The Effect of Dexlansoprazole on Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Databases and Search Strategy
2.4. Study Selection and Data Extraction
2.5. Kappa Analysis
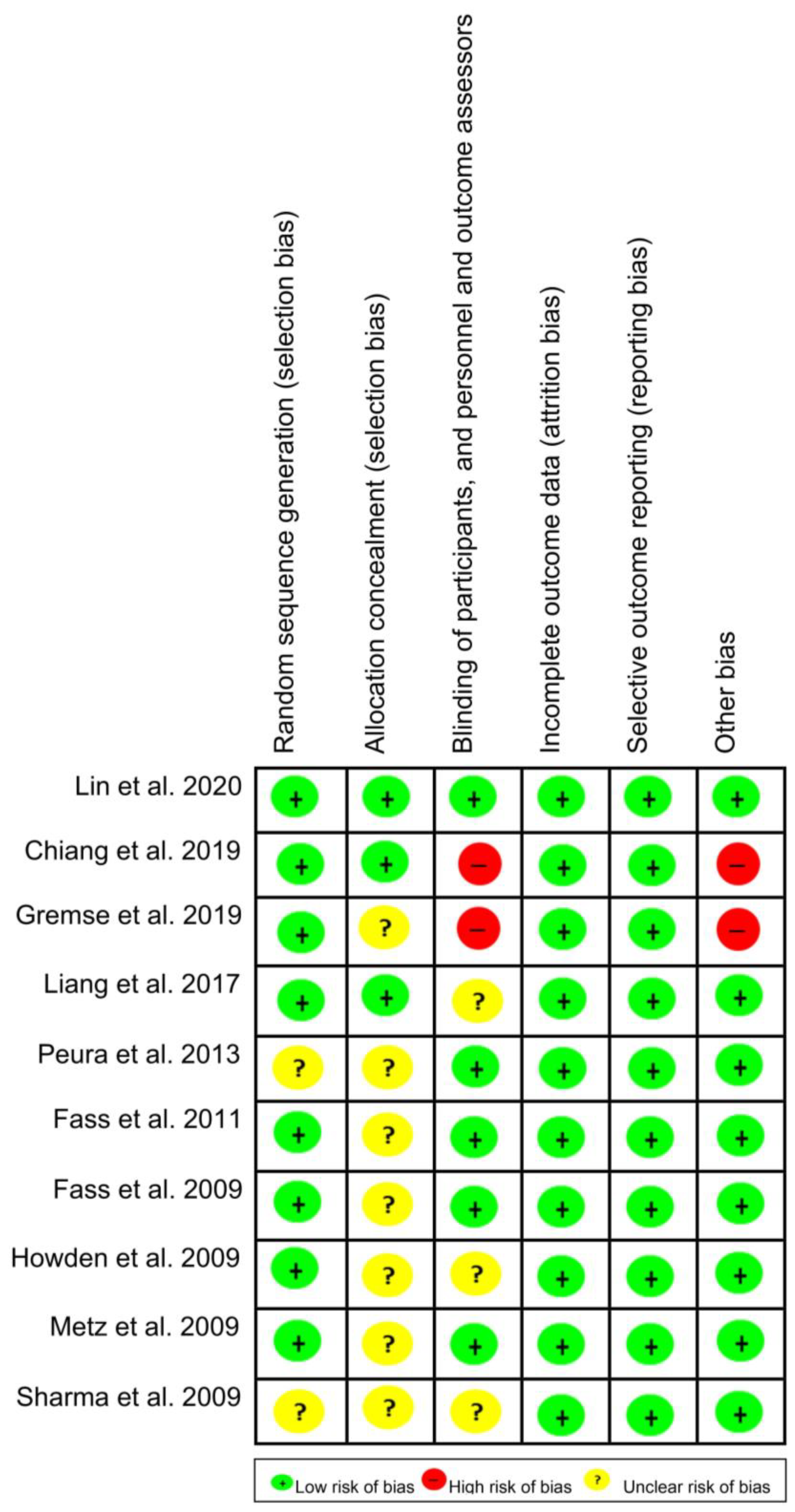
2.6. Bias Risk Assessment
2.7. Quality of Evidence
2.8. Synthesis of Results
3. Results
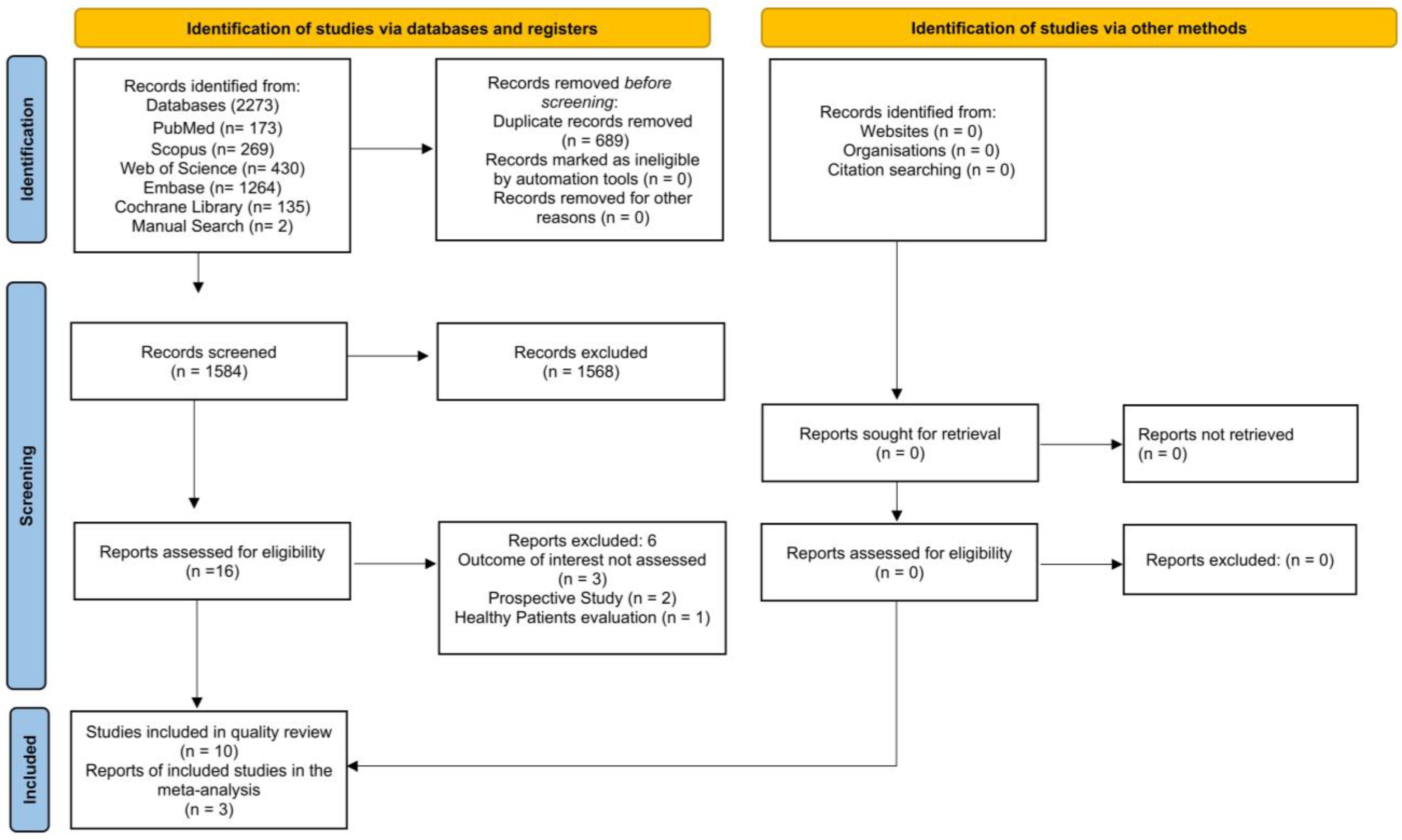
3.1. Bibliographic Search
3.2. Study Description
3.3. Outcome Results in the Treatment of GERD
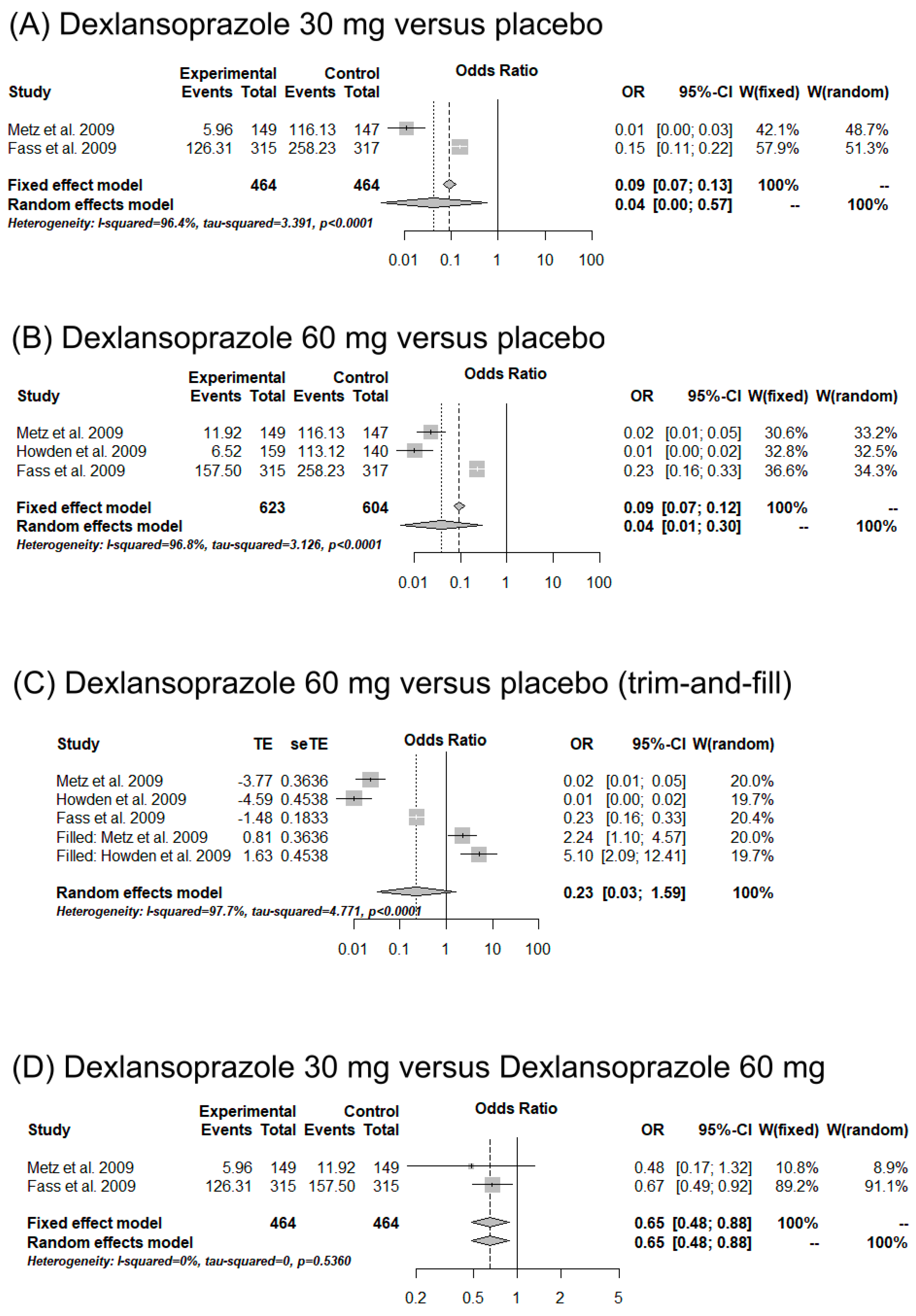
3.3.1. Efficacy of Dexlansoprazole versus Placebo Group
3.3.2. Efficacy of Dexlansoprazole versus Another PPI
3.4. Bias Risk and Quality of Evidence
3.5. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Search Strategy |
|---|
| PUBMED: 173 |
| ((Gastroesophageal Reflux) OR (Gastric Acid Reflux) OR (Acid Reflux, Gastric) OR (Reflux, Gastric Acid) OR (Gastric Acid Reflux Disease) OR (Gastro-Esophageal Reflux) OR (Gastro Esophageal Reflux) OR (Reflux, Gastro-Esophageal) OR (Gastroesophageal Reflux Disease) OR GERD OR (Reflux, Gastroesophageal) OR (Esophageal Reflux) OR (Gastro-oesophageal Reflux) OR (Gastro oesophageal Reflux) OR (Reflux, Gastro-oesophageal) OR (gastro-oesophageal reflux disease) OR (reflux disease) OR esophagitis OR heartburn OR Regurgitation)) AND ((Dexlansoprazole) OR (Lansoprazole, R-Isomer) OR (Lansoprazole, R Isomer) OR (R-Isomer Lansoprazole) OR (R-Lansoprazole) OR (R Lansoprazole) OR (Dexlansoprazole Sesquihydrate) OR (TAK 390MR) OR (TAK390MR) OR (TAK-390MR) OR (TAK-390) OR (TAK 390) OR (TAK390) OR Dexilant OR (T-168390) OR (T 168390) OR (T168390) |
| SCOPUS: 269 |
| (TITLE-ABS-KEY (((gastroesophageal AND reflux) OR (gastric AND acid AND reflux) OR (acid AND reflux, AND gastric) OR (reflux, AND gastric AND acid) OR (gastric AND acid AND reflux AND disease) OR (gastro-esophageal AND reflux) OR (gastro AND esophageal AND reflux) OR (reflux, AND gastro-esophageal) OR (gastroesophageal AND reflux AND disease) OR gerd OR (reflux, AND gastroesophageal) OR (esophageal AND reflux) OR (gastro-oesophageal AND reflux) OR (gastro AND oesophageal AND reflux) OR (reflux, AND gastro-oesophageal) OR (gastro-oesophageal AND reflux AND disease) OR (reflux AND disease) OR esophagitis OR heartburn OR regurgitation)) AND TITLE-ABS-KEY ((dexlansoprazole) OR (lansoprazole, AND r-isomer) OR (lansoprazole, AND r AND isomer) OR (r-isomer AND lansoprazole) OR (r-lansoprazole) OR (r AND lansoprazole) OR (dexlansoprazole AND sesquihydrate))) |
| EMBASE: 1264 |
