The Influence of HLA Polymorphisms on the Severity of COVID-19 in the Romanian Population
Abstract
1. Introduction
2. Results
2.1. Characterization of the Study Group
2.2. Allele Analysis
2.3. Haplotype Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design, Setting, and Participants
4.2. Variables
4.3. DNA Extraction
4.4. HLA Typing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6, Erratum in Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Langton, D.J.; Bourke, S.C.; Lie, B.A.; Reiff, G.; Natu, S.; Darlay, R.; Burn, J.; Echevarria, C. The influence of HLA genotype on the severity of COVID-19 infection. HLA 2021, 98, 14–22. [Google Scholar] [CrossRef]
- Sanchez-Mazas, A.; Lemaître, J.F.; Currat, M. Distinct evolutionary strategies of human leucocyte antigen loci in pathogen-rich environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 830–839. [Google Scholar] [CrossRef][Green Version]
- Pareek, M.; Bangash, M.N.; Pareek, N.; Pan, D.; Sze, S.; Minhas, J.S.; Hanif, W.; Khunti, K. Ethnicity and COVID-19: An urgent public health research priority. Lancet 2020, 395, 1421–1422. [Google Scholar] [CrossRef]
- de Sousa, E.; Ligeiro, D.; Lérias, J.R.; Zhang, C.; Agrati, C.; Osman, M.; El-Kafrawy, S.A.; Azhar, E.I.; Ippolito, G.; Wang, F.S.; et al. Mortality in COVID-19 disease patients: Correlating the association of major histocompatibility complex (MHC) with severe acute respiratory syndrome 2 (SARS-CoV-2) variants. Int. J. Infect. Dis. 2020, 98, 454–459. [Google Scholar] [CrossRef]
- Muñiz-Castrillo, S.; Vogrig, A.; Honnorat, J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Auto Immun. Highlights 2020, 11, 2. [Google Scholar] [CrossRef]
- Shiina, T.; Hosomichi, K.; Inoko, H.; Kulski, J.K. The HLA genomic loci map: Expression, interaction, diversity and disease. J. Hum. Genet. 2009, 54, 15–39. [Google Scholar] [CrossRef]
- Martin, M.P.; Carrington, M. Immunogenetics of viral infections. Curr. Opin. Immunol. 2005, 17, 510–516. [Google Scholar] [CrossRef]
- Matei, H.V.; Vica, M.L.; Siserman, C.V. Association between HLA class II alleles and hepatitis B virus infection in Transylvania, Romania. Immunol. Investig. 2018, 47, 735–744. [Google Scholar] [CrossRef]
- Mosaad, Y.M.; Farag, R.E.; Arafa, M.M.; Eletreby, S.; El-Alfy, H.A.; Eldeek, B.S.; Tawhid, Z.M. Association of human leucocyte antigen Class I (HLA-A and HLA-B) with chronic hepatitis C virus infection in Egyptian patients. Scand. J. Immunol. 2010, 72, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.L.; Kikuchi, M.; Vu, T.Q.H.; Do, Q.H.; Tran, T.T.; Vo, D.T.; Ha, M.T.; Vo, V.T.; Cao, T.P.N.; Tran, V.D.; et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl. Trop. Dis. 2008, 2, e304. [Google Scholar]
- Dutta, M.; Dutta, P.; Medhi, S.; Borkakoty, B.; Biswas, D. Polymorphism of HLA class I and class II alleles in influenza A(H1N1)pdm09 virus infected population of Assam, Northeast India. J. Med. Virol. 2018, 90, 854–860. [Google Scholar] [CrossRef]
- Luckey, D.; Weaver, E.A.; Osborne, D.G.; Billadeau, D.D.; Taneja, V. Immunity to Influenza is dependent on MHC II polymorphism: Study with 2 HLA transgenic strains. Sci. Rep. 2019, 9, 19061. [Google Scholar] [CrossRef] [PubMed]
- Harishankar, M.; Selvaraj, P.; Bethunaickan, R. Influence of genetic polymorphism towards pulmonary tuberculosis susceptibility. Front. Med. 2018, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Cutler, S.; Davis, I.; Lu, R.; Soghoian, D.Z.; Qi, Y.; Sidney, J.; Kranias, G.; Flanders, M.D.; Lindqvist, M.; et al. Association of HLA-DRB1-restricted CD4+ T cell responses with HIV immune control. Nat. Med. 2013, 19, 930–933. [Google Scholar] [CrossRef]
- da Costa Lima-Junior, J.; Pratt-Riccio, L.R. Major Histocompatibility Complex and Malaria: Focus on Plasmodium vivax Infection. Front. Immunol. 2016, 7, 13. [Google Scholar]
- Janice Oh, H.L.; Ken-En Gan, S.; Bertoletti, A.; Tan, Y.J. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 2012, 1, e23. [Google Scholar] [CrossRef]
