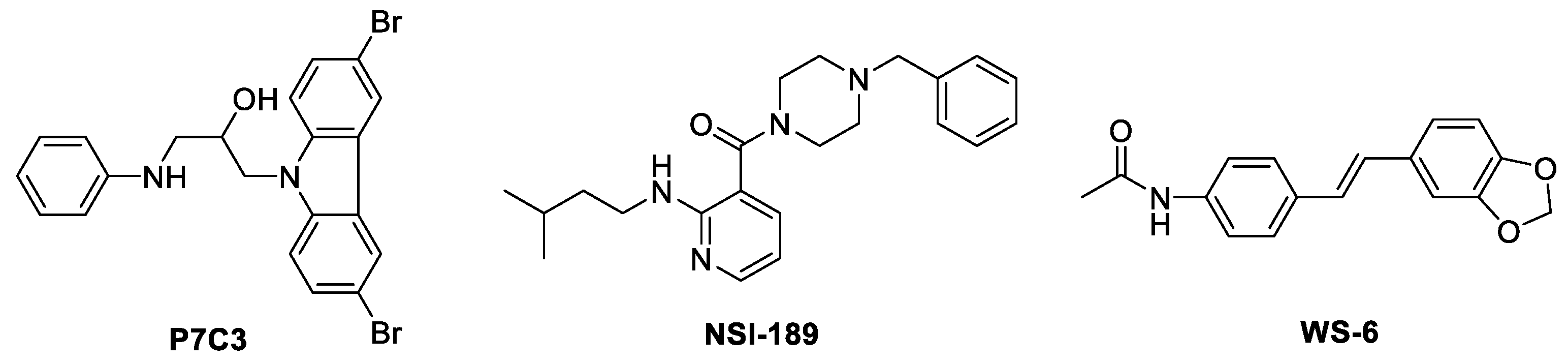
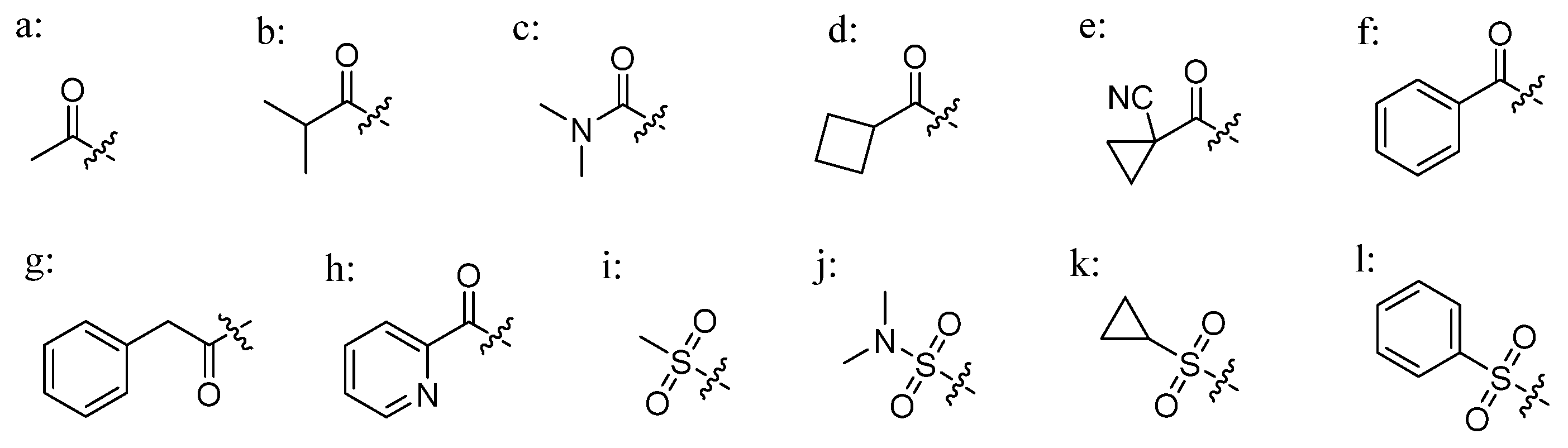