| (‘gastroesophageal reflux’/exp OR ‘gastroesophageal reflux’ OR (gastroesophageal AND reflux) OR ‘gastric acid reflux’ OR (gastric AND (‘acid’/exp OR acid) AND reflux) OR ‘acid reflux, gastric’ OR ((‘acid’/exp OR acid) AND reflux, AND gastric) OR ‘reflux, gastric acid’ OR (reflux, AND gastric AND (‘acid’/exp OR acid)) OR ‘gastric acid reflux disease’ OR (gastric AND (‘acid’/exp OR acid) AND reflux AND (‘disease’/exp OR disease)) OR ‘gastro-esophageal reflux’/exp OR ‘gastro-esophageal reflux’ OR (‘gastro esophageal’ AND reflux) OR ‘gastro esophageal reflux’/exp OR ‘gastro esophageal reflux’ OR ((‘gastro’/exp OR gastro) AND esophageal AND reflux) OR ‘reflux, gastro-esophageal’ OR (reflux, AND ‘gastro esophageal’) OR ‘gastroesophageal reflux disease’/exp OR ‘gastroesophageal reflux disease’ OR (gastroesophageal AND reflux AND (‘disease’/exp OR disease)) OR gerd OR ‘reflux, gastroesophageal’/exp OR ‘reflux, gastroesophageal’ OR (reflux, AND gastroesophageal) OR ‘esophageal reflux’/exp OR ‘esophageal reflux’ OR (esophageal AND reflux) OR ‘gastro-oesophageal reflux’/exp OR ‘gastro-oesophageal reflux’ OR (‘gastro oesophageal’ AND reflux) OR ‘gastro oesophageal reflux’/exp OR ‘gastro oesophageal reflux’ OR ((‘gastro’/exp OR gastro) AND oesophageal AND reflux) OR ‘reflux, gastro-oesophageal’ OR (reflux, AND ‘gastro oesophageal’) OR ‘gastro-oesophageal reflux disease’ OR (‘gastro oesophageal’ AND reflux AND (‘disease’/exp OR disease)) OR ‘reflux disease’ OR (reflux AND (‘disease’/exp OR disease)) OR ‘esophagitis’/exp OR esophagitis OR ‘heartburn’/exp OR heartburn OR ‘regurgitation’/exp OR regurgitation) AND (dexlansoprazole OR (lansoprazole, AND ‘r isomer’) OR (lansoprazole, AND r AND isomer) OR (‘r isomer’ AND lansoprazole) OR ‘r lansoprazole’ OR (r AND lansoprazole) OR (dexlansoprazole AND sesquihydrate) OR (tak AND 390mr) OR tak390mr OR ‘tak 390mr’ OR ‘tak 390’ OR (tak AND 390) OR tak390 OR dexilant OR ‘t 168390’ OR (t AND 168390) OR t168390) AND [embase]/lim |
| WEB OF SCIENCE: 430 |
| ((Gastroesophageal Reflux) OR (Gastric Acid Reflux) OR (Acid Reflux, Gastric) OR (Reflux, Gastric Acid) OR (Gastric Acid Reflux Disease) OR (Gastro-Esophageal Reflux) OR (Gastro Esophageal Reflux) OR (Reflux, Gastro-Esophageal) OR (Gastroesophageal Reflux Disease) OR (GERD) OR (Reflux, Gastroesophageal) OR (Esophageal Reflux) OR (Gastro-oesophageal Reflux) OR (Gastro oesophageal Reflux) OR (Reflux, Gastro-oesophageal) OR (gastro-oesophageal reflux disease) OR (reflux disease) OR esophagitis OR heartburn OR Regurgitation) (All Fields) AND (Dexlansoprazole) OR (Lansoprazole, R-Isomer) OR (Lansoprazole, R Isomer) OR (R-Isomer Lansoprazole) OR (R-Lansoprazole) OR (R Lansoprazole) OR (Dexlansoprazole Sesquihydrate) OR (TAK 390m) OR (TAK390MR) OR (TAK-390m) OR (TAK-390) OR (TAK 390) OR (ta3090) OR (derivant) OR (T-168490) OR (T 168490) OR (t11839) (Tópico) |