- Hajeer, A.H.; Balkhy, H.; Johani, S.; Yousef, M.Z.; Arabi, Y. Association of human leukocyte antigen class II alleles with severe Middle East respiratory syndrome-coronavirus infection. Ann. Thorac. Med. 2016, 11, 211–213. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Zhang, J.; He, J.; Zhu, F. Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). HLA 2020, 96, 194–196. [Google Scholar] [CrossRef]
- Warren, R.L.; Birol, I. HLA predictions from the bronchoalveolar lavage fluid samples of five patients at the early stage of the wuhan seafood market COVID-19 outbreak. arXiv 2020, arXiv:2004.07108v3. [Google Scholar]
- Abdelhafiz, A.S.; Ali, A.; Fouda, M.A.; Sayed, D.M.; Kamel, M.M.; Kamal, L.M.; Khalil, M.A.; Bakry, R.M. HLA-B*15 predicts survival in Egyptian patients with COVID-19. Hum. Immunol. 2022, 83, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, A.; Magistroni, P.; Vespasiano, F.; Bella, A.; Bellino, S.; Puoti, F.; Alizzi, S.; Vaisitti, T.; Boros, S.; Grossi, P.A.; et al. HLA and AB0 Polymorphisms may influence SARS-CoV-2 infection and COVID-19 severity. Transplantation 2021, 105, 193–200. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Carrega, P.; Venanzi Rullo, E.; Ducatelli, R.; Falco, M.; Freni, J.; Miceli, M.; Cavaliere, R.; Fontana, V.; Versace, A.; et al. HLA-C*17 in COVID-19 patients: Hints for associations with severe clinical outcome and cardiovascular risk. Immunol. Lett. 2021, 234, 44–46. [Google Scholar] [CrossRef]
- Khor, S.S.; Omae, Y.; Nishida, N.; Sugiyama, M.; Kinoshita, N.; Suzuki, T.; Suzuki, M.; Suzuki, S.; Izumi, S.; Hojo, M.; et al. HLA-A*11:01:01:01, HLA-C*12:02:02:01-HLA-B*52:01:02:02, age and sex are associated with severity of Japanese COVID-19 with respiratory failure. Front. Immunol. 2021, 12, 658570. [Google Scholar] [CrossRef] [PubMed]
- Ben Shachar, S.; Barda, N.; Manor, S.; Israeli, S.; Dagan, N.; Carmi, S.; Balicer, R.; Zisser, B.; Louzoun, Y. MHC haplotyping of SARS-CoV-2 patients: HLA subtypes are not associated with the presence and severity of COVID-19 in the Israeli population. J. Clin. Immunol. 2021, 41, 1154–1161. [Google Scholar] [CrossRef]
- Castelli, E.C.; de Castro, M.V.; Naslavsky, M.S.; Scliar, M.O.; Silva, N.S.B.; Andrade, H.S.; Souza, A.S.; Pereira, R.N.; Castro, C.F.B.; Mendes-Junior, C.T.; et al. MHC variants associated with symptomatic versus asymptomatic SARS-CoV-2 infection in highly exposed individuals. Front. Immunol. 2021, 12, 742881. [Google Scholar] [CrossRef]
- Cheranev, V.; Bulusheva, I.; Vechorko, V.; Korostin, D.; Rebrikov, D. The search of association of HLA class I and class II alleles with COVID-19 mortality in the Russian cohort. Int. J. Mol. Sci. 2023, 24, 3068. [Google Scholar] [CrossRef]
- Detsika, M.G.; Giatra, C.; Kitsiou, V.; Jahaj, E.; Athanassiades, T.; Kouniaki, D.; Orfanos, S.E.; Dimopoulou, I.; Pagoni, M.; Tarassi, K.; et al. Demographic, clinical and immunogenetic profiles of a Greek cohort of COVID-19 patients. Life 2021, 11, 1017. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Ghasemi-Basir, H.R.; Majzoobi, M.M.; Rasouli-Saravani, A.; Hajilooi, M.; Solgi, G. HLA-DRB1*04 may predict the severity of disease in a group of Iranian COVID-19 patients. Hum. Immunol. 2021, 82, 719–725. [Google Scholar] [CrossRef]
- Iturrieta-Zuazo, I.; Rita, C.G.; García-Soidán, A.; de Malet Pintos-Fonseca, A.; Alonso-Alarcón, N.; Pariente-Rodríguez, R.; Tejeda-Velarde, A.; Serrano-Villar, S.; Castañer-Alabau, J.L.; Nieto-Gañán, I. Possible role of HLA class-I genotype in SARS-CoV-2 infection and progression: A pilot study in a cohort of COVID-19 Spanish patients. Clin. Immunol. 2020, 219, 108572. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.L.; Birol, I. HLA alleles measured from COVID-19 patient transcriptomes reveal associations with disease prognosis in a New York cohort. PeerJ 2021, 9, e12368. [Google Scholar] [CrossRef] [PubMed]
- Naemi, F.M.A.; Al-Adwani, S.; Al-Khatabi, H.; Al-Nazawi, A. Frequency of HLA alleles among COVID-19 infected patients: Preliminary data from Saudi Arabia. Virology 2021, 560, 1–7. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of the Republic of Moldova. Population and Housing Census in the Republic of Moldova. 2014. Available online: https://statistica.gov.md/en/population-and-housing-census-in-2014-122.html (accessed on 16 July 2023).