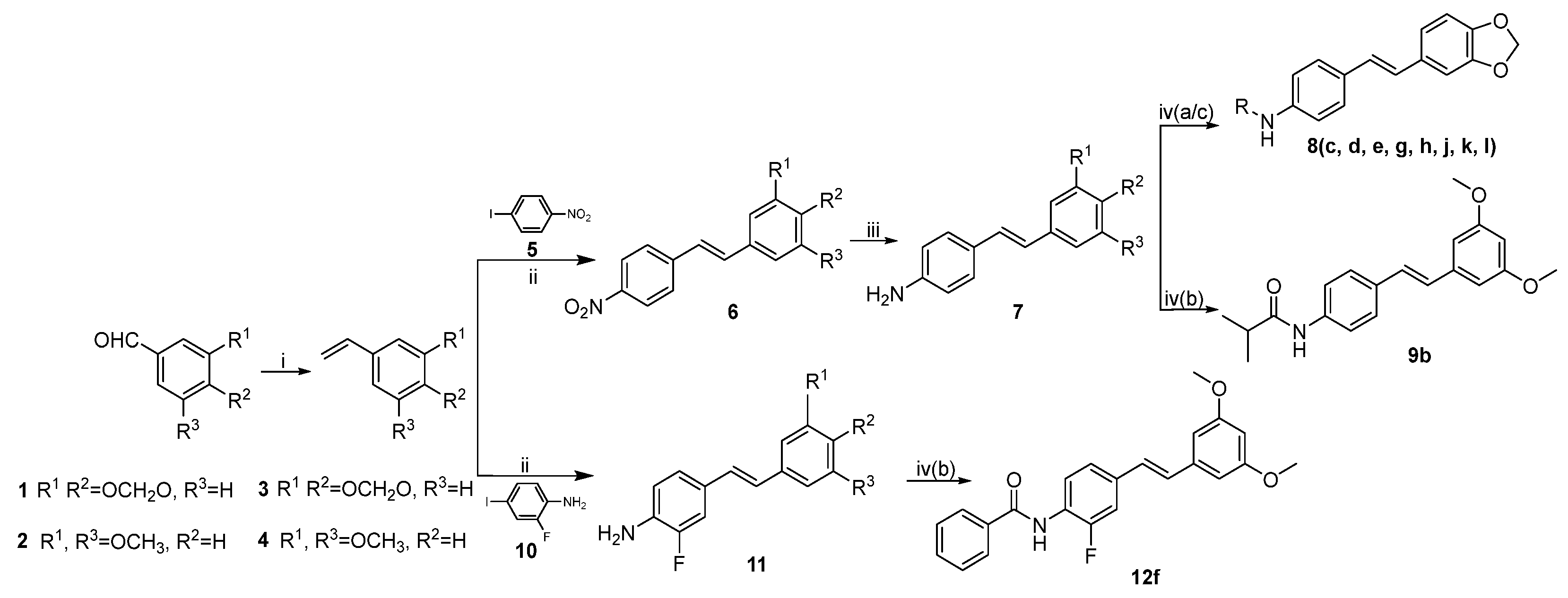