| LIBRARY COCHRANE: 135 |
| (Gastroesophageal Reflux) OR (Gastric Acid Reflux) OR (Acid Reflux, Gastric) OR (Reflux, Gastric Acid) OR (Gastric Acid Reflux Disease) OR (Gastro-Esophageal Reflux) OR (Gastro Esophageal Reflux) OR (Reflux, Gastro-Esophageal) OR (Gastroesophageal Reflux Disease) OR (GERD) OR (Reflux, Gastroesophageal) OR (Esophageal Reflux) OR (Gastro-oesophageal Reflux) OR (Gastro oesophageal Reflux) OR (Reflux, Gastro-oesophageal) OR (gastro-oesophageal reflux disease) OR (reflux disease) OR esophagitis OR heartburn OR Regurgitation in Title Abstract Keyword AND (Dexlansoprazole) OR (Lansoprazole, R-Isomer) OR (Lansoprazole, R Isomer) OR (R-Isomer Lansoprazole) OR (R-Lansoprazole) OR (R Lansoprazole) OR (Dexlansoprazole Sesquihydrate) in Title Abstract Keyword |
Appendix B
References
- Slater, B.J.; Collings, A.; Dirks, R.; Gould, J.C.; Qureshi, A.P.; Juza, R.; Rodríguez-Luna, M.R.; Wunker, C.; Kohn, G.P.; Kothari, S.; et al. Multi-society consensus conference and guideline on the treatment of gastroesophageal reflux disease (GERD). Surg. Endosc. 2023, 37, 781–806. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hasak, S.; Nix, B.D.; Sayuk, G.S.; Newberry, R.D.; Gyawali, C.P. Genetic risk factors for perception of symptoms in GERD: An observational cohort study. Aliment. Pharmacol. Ther. 2018, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 6, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Coyne, K.; Wiklund, I. The gastro-oesophageal reflux disease impact scale: A patient management tool for primary care. Aliment. Pharmacol. Ther. 2007, 25, 1451–1459. [Google Scholar] [CrossRef]
- Quigley, E.M.; Hungin, A.P. Review article: Quality-of-life issues in gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2005, 22, 41–47. [Google Scholar] [CrossRef]
- Kellerman, R.; Kintanar, T. Gastroesophageal Reflux Disease. Prim. Care 2017, 44, 561–573. [Google Scholar] [CrossRef]
- Patti, M.G. An Evidence-Based Approach to the Treatment of Gastroesophageal Reflux Disease. JAMA Surg. 2016, 151, 73–78. [Google Scholar] [CrossRef]
- Chinzon, D.; Rossini, A.R.A.; Kiburd, B.; Navarro-Rodrigues, T.; Barbuti, R.C.; Hashimoto, C.L.; Eisig, J.N.; Moraes-Filho, J.P. Refluxo gastroesofágico diagnóstico e tratamento. Rev. Assoc. Médica Bras. Cons. Fed. Med. 2006, 50, 251–263. [Google Scholar]