- Nistor, I. (Ed.) Populațiunea Basarabiei. In Istoria Basarabiei, 1st ed.; Humanitas: București, Romania, 2017; pp. 212–228. [Google Scholar]
- Constantinescu, I.; Boșcaiu, V.; Cianga, P.; Dinu, A.A.; Gai, E.; Melinte, M.; Moise, A. The frequency of HLA alleles in the Romanian population. Immunogenetics 2016, 68, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Matei, H.V.; Bondor, C.I.; Nicula, G.Z.; Siserman, C.V.; Loga, L.; Dican, L. HLA Polymorphisms and haplotype diversity in Transylvania, Romania. J. Immunol. Res. 2019, 2019, 1342762. [Google Scholar] [CrossRef]
- Matei, H.V.; Vică, M.L.; Ciucă, I.; Coman, H.G.; Nicula, G.Z.; Siserman, C.V. Correlations Among the HLA-DQB1 Alleles and Suicidal Behavior. J. Forensic Sci. 2020, 65, 166–169. [Google Scholar] [CrossRef]
- Vică, M.L.; Delcea, C.; Dumitrel, G.A.; Vușcan, M.E.; Matei, H.V.; Teodoru, C.A.; Siserman, C.V. The influence of HLA alleles on the affective distress profile. Int. J. Environ. Res. Public Health 2022, 19, 12608. [Google Scholar] [CrossRef]
- Vuscan, M.E.; Vica, M.L.; Balici, S.; Nicula, G.Z.; Rusu, S.I.; Siserman, C.V.; Coman, H.G.; Matei, H.V. Association of HLA class II alleles with suicidal behavior in a Transylvanian population. Rev. Romana Med. Lab. 2023, 31, 15–23. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef]
- Distante, C.; Piscitelli, P.; Miani, A. COVID-19 outbreak progression in Italian regions: Approaching the peak by the end of march in Northern Italy and first week of april in Southern Italy. Int. J. Environ. Res. Public Health 2020, 17, 3025. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020, 323, 1574–1581, Erratum in JAMA 2021, 325, 2120. [Google Scholar] [CrossRef]
- Novelli, A.; Andreani, M.; Biancolella, M.; Liberatoscioli, L.; Passarelli, C.; Colona, V.L.; Rogliani, P.; Leonardis, F.; Campana, A.; Carsetti, R.; et al. HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. HLA 2020, 96, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, R.; Ajam, A.; Karbalai, S.; Ashraf, H.; Dehaghi, M.R.O.; Tabriz, H.M.; Pazoki, M.; Khalili, F. Association between Human Leukocyte Antigen and COVID-19 severity. Acta Med. Iran 2021, 59, 400–405. [Google Scholar] [CrossRef]
- Schindler, E.; Dribus, M.; Duffy, B.F.; Hock, K.; Farnsworth, C.W.; Gragert, L.; Liu, C. HLA genetic polymorphism in patients with Coronavirus Disease 2019 in Midwestern United States. HLA 2021, 98, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Littera, R.; Campagna, M.; Deidda, S.; Angioni, G.; Cipri, S.; Melis, M.; Firinu, D.; Santus, S.; Lai, A.; Porcella, R.; et al. Human Leukocyte Antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. The Sardinian experience. Front. Immunol. 2020, 11, 605688. [Google Scholar] [CrossRef] [PubMed]
- Shkurnikov, M.; Nersisyan, S.; Jankevic, T.; Galatenko, A.; Gordeev, I.; Vechorko, V.; Tonevitsky, A. Association of HLA class I genotypes with severity of Coronavirus Disease-19. Front. Immunol. 2021, 12, 641900. [Google Scholar] [CrossRef] [PubMed]
