhang, Z.; Liu, T. Cytochrome P450 gene, CYP4G51, modulates hydrocarbon production in the pea aphid, Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2016, 76, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Finet, C.; Slavik, K.; Pu, J.; Carroll, S.B.; Chung, H. Birth-and-Death Evolution of the Fatty Acyl-CoA Reductase (FAR) Gene Family and Diversification of Cuticular Hydrocarbon Synthesis in Drosophila. Genome Biol. Evol. 2019, 11, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, D.; Dulbecco, A.; Juarez, M.; Calderon Fernandez, G. A fatty acid synthase gene (FASN3) from the integument tissue of Rhodnius prolixus contributes to cuticle water loss regulation. Insect Mol. Biol. 2019, 28, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Chen, N.; Bai, Y.; Qiao, J.; Li, S.; Fan, Y.; Liu, T. BgFas1: A fatty acid synthase gene required for both hydrocarbon and cuticular fatty acid biosynthesis in the German cockroach, Blattella germanica (L.). Insect Biochem. Mol. Biol. 2019, 112, 103203. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Fan, Y.; Bai, T.; Wu, B.; Pei, X.; Wang, D.; Liu, T. Lipophorin receptor regulates the cuticular hydrocarbon accumulation and adult fecundity of the pea aphid Acyrthosiphon pisum. Insect Sci. 2021, 28, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.; Bertagnolli, N.; Evans, J.; Sieber, M.; Cox, J.; Thummel, C. Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 2014, 4, 839–850. [Google Scholar] [CrossRef]
- Emile, V. Fuel Metabolism of the Mosquito (Culex quinquefasciatus) Embryo. J. Insect Physiol. 1993, 39, 831–833. [Google Scholar]
- Merzendorfer, H.; Kim, H.; Chaudhari, S.; Kumari, M.; Specht, C.; Butcher, S.; Brown, S.; Manak, J.; Beeman, R.; Kramer, K.; et al. Genomic and proteomic studies on the effects of the insect growth regulator diflubenzuron in the model beetle species Tribolium castaneum. Insect Biochem. Mol. Biol. 2012, 42, 264–276. [Google Scholar] [CrossRef]
- Pavlidi, N.; Kampouraki, A.; Tseliou, V.; Wybouw, N.; Dermauw, W.; Roditakis, E.; Nauen, R.; Van Leeuwen, T.; Vontas, J. Molecular characterization of pyrethroid resistance in the olive fruit fly Bactrocera oleae. Pestic. Biochem. Physiol. 2018, 148, 1–7. [Google Scholar] [CrossRef]
- Misra, J.; Horner, M.; Lam, G.; Thummel, C. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef]
- Hsu, J.; Lin, Y.; Chang, C.; Hua, K.; Chen, M.; Huang, L.; Chen, C. Discovery of Organophosphate Resistance-Related Genes Associated With Well-known Resistance Mechanisms of Plutella xylostella (L.) (Lepidoptera: Plutellidae) by RNA-Seq. J. Econ. Entomol. 2016, 109, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Zamora, L.; Prospero, J.; Hansell, D.; Trapp, J. Atmospheric P deposition to the subtropical North Atlantic: Sources, properties, and relationship to N deposition. J. Geophys. Res. Atmos. 2013, 118, 1546–1562. [Google Scholar] [CrossRef]


| Primer Sequence (5′-3′) | |
|---|---|
| For gene cloning | |
| PcABCG23-F | TTTCGTCACCGATCCAGTCC |
| PcABCG23-R | TGACACGACTTAGGGCGAAC |
| PcABCG23-3′F1 | TCGTGTCCGCTCTCTGTGA |
| PcABCG23-3′F2 | CTTATTTCCGTTGGCTGTG |
| PcABCG23-5′R1 | CGAAGTGCTGCTGATGGTGAT |
| PcABCG23-5′R2 | TGCTGCTGATGGTGATTGC |
| UPM long | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT |
| UPM short | CTAATACGACTCACTATAGGGC |
| NUP | AAGCAGTGGTAACAACGCAGAGT |
| For qRT-PCR | |
| PcGAPDH -F | CTTTGGCCAAGGTCATCAAT |
| PcGAPDH -R | CGGTAGCGGCAGGTATAATG |
| PcELF1A -F | GGCACTTCGTCTTCCACTTC |
| PcELF1A -R | ATGATTCGTGGTGCATCTCA |
| PcABCG23-qF | TATGGAATGGTCGTGTCCGC |
| PcABCG23-qR | CGCGTATTCACACAGCCAAC |
| For ABCG23 dsRNA synthesis | |
| T7 GFP ds-F | TAATACGACTCACTATAGGGCCACAAGTTCAGCGTGTCCG |
| T7 GFP ds-R | TAATACGACTCACTATAGGGTGGGTGCTCAGGTAGTGGTTGT |
| T7 ABCG23 ds-F | TAATACGACTCACTATAGGGCGGTTGTCTTCGACCTCAAT |
| T7 ABCG23 ds-R | TAATACGACTCACTATAGGGCTTGTCTCATGAAGCCCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, H.; Xin, T.; Xia, B. Knockdown of the ABCG23 Gene Disrupts the Development and Lipid Accumulation of Panonychus citri (Acari/Tetranychidae). Int. J. Mol. Sci. 2024, 25, 827. https://doi.org/10.3390/ijms25020827
Wang H, Wang H, Xin T, Xia B. Knockdown of the ABCG23 Gene Disrupts the Development and Lipid Accumulation of Panonychus citri (Acari/Tetranychidae). International Journal of Molecular Sciences. 2024; 25(2):827. https://doi.org/10.3390/ijms25020827
Chicago/Turabian StyleWang, Hongyan, Haifeng Wang, Tianrong Xin, and Bin Xia. 2024. "Knockdown of the ABCG23 Gene Disrupts the Development and Lipid Accumulation of Panonychus citri (Acari/Tetranychidae)" International Journal of Molecular Sciences 25, no. 2: 827. https://doi.org/10.3390/ijms25020827
APA StyleWang, H., Wang, H., Xin, T., & Xia, B. (2024). Knockdown of the ABCG23 Gene Disrupts the Development and Lipid Accumulation of Panonychus citri (Acari/Tetranychidae). International Journal of Molecular Sciences, 25(2), 827. https://doi.org/10.3390/ijms25020827