- Iwakiri, K.; Fujiwara, Y.; Manabe, N.; Ihara, E.; Kuribayashi, S.; Akiyama, J.; Kondo, T.; Yamashita, H.; Ishimura, N.; Kitasako, Y.; et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease. J. Gastroenterol. 2022, 57, 267–285. [Google Scholar] [CrossRef]
- Böhmer, A.C.; Schumacher, J. Insights into the genetics of gastroesophageal reflux disease (GERD) and GERD-related disorders. Neurogastroenterol. Motil. 2017, 29, e13017. [Google Scholar] [CrossRef]
- Wiesner, A.; Zwolińska-Wcisło, M.; Paśko, P. Effect of Food and Dosing Regimen on Safety and Efficacy of Proton Pump Inhibitors Therapy-A Literature Review. Int. J. Environ. Res. Public. Health. 2021, 18, 3527. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Sachs, G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef]
- Hershcovici, T.; Jha, L.K.; Fass, R. Dexlansoprazole MR: A review. Ann. Med. 2011, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Radwan, P. Dexlansoprazole—A new-generation proton pump inhibitor. Prz. Gastroenterol. 2015, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Emerson, C.R.; Marzella, N. Dexlansoprazole: A proton pump inhibitor with a dual delayed-release system. Clin. Ther. 2010, 32, 1578–1596. [Google Scholar] [CrossRef]
- Grady, H.; Murakawa, Y.; Mulford, D.; Kukulka, M. Development of Dexlansoprazole Delayed-Release Capsules, a Dual Delayed-Release Proton Pump Inhibitor. J. Pharm. Sci. 2019, 108, 3496–3501. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chalub, L.O.; Nunes, G.P.; Ferrisse, T.M.; Strazzi-Sahyon, H.B.; Dos Santos, P.H.; Gomes-Filho, J.E.; Cintra, L.T.A.; Sivieri-Araujo, G. Postoperative pain in root canal treatment with ultrasonic versus conventional irrigation: A systematic review and meta-analysis of randomized controlled trials. Clin. Oral Investig. 2022, 26, 3343–3356. [Google Scholar] [CrossRef]
- Nunes, G.P.; Pirovani, B.O.; Nunes, L.P.; Silva, A.N.A.; Morábito, M.J.S.D.; Nunes-Júnior, N.A.; Delbem, A.C.B.; Ferrisse, T.M. Does oral lichen planus aggravate the state of periodontal disease? A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3357–3371. [Google Scholar] [CrossRef]
- Nunes, G.P.; Delbem, A.C.B.; Gomes, J.M.L.; Lemos, C.A.A.; Pellizzer, E.P. Postoperative pain in endodontic retreatment of one visit versus multiple visits: A systematic review and meta-analysis of randomized controlled trials. Clin. Oral Investig. 2021, 25, 455–468. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane, 2022. Available online: www.training.cochrane.org/handbook (accessed on 13 August 2023).
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence. 2019. Available online: https://cccrg.cochrane.org/author-resources (accessed on 7 November 2023).