- Dobrijević, Z.; Gligorijević, N.; Šunderić, M.; Penezić, A.; Miljuš, G.; Tomić, S.; Nedić, O. The association of human leucocyte antigen (HLA) alleles with COVID-19 severity: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2378. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Franco, A.; Barrios, Y.; Cáceres, J.J.; Solé-Violán, J.; Perez, A.; Marcos Y Ramos, J.A.; Ramos-Gómez, L.; Ojeda, N.; et al. HLA genetic polymorphisms and prognosis of patients with COVID-19. Polimorfismos genéticos de los HLA y pronóstico de pacientes con COVID-19. Med. Intensiv. Engl. Ed. 2021, 45, 96–103. [Google Scholar] [CrossRef]
- Hernández-Doño, S.; Sánchez-González, R.A.; Trujillo-Vizuet, M.G.; Zamudio-Castellanos, F.Y.; García-Silva, R.; Bulos-Rodríguez, P.; Vazquez-Guzmán, C.A.; Cárdenas-Ramos, X.; de León Rodríguez, D.; Elías, F.; et al. Protective HLA alleles against severe COVID-19: HLA-A*68 as an ancestral protection allele in Tapachula-Chiapas, Mexico. Clin. Immunol. 2022, 238, 108990. [Google Scholar] [CrossRef]
- Gambino, C.M.; Aiello, A.; Accardi, G.; Caruso, C.; Candore, G. Autoimmune diseases and 8.1 ancestral haplotype: An update. HLA 2018, 92, 137–143. [Google Scholar] [CrossRef]
- Pisanti, S.; Deelen, J.; Gallina, A.M.; Caputo, M.; Citro, M.; Abate, M.; Sacchi, N.; Vecchione, C.; Martinelli, R. Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of COVID-19. J. Transl. Med. 2020, 18, 352. [Google Scholar] [CrossRef]
- World Health Organisation. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 18 August 2023).
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.M.; Cui, L.; Toh, M.P.H.S.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and virological features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) variants of concern: A retrospective cohort study comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 2022, 75, e1128–e1136. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhang, W.; Fang, X.; Yu, D.; Wang, X. Challenges of SARS-CoV-2 Omicron variant and appropriate countermeasures. J. Microbiol. Immunol. Infect. 2022, 55, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; David, J.K.; Maden, S.K.; Wood, M.A.; Weeder, B.R.; Nellore, A.; Thompson, R.F. Human Leukocyte Antigen susceptibility map for Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 2020, 94, e00510-20. [Google Scholar] [CrossRef]
- Campbell, K.M.; Steiner, G.; Wells, D.K.; Ribas, A.; Kalbasi, A. Prioritization of SARS-CoV-2 epitopes using a pan-HLA and global population inference approach. Preprint. bioRxiv 2020. [Google Scholar] [CrossRef]
- Enayatkhani, M.; Hasaniazad, M.; Faezi, S.; Gouklani, H.; Davoodian, P.; Ahmadi, N.; Einakian, M.A.; Karmostaji, A.; Ahmadi, K. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: An in silico study. J. Biomol. Struct. Dyn. 2021, 39, 2857–2872. [Google Scholar] [CrossRef]
- Pappas, A.G.; Chaliasou, A.-L.; Panagopoulos, A.; Dede, K.; Daskalopoulou, S.; Moniem, E.; Polydora, E.; Grigoriou, E.; Psarra, K.; Tsirogianni, A.; et al. Kinetics of Immune Subsets in COVID-19 Patients Treated with Corticosteroids. Viruses 2023, 15, 51. [Google Scholar] [CrossRef]
- National Institut of the Public Health. Risk Assessement. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/infectia-cu-noul-coronavirus-sars-cov-2/evaluare-de-risc/ (accessed on 30 July 2023).
- Center for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 30 July 2023).
- HLA-FluoGene®–The Fluorescence PCR. Available online: https://www.inno-train.de/en/products/hla-typing/hla-real-time-pcr/ (accessed on 28 March 2023).