- Han, S.; Choi, H.Y.; Kim, Y.H.; Choi, S.; Kim, S.; Nam, J.Y.; Kim, B.; Song, G.S.; Lim, H.-S.; Bae, K.-S. Comparison of Pharmacodynamics between Tegoprazan and Dexlansoprazole Regarding Nocturnal Acid Breakthrough: A Randomized Crossover Study. Gut Liver 2023, 17, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.D.; Pilmer, B.; Kierkuś, J.; Hunt, B.; Perez, M.C.; Gremse, D. Dexlansoprazole for Heartburn Relief in Adolescents with Symptomatic, Nonerosive Gastro-esophageal Reflux Disease. Dig. Dis. Sci. 2017, 62, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Peura, D.A.; Pilmer, B.; Hunt, B.; Mody, R.; Perez, M.C. The effects of increasing body mass index on heartburn severity, frequency and response to treatment with dexlansoprazole or lansoprazole. Aliment. Pharmacol. Ther. 2013, 37, 810–818. [Google Scholar] [CrossRef]
- Fass, R.; Inadomi, J.; Han, C.; Mody, R.; O’Neil, J.; Perez, M.C. Maintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified release. Clin. Gastroenterol. Hepatol. 2012, 10, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Peura, D.A.; Metz, D.C.; Dabholkar, A.H.; Paris, M.M.; Yu, P.; Atkinson, S.N. Safety profile of dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed release formulation: Global clinical trial experience. Aliment. Pharmacol. Ther. 2009, 30, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, J.; Atkinson, S.N. Effects of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor, on plasma gastrin levels in healthy subjects. J. Clin. Pharmacol. 2009, 49, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Luo, J.C.; Ting, P.H.; Chang, T.; Huang, Y.; Hou, M.; Lee, F. Comparison of the efficiency of two different proton pump inhibitor formula in treatment of patients with atypical gastroesophageal reflux disease: A prospective randomized study. J. Gastroenterol. Hepatol. 2020, 35, 2096–2102. [Google Scholar] [CrossRef]
- Chiang, H.H.; Wu, D.C.; Hsu, P.I.; Kuo, C.-H.; Tai, W.-C.; Yang, S.-C.; Wu, K.-L.; Yao, C.-C.; Tsai, C.-E.; Liang, C.-M.; et al. Clinical efficacy of 60-mg dexlansoprazole and 40-mg esomeprazole after 24 weeks for the on-demand treatment of gastroesophageal reflux disease grades A and B: A prospective randomized trial. Drug Des. Dev. Ther. 2019, 13, 1347–1356. [Google Scholar] [CrossRef]
- Gremse, D.; Gold, B.D.; Pilmer, B.; Hunt, B.; Korczowski, B.; Perez, M.C. Dual Delayed-Release Dexlansoprazole for Healing and Maintenance of Healed Erosive Esophagitis: A Safety Study in Adolescents. Dig. Dis. Sci. 2019, 64, 493–502. [Google Scholar] [CrossRef]
- Liang, C.M.; Kuo, M.T.; Hsu, P.I.; Kuo, C.-H.; Tai, W.-C.; Yang, S.-C.; Wu, K.-L.; Wang, H.-M.; Yao, C.-C.; Tsai, C.-E.; et al. First-week clinical responses to dexlansoprazole 60 mg and esomeprazole 40 mg for the treatment of grades A and B gastroesophageal reflux disease. World J. Gastroenterol. 2017, 23, 8395–8404. [Google Scholar] [CrossRef]
- Peura, D.A.; Pilmer, B.; Hunt, B.; Mody, R.; Perez, M.C. Distinguishing the impact of dexlansoprazole on heartburn vs. regurgitation in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2013, 38, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Johnson, D.A.; Orr, W.C.; Han, C.; Mody, R.; Stern, K.N.; Pilmer, B.L.; Perez, C.M. The effect of dexlansoprazole MR on nocturnal heartburn and GERD-related sleep disturbances in patients with symptomatic GERD. Am. J. Gastroenterol. 2011, 106, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Chey, W.D.; Zakko, S.F.; Andhivarothai, N.; Palmer, R.N.; Perez, M.C.; Atkinson, S.N. Clinical trial: The effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux disease. Aliment. Pharmacol. Ther. 2009, 29, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Howden, C.W.; Larsen, L.M.; Perez, M.C.; Palmer, R.; Atkinson, S.N. Clinical trial: Efficacy and safety of dexlansoprazole MR 60 and 90 mg in healed erosive oesophagitis—Maintenance of healing and symptom relief. Aliment. Pharmacol. Ther. 2009, 30, 895–907. [Google Scholar] [CrossRef]
- Metz, D.C.; Howden, C.W.; Perez, M.C.; Larsen, L.; O’Neil, J.; Atkinson, S.N. Clinical trial: Dexlansoprazole MR, a proton pump inhibitor with dual delayed-release technology, effectively controls symptoms and prevents relapse in patients with healed erosive oesophagitis. Aliment. Pharmacol. Ther. 2009, 29, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shaheen, N.J.; Perez, M.C.; Pilmer, B.L.; Lee, M.; Atkinson, S.N.; Peura, D. Clinical trials: Healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation--results from two randomized controlled studies. Aliment. Pharmacol. Ther. 2009, 29, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; El-Serag, H. Systematic review: The association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2011, 34, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Kyte, D.G.; Draper, H.; Ives, J.; Liles, C.; Gheorghe, A.; Calvert, M. Patient reported outcomes (PROs) in clinical trials: Is ’in-trial’ guidance lacking? a systematic review. PLoS ONE 2013, 8, e60684. [Google Scholar] [CrossRef]
- Ghisa, M.; Barberio, B.; Savarino, V.; Marabotto, E.; Ribolsi, M.; Bodini, G.; Zingone, F.; Frazzoni, M.; Savarino, E. The Lyon Consensus: Does It Differ from the Previous Ones? J. Neurogastroenterol. Motil. 2020, 26, 311–321. [Google Scholar] [CrossRef]
- Katz, P.O.; Gerson, L.B.; Vela, M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2013, 108, 308–329. [Google Scholar] [CrossRef]
- Peura, D.A.; Lee, C.; Siepman, N.; Kovacs, T.O. Long-term efficacy of symptom-based, titrated dose lansoprazole in preventing relapse of erosive reflux esophagitis in subjects with a recent history of erosive reflux esophagitis. Gastroenterology 2005, 128, 527–528. [Google Scholar]
- Freston, J.W.; Rose, P.A.; Heller, C.A.; Haber, M.; Jennings, D. Safety profile of Lansoprazole: The US clinical trial experience. Drug Saf. 1999, 20, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Aciphex® (Rabeprazole Sodium). Full Prescribing Information. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020973s035204736s005lbl.pdf (accessed on 13 November 2023).
- Nexium® (Esomeprazole Magnesium). Full Prescribing Information. Wilmington, DE: AstraZeneca LP. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022101s014021957s017021153s050lbl.pdf (accessed on 8 November 2023).
- Prilosec® (Omeprazole). Full Prescribing Information. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf (accessed on 10 November 2023).
- Lee, R.D.; Vakily, M.; Mulford, D.; Wu, J.; Atkinson, S.N. Clinical trial: The effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel Dual Delayed Release formulation of a proton pump inhibitor—Evidence for dosing flexibility. Aliment. Pharmacol. Ther. 2009, 29, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Shapiro, M.; Dekel, R.; Sewell, J. Systematic review: Proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment. Pharmacol. Ther. 2005, 22, 79–94. [Google Scholar] [CrossRef]
- Hungin, A.P.; Rubin, G.; O’Flanagan, H. Factors influencing compliance in long-term proton pump inhibitor therapy in general practice. Br. J. Gen. Pract. 1999, 49, 463–464. [Google Scholar]

| Reasons | References |
|---|---|
| Prospective Study | Fass et al., 2012 [26] and Gold et al., 2017 [24] |
| Outcome of interest not assessed | Peura et al., 2009 [27], Zhang et al., 2009 [28], and Peura et al., 2013 [25] |
| Healthy patients (absence of GERD) | Han et al., 2023 [23] |
| Authors, Year (Local) | G1: Dexlansoprazole G2: Comparison Group (Placebo or Another PPI) Number of Patients (n), and Sex (M/F): | Mean Age | Characteristics of the Samples | Use of Medication and Systemic Disturbs | Method of Analysis | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|
| Lin et al., 2020 [29] (Taipei, Taiwan) | G1: Dexlansoprazole 60 mg; n = 116 (M: 44; F: 67) G2: Lansoprazole 30 mg; n = 116 (M: 36; F: 75) | G1: 58.1 ± 12.4 G2: 56.4 ± 11.5 | n (%) Acid regurgitation G1: 64 (55.2%) G2: 76 (65.5%) Erosive esophagitis G1: 28 (24.1%) G2: 32 (27.6%) Los Angeles Score: grades A, B, C, D. Heartburn G1: 52 (44.8%) G2: 47 (40.5%) Typical symptom G1: 100 (86.2%) G2: 107 (92.2%) Cough G1: 51 (43.9%) G2: 50 (43.1%) Globus G1: 66 (56.9%) G2: 78 (67.2%) Hoarseness G1: 21 (18.1%) G2: 20 (17.2%) | Diabetes and Dyslipidemia | Endoscopy and questionnaire designed using reflux symptom index (RSI) | 8 weeks | Therapeutic response rate Typical symptoms, n/N (%) G1: 93/100 (93.0%) G2: 87/107 (81.3%) p = 0.014 Acid regurgitation, n/N (%) G1: 56/64 (87.5%) G2: 62/76 (81.6%) Heartburn, n/N (%) G1: 48/52 (92.3%) G2: 35/47 (74.5%) p = 0.027 Atypical symptom, n/N (%) G1: 78/116 (67.2%) G2: 44/116 (37.9%) < 0.001 Cough, n/N (%) G1: 39/51 (76.5%) G2: 19/50 (38.0%) < 0.001 Globus, n/N (%) G1: 46/66 (69.7%) G2: 24/78 (30.8%) < 0.001 Hoarseness, n/N (%) G1: 11/21 (52.4%) G2: 6/20 (30.0%) |
| Chiang et al., 2019 [30] (Kaohsiung, Taiwan) | G1: Dexlansoprazole 60 mg; n = 43 (M: 20; F: 23) G2: Esomeprazole 40 mg; n = 43 (M: 20; F23) | G1: 46.9 G2: 50.5 | G1: GERD Los Angeles grades A and B G2: GERD Los Angeles grades A and B | n (%) Smoking G1: 9 (22.5) G2: 5 (12.2) Alcohol use G1: 14 (35.0) G2: 15 (36.6) Metabolic Syndrome G1: 24 (60.0) G2: 23(56.1) PPI dependence G1: 33 (82,5) G2: 38 (92,7) H. pylori infection G1: 6 (15.0) G2: 6 (14.6) | Endoscopy, Gastroesophageal Reflux Questionnaire (GERDQ) | Clinical GERDQ at 4, 8, 12, 16, 20 weeks and endoscopy at 24 weeks. | Changes in GERDQ scores G1/G2 (Mean ± SD) Week 0: 23.2 ± 3.7/23.7 ± 4.7 Week 4: 17.1 ± 3.7/18.0 ± 4.1 Week 8: 16.4 ± 3.6/16.9 ± 3.7 Week 12: 16.3 ± 4.0/17.4 ± 4.7 Week 16: 14.7 ± 4.4/16.2 ± 4.7 Week 20: 13.7 ± 3.2/15.0 ± 4.8 Week 24: 13.1 ± 3.8/16.5 ± 10.9 Days to symptom resolution G1: 9.2 ± 14.4/G2: 10.5 ± 16.2 p = 0.700 Improvement in the GERDQ score in the on-demand period (8 vs. 24 week) G1: p < 0.0001/G2: p = 0.846 Number of days with reflux symptoms: G1: 37.3 ± 37.8 / G2: 53.9 ± 54.2; p = 0.008 |
| Gremse et al., 2019 [31] (USA, Poland, Mexico and Portugal) | G1: Dexlansoprazole MR 30 mg; n = 25 (M: 14; F:11) Dexlansoprazole MR 60 mg; n = 62 (M: 38; F: 24) G2: Placebo; n = 26 (M:16; F:10) | G1: 30 mg: 14.6 60 mg: 14.8 G2: 14.8 | Patients with GERD and esophagitis (Los Angeles Score) severity grades A, B, C, or D: G1.1: 30 mg: A (14), B (11), C (0), D (0) G1.2: 60 mg: A (34), B (26), C (1) e D (1) G2: A (16), B (9), C (1), D (0) | Smoker G1.1: 1 (4.0) G1.2: 1 (1.6) | Endoscopy, eDiary entry, and investigator assessment of GERD | Treatment follow-up 3 months later. | EE Healing Phase After 8 weeks of treatment G1.2: 88% EE Healing Phase After 16-weeks, double-blind G1.1: 82%/G2: 55% Absence of Heartburn G1:86.6% of days G2: 68.1% of days Treatment-Free Follow-up Phase: 3 months: Absence of heartburn on average days G1: 86.3% G2: 83.6% Absence of rescue medication G1: 99.1% of days G2: 97.7% of days |