- Schaid, D.J.; Rowland, C.M.; Tines, D.E.; Jacobson, R.M.; Poland, G.A. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am. J. Hum. Genet. 2002, 70, 425–434. [Google Scholar] [CrossRef]
- Sinnwell, J.P.; Schaid, D.J. Haplo.stats: Statistical Analysis of Haplotypes with Traits and Covariates when Linkage Phase Is Ambiguous, v1.6.0. (Version 1.6.0) 23 July 2018. Available online: https://cran.r-project.org/web/packages/haplo.stats/index.html (accessed on 28 July 2023).
| Group | Control (n = 169) | Severe (n = 130) | p-Value |
|---|---|---|---|
| Age (years), median (IQR 1) | 35 (29–40) | 63 (53–70.75) | <0.001 |
| Sex (F), no (%) | 82 (48.52) | 67 (51.54) | 0.605 |
| Characteristics | Not Deceased (n = 86) | Deceased (n = 44) | p-Value |
|---|---|---|---|
| Age (years), median (IQR 1) | 60.5 (51–67) | 67 (59.5–77) | <0.001 |
| Sex | |||
| Female, n (%) | 57 (66.28) | 10 (22.73) | <0.001 |
| Male, n (%) | 29 (33.72) | 34 (77.27) | |
| Comorbidities | |||
| Pulmonary, n (%) | 8 (9.3) | 6 (13.64) | 0.552 |
| Cardiovascular, n (%) | 9 (10.47) | 32 (72.73) | <0.001 |
| Obesity, n (%) | 6 (4.11) | 10 (11.63) | 0.029 |
| Diabetes, n (%) | 38 (26.03) | 10 (11.63) | 0.009 |
| Hepatic, n (%) | 6 (6.98) | 13 (29.55) | <0.001 |
| Renal, n (%) | 1 (1.16) | 14 (31.82) | <0.001 |
| Cancers, n (%) | 1 (1.16) | 2 (4.55) | 0.264 |
| Vaccinated, n (%) | 28 (40.58) | 1 (4) | <0.001 |
| Oxygen therapy, n (%) | 49 (56.98) | 23 (71.88) | 0.14 |
| ICU 2, n (%) | 26 (31.33) | 18 (56.25) | 0.014 |
| Characteristics | Not Deceased/ICU 1 (n = 57) | Deceased/ICU 1 (n = 73) | p-Value |
|---|---|---|---|
| Age (years), median (IQR 2) | 57 (46–63) | 67 (59–77) | <0.001 |
| Sex | <0.001 | ||
| Female, n (%) | 42 (73.68) | 25 (34.25) | |
| Male, n (%) | 15 (26.32) | 48 (65.75) | |
| Comorbidities | |||
| Pulmonary, n (%) | 2 (3.51) | 12 (16.44) | 0.018 |
| Cancers, n (%) | 1 (1.75) | 40 (54.79) | <0.001 |
| Cardiovascular, n (%) | 1 (1.75) | 40 (54.79) | <0.001 |
| Obesity, n (%) | 0 (0) | 16 (11.27) | <0.001 |
| Diabetes, n (%) | 24 (26.67) | 24 (16.9) | 0.074 |
| Hepatic, n (%) | 2 (3.51) | 17 (23.29) | 0.002 |
| Renal, n (%) | 0 (0) | 15 (20.55) | <0.001 |
| Vaccinated, n (%) | 23 (44.23) | 6 (14.29) | 0.002 |
| Characteristics | Without Oxygen (n = 46) | With Oxygen (n = 72) | p-Value |