| Liang et al., 2017 [32] (Taiwan) | G1: Dexlansoprazole 60 mg; n = 81 (M: 34; F: 47) G2: Esomeprazole 40 mg; n = 81 (M: 43; F: 38) | G1: 50.6 ± 13.3 G2: 49.9 ± 12.8 | Patients with clinical symptoms of acid regurgitation, heartburn, and a feeling of acidity in the stomach and who had endoscopy-confirmed LA grades A or B erosive esophagitis | N (%) H. pylori infection Previous history G1:10 (12.3) G2:15 (18.5) Current infection G1:10 (12.3) G2: 12 (14.8) Hiatal hernia G1:10 (12.3) G2: 15 (18.5) GEFV (grade 3 or 4) G1: 7 (8.6) G2: 8 (9.9) Esophagitis grade B G1: 15 (18.5) G2: 13 (16.0) | Endoscopy, GERDQ | The study continued for 1 week with evaluations on days 1, 3 and 7. | n (%) G1/G2 CSR Day 1: 21 (25.9)/23 (28.4) CSR Day 3: 27 (33.3)/26 (32.1) CSR Day 7: 42 (51.9)/39 (48.1) Night reflux: 45 (76.3)/40 (74.1) Night heart burn: 20 (33.9)/18 (33.3) Night acid reflux: 20 (33.9)/19 (35.2) Frequency of night symptoms: 2.7 ± 2.0/2.7 ± 2.4 |
| Peura et al., 2013 [33] (Virginia, USA) | GERD non-erosive G1: G1.1: 30 mg (n = 217); G1.2: 60 mg (n = 225) n = 442 (M: 130; F: 312) G2: Palacebo; n = 219 (M: 54; F: 165) EE G1: Dexlansoprazole 60 mg; n = 925 (M: 478; F: 447) G2: Lansoprazole 30 mg; n = 984 (M:507; F: 477) | GERD G1: Dexlansoprazole 30 mg (48.0) 60 mg (46.5) G2: Placebo (46.3) EE G1: Dexlansoprazole 60 mg (48.3) G2: Lansoprazole 30 mg (46.8) | 2 studies were performed: the first with patients with a history of heartburn for ≥6 months and a diagnosis of GERD experiencing heartburn ≥4 of 7 days, and the second with endoscopically confirmed EE (LA grades A, B, C, and D): G1: A (283), B (348), C (235) e D (59) G2: A (303), B (378), C (239) e C (64) | GERD: n (%) Smoker G1.1: 43 (19.8) G1.2: 37 (16.4) G2: 40 (18.3) Alcohol drinker G1.1: 105 (48.4) G1.2: 129 (57.3) G2: 131 (59.8) Positive H. pylori G1.1: 67 (30.9) G1.2: 64 (28.4) G2: 64 (29.2) EE: n (%) Smoker G1:234 (25.3) G2: 258 (26.2) Alcohol drinker G1: 534 (57.7) G2: 528 (53.7) Positive H. pylori G1: 9 (1.0) G2: 11 (1.1) | Endoscopy, Symptom Severity Index (PAGI-SYM) | Evaluation performed in weeks 2, 4, and 8. | Mean PAGI-SYM NERD Baseline/week 2/week 4 Heartburn/regurgitation subscale G1.1: 2.66/1.20/0.92 G1.2: 2.68/1.07/0.87 G2: 2.71/1.74/1.50 p ≤ 0.00001 Heartburn only G1.1: 3.12/1.40/1.03 G1.2: 3.18/1.29/1.02 G2: 3.16/2.18/1.85 Regurgitation only G1.1: 2.61/1.16/0.93 G1.2: 2.64/1.01/0.85 G2: 2.63/1.62/1.46 Mean PAGI-SYM EE Baseline/week 4/week 8 Heartburn/regurgitation subscale G1: 2.71/0.69/0.56 G2: 2.64/0.76/0.67 p < 0.05 Heartburn only G1: 3.30/0.79/0.68 G2: 3.23/0.92/0.81 Regurgitation only G1: 2.63/0.68/0.55 G2: 2.56/0.73/0.65 |
| Fass et al., 2011 [34] (Arizona, USA) | G1: Dexlansoprazole MR 30 mg; n = 152 (M:55; F: 97) G2: Placebo; n = 153 (M: 55; F: 98) | G1: 44.6 ± 11.29 G2: 43.9 ± 12.45 | Patients with a history of symptomatic GERD with or without a history of erosive esophagitis diagnosed >6 months before screening | PPI use within 6 months of randomization G1:79 (52.0) G2:79 (51.6) Alcohol drinker G1: 86 (56.6) G2: 81 (52.9) Smoker G1: 35 (23.0) G2: 42 (27.5) | Endoscopy, PSQI Pittsburgh Sleep Quality Index (PSQI), Nocturnal Gastroesophageal Reflux Disease Symptom Severity and Impact Questionnaire (N-GSSIQ), Work Productivity and Activity Impairment (WPAI) | Endoscopy evaluation 4 days before day 1. Period of study therapy: 4 weeks. Evaluations on day 1 and week 4. | (%) Nights without heartburn G1: 73.1/G2: 35.7 Patients with relief of nocturnal heartburn during the last 7 days of treatment: G1: 47.5/G2: 19.6 Patients with relief of GERD-related sleep disturbances during the last 7 days of treatment G1: 69.7/G2: 47.9 Baseline/4 week/changes (mean and SD): Nocturnal GERD symptom severity subscale mean and SD Baseline/4 week/changes G1: 29.20 ± 12.13/10.85 ± 13.03/ −18.35 ± 13.51 G2: 30.33 ± 11.29/18.45 ± 14.67/ −11.88 ± 13.01 |
| Fass et al., 2009 [35] (Arizona, USA) | G1: n = 630 (M: 190; F: 440) G1.1: Dexlansoprazole MR 30 mg; n = 315 (M: 84; F: 231) G1.2: Dexlansoprazole MR 60 mg; n = 315 (M: 106; F: 209) G2: Placebo; n = 317 (M: 84; F: 233) | G1: 47.6 (13.6) G2: 47.6 (14.4) | Patients with NERD who displayed normal mucosa (no EO) at the screening endoscopy. | BMI G1: 627/G2: 317 Helicobacter pylori status, n Positive G1: 185/G2: 89 Alcohol use, n Drinker G1: 343/G2: 182 Smoking status, n Smoker G1: 129/G2: 52 | Endoscopy, PAGI-SYM | After 4 weeks of self-administration of the drug, all patients were examined and submitted to laboratory evaluations. | Median percentage of 24 h heartburn-free days G1.1: 54.9 G1.2: 50.0 G2: 18.54 Median percentage of nights without heartburn G1.1: 80.8 G1.2: 76.9 G2: 51.7 |