|---|---|---|---|
| Age (years), median (IQR 1) | 53 (42.5–62.75) | 64 (59.75–73.5) | <0.001 |
| Sex | 0.077 | ||
| Female, n (%) | 30 (65.22) | 35 (48.61) | |
| Male, n (%) | 16 (34.78) | 37 (51.39) | |
| Comorbidities | |||
| Pulmonary, n (%) | 1 (2.17) | 12 (16.67) | 0.014 |
| Cancers, n (%) | 0 (0) | 2 (2.78) | 0.52 |
| Cardiovascular, n (%) | 8 (17.39) | 25 (34.72) | 0.041 |
| Obesity, n (%) | 2 (2.86) | 12 (8.7) | 0.147 |
| Diabetes, n (%) | 10 (14.29) | 38 (27.54) | 0.032 |
| Hepatic, n (%) | 6 (13.04) | 9 (12.5) | 0.931 |
| Renal, n (%) | 6 (13.04) | 5 (6.94) | 0.335 |
| Vaccinated, n (%) | 20 (46.51) | 9 (19.15) | 0.006 |
| Deceased, n (%) | 9 (19.57) | 23 (31.94) | 0.14 |
| HLA 1 Allele n 2 (%) | Severe (n = 260) | Control (n = 338) | OR 3 Unadjusted (95% CI 4) | p-Value | OR Adjusted (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| A | n = 260 | n = 318 | ||||
| A*02 | 77 (29.62) | 70 (22.01) | 1.49 (1.02–2.17) | 0.037 | 1.6 (0.88–2.9) | 0.121 |
| A*33 | 1 (0.38) | 11 (3.46) | 0.11 (0.01–0.84) | 0.010 | 0.03 (0–0.3) | 0.006 |
| B | n = 260 | n = 318 | ||||
| B*27 | 22 (8.46) | 14 (4.4) | 2.01 (1.01–4.01) | 0.045 | 4.63 (1.57–13.78) | 0.005 |
| B*40 | 10 (3.85) | 26 (8.18) | 0.45 (0.21–0.95) | 0.032 | 0.48 (0.12–1.71) | 0.278 |
| B*41 | 1 (0.38) | 9 (2.83) | 0.13 (0.02–1.05) | 0.027 | 0.33 (0.01–5.07) | 0.526 |
| B*50 | 10 (3.85) | 2 (0.63) | 6.32 (1.37–29.11) | 0.007 | 7.94 (1.25–70.14) | 0.037 |
| B*58 | 0 (0) | 6 (1.89) | 0 (0–NC 5) | 0.035 | 0 (NA–3.8 × 1030) | 0.982 |
| C | n = 260 | n = 318 | ||||
| C*15 | 8 (3.08) | 25 (7.86) | 0.37 (0.16–0.84) | 0.014 | 0.13 (0.03–0.53) | 0.004 |
| DRB1 | n = 260 | n = 304 | ||||
| DRB1*15 | 17 (6.54) | 37 (12.17) | 0.5 (0.28–0.92) | 0.023 | 0.88 (0.33–2.17) | 0.793 |
| DQB1 | n = 260 | n = 304 | ||||
| DQB1*06 | 33 (12.69) | 63 (20.72) | 0.56 (0.35–0.88) | 0.011 | 0.69 (0.32–1.41) | 0.314 |
| HLA 1 Allele | Deceased (n 2 = 88) | Not Deceased (n = 172) | OR 3 Unadjusted (95% CI 4) | p-Value | OR Adjusted (95% CI) | p-Value | Hosmer Lemeshow Test | AUROC 5 (95% CI) |
|---|---|---|---|---|---|---|---|---|
| A*30 | 7 (7.95) | 3 (1.74) | 4.87 (1.23–19.32) | 0.034 | 14.05 (0.59–238.42) | 0.073 | 0.430 | 0.94 (0.90–0.97) |
| B*18 | 16 (18.18) | 12 (6.98) | 2.96 (1.33–6.58) | 0.006 | 1.46 (0.36–5.98) | 0.597 | 0.032 | 0.94 (0.90–0.97) |