| Howden et al., 2009 [36] (Illinois, USA) | G1: n = 311 (M: 165; F: 146); G1.1 Dexlansoprazole MR 60 mg 159 (M: 83; F: 76) G1.2 Dexlansoprazole MR 90 mg 152 (M: 82; F:70) G2: Placebo; n = 140 (M:70; F: 70) | G1: G1.1—60 mg (49.7) G1.2—90 mg (48.8) G2: 48.2 | Patients with erosive esophagitis (EO severity by LA classification—A, B, C, D): G1: A (114), B (119), C (65) e D (13) G2: A (58), B (48), C (28) e D (6) | None | Endoscopy, PAGI-SYM, Disorders Quality-of-Life Index Questionnaire (PAGI-QOL) | Endoscopy before the therapy and 4 or 8 weeks after the therapy. Return visits after 1, 3, and 6 months. | Median days without heartburn during treatment, % 24 h days/Nights G1.1: 95.8/98.3; G1.2: 94.4/97.1; G2: 19.2/50.0 Median of mean severity of heartburn during treatment, % 24 h days/Nights G1.1: 0.03/0.02; G1.2: 0.04/0.04; G2: 1.00/0.83 Median days without rescue medication during treatment, % G1.1: 94.9/G1.2: 93.6/G2: 27.5 |
| Metz et al., 2009 [37] (75 Centers in USA, 19 in non-USA sites) | G1: n = 298 (M: 143; F: 155); G1.1 Dexlansoprazole 30 mg; G1.2: Dexlansoprazole MR 60 mg G2: Placebo; n = 147 (M:72; F: 75) | G1: 30 mg: 47.1 60 mg: 47.9 G2: 49.5 | Patients with GERD and EE: EO severity by LA classification—A, B, C, D: G1: A (109), B (103), C (70) e D (16) G2: A (51), B (57), C (34) e D (5) | None | Endoscopy, PAGI-QOL, PAGI-SYM | Outcomes were recorded on day 1 and at months 1, 3, and 6. | Median percentage of 24 h heartburn-free days and median percentage of nights without heartburn during treatment: 24 h heartburn-free days G1.1: 96%/G1.2: 91%/G2: 29% Nights without heartburn G1.1: 99%/G1.2: 96%/G2: 72% |
| Sharma et al., 2009 [38] (Kansas, USA) | Study 1: G1: n = 1348 (M: 746; F: 602); G1.1 Dexlansoprazole MR 60 mg G1.2 Dexlansoprazole MR 90 mg G2: n = 690 (M: 365; F: 237); Lansoprazole MR 30 mg Study 2: G1: n = 1381 (M: 733; F: 648); Dexlansoprazole MR 60 mg G1.2 Dexlansoprazole MR 90 mg G2: Lansoprazole MR 30 mg; n = 673 (M: 362; F: 275) | Study 1 G1: 60 mg (47.8) 90 mg (47.3) G2: 47.3 Study 2 G1: 60 mg (48.7) 90 mg (47.7) G2: (47.3) | Adult patients’ EO (EO severity by LA classification—A, B, C, D): Study 1 G1: A (478), B (480), C (311) e D (78) G2: A (231), B (248), C (170) e D (40) Study 2 G1: A (505), B (478), C (308) e D (88) G2: A (222), B (257), C (150) e D (44) | None | Endoscopy, patient responses in Daily Diaries—The Gastro Symptom Rating Scale (GSRS) | The screening period lasted up to 21 days. On the week 4 visit and on the week 8 or final visit (if not healed by week 4). | (%) 24 h heartburn-free days: Study 1: G1.1: 82.1/G1.2: 84.2/G2: 80.0; p > 0.05 Study 2: G1.1: 83.0/G1.2: 80.8/G2: 78.3 Patients with complete EE healing by week 8 Life table/crude rate Study 1 G1.1: 92.3/85.3—G1.2: 92.2/85.8—G2: 86.1/79.0; p > 0.025 Study 2 G1.1: 93.1/86.9; G1.2: 94.9/89.4; G2: 91.5/84.6; p < 0.05 Patients with baseline grades C or D EE-healed by week 8 Life table/crude rate Study 1: G1.1: 88.9/79.7—G1.2: 83.8/74.1; G2: 74.5/65.0 Study 2: G1.1: 87.6/77.8; G1.2: 93.3/86.3; G2: 87.7/78.9 |
| Quality Assessment | |||||||
|---|---|---|---|---|---|---|---|
| Nº of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Certainty |
| 10 | Randomized trials | Not serious | Serious a,b | Not serious | Not serious | All plausible residual confounding variables would reduce the demonstrated effect. | ⨁⨁⨁◯ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, G.P.; Silveira, T.C.; Marciano, J.V.S.; dos Reis-Prado, A.H.; Ferrisse, T.M.; dos Anjos, E.B.; Fernandes, M.H. The Effect of Dexlansoprazole on Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 1247. https://doi.org/10.3390/ijms25021247
Nunes GP, Silveira TC, Marciano JVS, dos Reis-Prado AH, Ferrisse TM, dos Anjos EB, Fernandes MH. The Effect of Dexlansoprazole on Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(2):1247. https://doi.org/10.3390/ijms25021247
Chicago/Turabian StyleNunes, Gabriel Pereira, Thayná Cerqueira Silveira, João Vítor Silveira Marciano, Alexandre Henrique dos Reis-Prado, Tulio Morandin Ferrisse, Evandro Barbosa dos Anjos, and Maria Helena Fernandes. 2024. "The Effect of Dexlansoprazole on Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 2: 1247. https://doi.org/10.3390/ijms25021247
APA StyleNunes, G. P., Silveira, T. C., Marciano, J. V. S., dos Reis-Prado, A. H., Ferrisse, T. M., dos Anjos, E. B., & Fernandes, M. H. (2024). The Effect of Dexlansoprazole on Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 25(2), 1247. https://doi.org/10.3390/ijms25021247