| C*07 | 33 (37.5) | 39 (22.67) | 2.05 (1.17–3.58) | 0.011 | 1.54 (0.49–4.85) | 0.456 | 0.230 | 0.94 (0.90–0.97) |
| DRB1*11 | 25 (28.41) | 30 (17.44) | 1.88 (1.02–3.45) | 0.040 | 0.89 (0.21–3.56) | 0.874 | 0.188 | 0.94 (0.90–0.97) |
| HLA 1 Allele | Deceased/ICU 2 (n 3 = 146) | Not Deceased/ICU 2 (n = 114) | OR 4 Unadjusted (95% CI 5) | p-Value | OR Adjusted (95% CI) | p-Value | Hosmer Lemeshow Test | AUROC 6 (95% CI) |
|---|---|---|---|---|---|---|---|---|
| A*03 | 10 (6.85) | 17 (14.91) | 0.42 (0.18–0.96) | 0.034 | 0.14 (0.02–0.77) | 0.036 | 0.123 | 0.92 (0.88–0.95) |
| C*04 | 16 (10.96) | 26 (22.81) | 0.42 (0.21–0.82) | 0.010 | 0.48 (0.15–1.5) | 0.218 | 0.116 | 0.91 (0.87–0.95) |
| DQB1*06 | 24 (16.44) | 9 (7.89) | 2.3 (1.02–5.16) | 0.040 | 3.2 (0.95–11.46) | 0.065 | 0.519 | 0.92 (0.88, 0.95) |
| HLA 1 Allele | With Oxygen (n 2 = 144) | Without Oxygen (n = 92) | OR 3 Unadjusted (95% CI 4) | p-Value | OR Adjusted (95% CI) | p-Value | Hosmer Lemeshow Test | AUROC 5 (95% CI) |
|---|---|---|---|---|---|---|---|---|
| A*03 | 11 (7.64) | 15 (16.3) | 0.42 (0.19–0.97) | 0.038 | 0.26 (0.07–0.85) | 0.032 | 0.933 | 0.82 (0.76–0.88) |
| DQB1*02 | 28 (19.44) | 31 (33.7) | 0.47 (0.26–0.86) | 0.014 | 0.31 (0.13–0.7) | 0.006 | 0.89 | 0.83 (0.77–0.89) |
| Severe | Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | DRB1 | DQB1 | Probability | A | B | C | DRB1 | DQB1 | Probability |
| 01 | 08 | 07 | 03 | 02 | 0.065 | 01 | 08 | 07 | 03 | 02 | 0.070 |
| 02 | 18 | 07 | 11 | 03 | 0.025 | 01 | 40 | 15 | 14 | 05 | 0.025 |
| 02 | 15 | 03 | 13 | 06 | 0.023 | 03 | 35 | 04 | 01 | 05 | 0.025 |
| 02 | 38 | 12 | 13 | 06 | 0.019 | 03 | 35 | 04 | 11 | 03 | 0.018 |
| 02 | 44 | 16 | 07 | 02 | 0.019 | 02 | 35 | 04 | 15 | 06 | 0.018 |
| 24 | 35 | 04 | 11 | 03 | 0.019 | 02 | 18 | 07 | 11 | 03 | 0.018 |
| 02 | 18 | 07 | 16 | 05 | 0.019 | 02 | 52 | 12 | 15 | 05 | 0.014 |
| 03 | 35 | 04 | 01 | 05 | 0.017 | 02 | 41 | 07 | 03 | 02 | 0.014 |
| 03 | 07 | 07 | 11 | 03 | 0.015 | 03 | 07 | 07 | 11 | 03 | 0.011 |
| 26 | 38 | 12 | 14 | 05 | 0.015 | 02 | 51 | 14 | 11 | 03 | 0.011 |
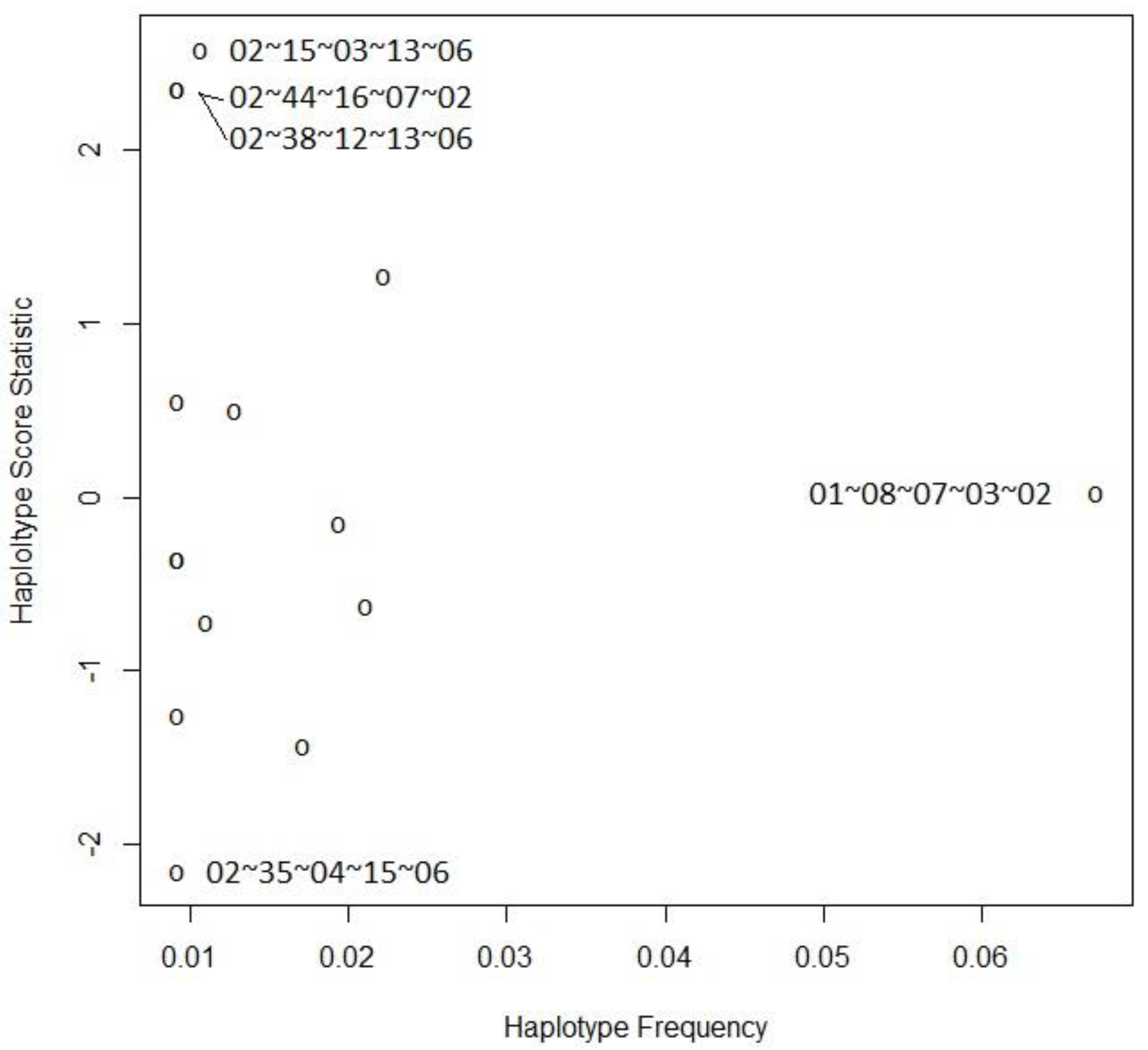
| A | B | C | DRB1 | DQB1 | Hap-Freq | Hap-Score | p-Value |
|---|---|---|---|---|---|---|---|
| 02 | 35 | 04 | 15 | 06 | 0.00919 | −2.15944 | 0.03082 |
| 01 | 40 | 15 | 14 | 05 | 0.01717 | −1.43421 | 0.15151 |
| 02 | 41 | 07 | 03 | 02 | 0.00919 | −1.25580 | 0.20919 |
| 11 | 52 | 12 | 15 | 06 | 0.01103 | −0.71707 | 0.47333 |
| 03 | 35 | 04 | 01 | 05 | 0.02108 | −0.62201 | 0.53393 |
| 02 | 52 | 12 | 15 | 05 | 0.00919 | −0.35216 | 0.72472 |
| 26 | 27 | 01 | 01 | 05 | 0.00919 | −0.35215 | 0.72472 |
| 24 | 35 | 04 | 11 | 03 | 0.01946 | −0.15135 | 0.87970 |
| 01 | 08 | 07 | 03 | 02 | 0.06722 | 0.02675 | 0.97866 |
| 03 | 07 | 07 | 11 | 03 | 0.01287 | 0.50167 | 0.61590 |
| 30 | 13 | 06 | 07 | 02 | 0.00919 | 0.55149 | 0.58130 |
| 02 | 18 | 07 | 11 | 03 | 0.02224 | 1.27490 | 0.20234 |
| 02 | 38 | 12 | 13 | 06 | 0.00919 | 2.35877 | 0.01834 |
| 02 | 44 | 16 | 07 | 02 | 0.00919 | 2.35877 | 0.01834 |
| 02 | 15 | 03 | 13 | 06 | 0.01070 | 2.58579 | 0.00972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vică, M.L.; Dobreanu, M.; Curocichin, G.; Matei, H.V.; Bâlici, Ș.; Vușcan, M.E.; Chiorean, A.D.; Nicula, G.Z.; Pavel Mironescu, D.C.; Leucuța, D.C.; et al. The Influence of HLA Polymorphisms on the Severity of COVID-19 in the Romanian Population. Int. J. Mol. Sci. 2024, 25, 1326. https://doi.org/10.3390/ijms25021326
Vică ML, Dobreanu M, Curocichin G, Matei HV, Bâlici Ș, Vușcan ME, Chiorean AD, Nicula GZ, Pavel Mironescu DC, Leucuța DC, et al. The Influence of HLA Polymorphisms on the Severity of COVID-19 in the Romanian Population. International Journal of Molecular Sciences. 2024; 25(2):1326. https://doi.org/10.3390/ijms25021326
Chicago/Turabian StyleVică, Mihaela Laura, Minodora Dobreanu, Ghenadie Curocichin, Horea Vladi Matei, Ștefana Bâlici, Mihaela Elvira Vușcan, Alin Dan Chiorean, Gheorghe Zsolt Nicula, Daniela Cristina Pavel Mironescu, Daniel Corneliu Leucuța, and et al. 2024. "The Influence of HLA Polymorphisms on the Severity of COVID-19 in the Romanian Population" International Journal of Molecular Sciences 25, no. 2: 1326. https://doi.org/10.3390/ijms25021326
APA StyleVică, M. L., Dobreanu, M., Curocichin, G., Matei, H. V., Bâlici, Ș., Vușcan, M. E., Chiorean, A. D., Nicula, G. Z., Pavel Mironescu, D. C., Leucuța, D. C., Teodoru, C. A., & Siserman, C. V. (2024). The Influence of HLA Polymorphisms on the Severity of COVID-19 in the Romanian Population. International Journal of Molecular Sciences, 25(2), 1326. https://doi.org/10.3390/ijms25021326