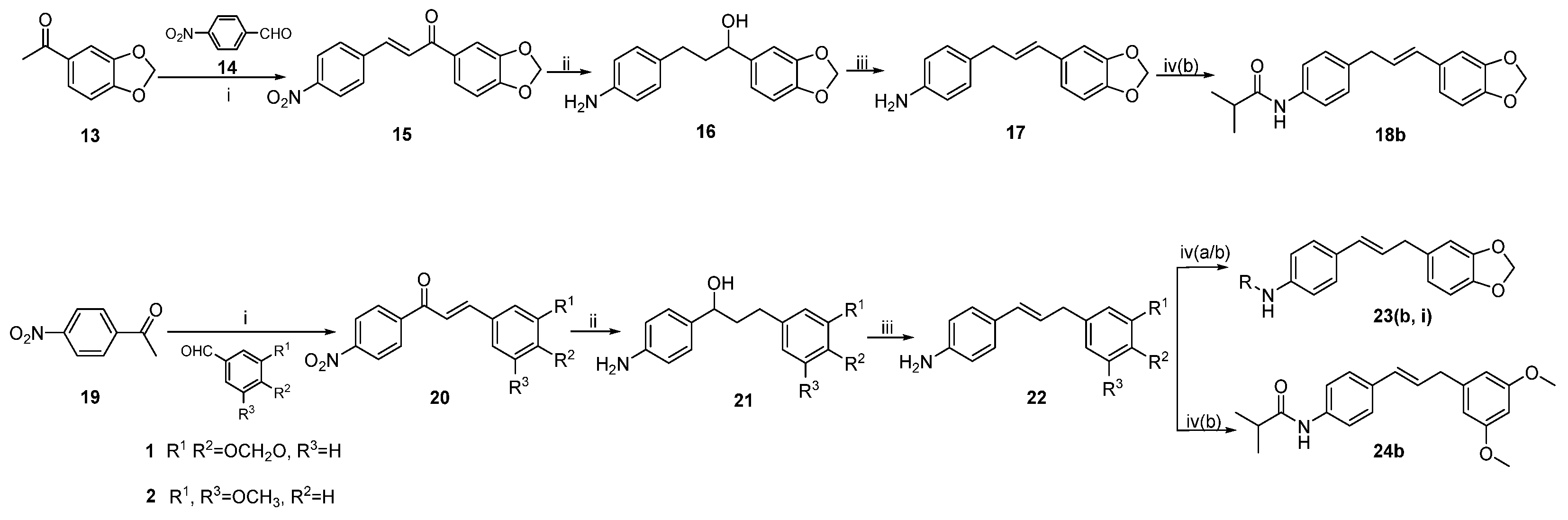
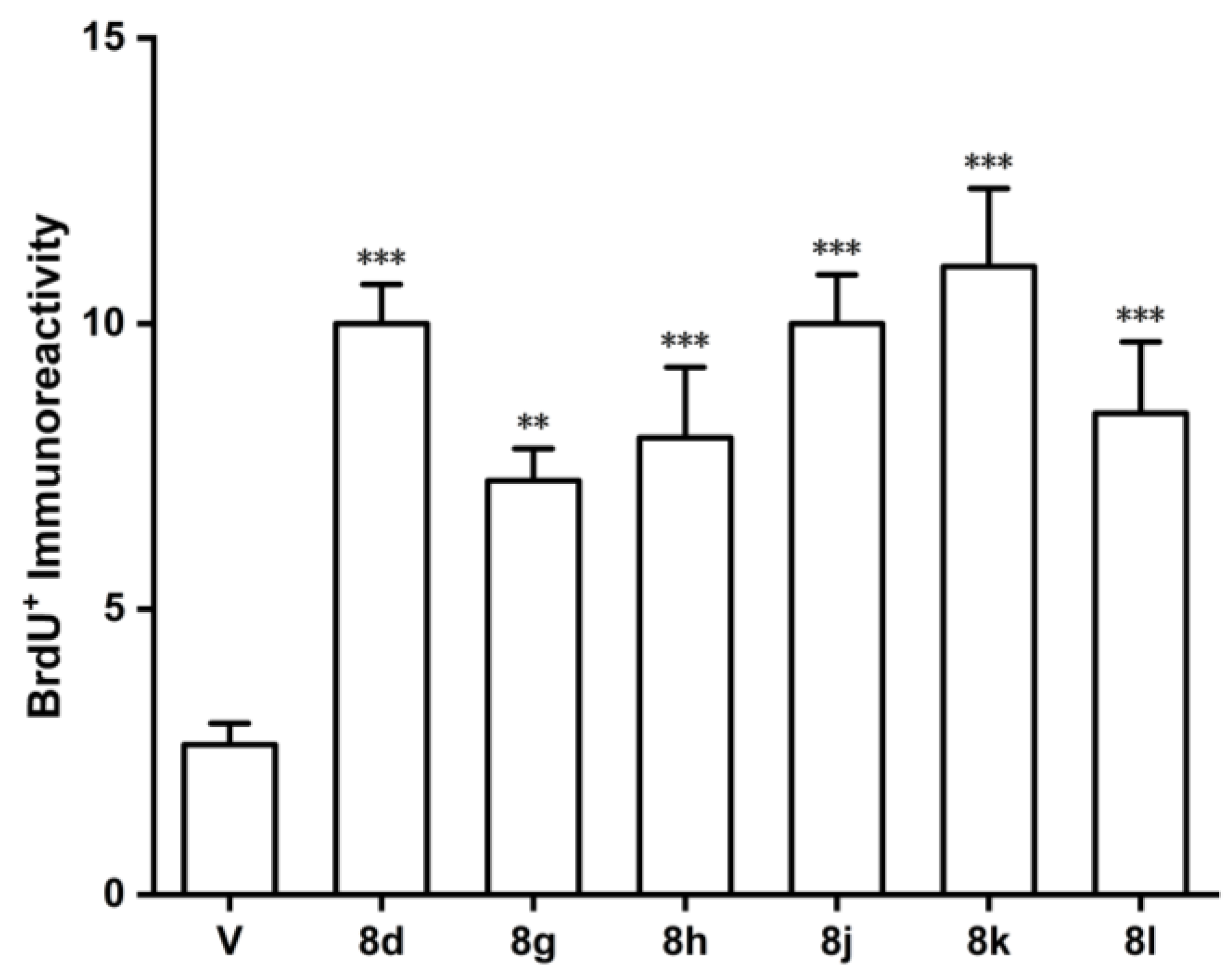
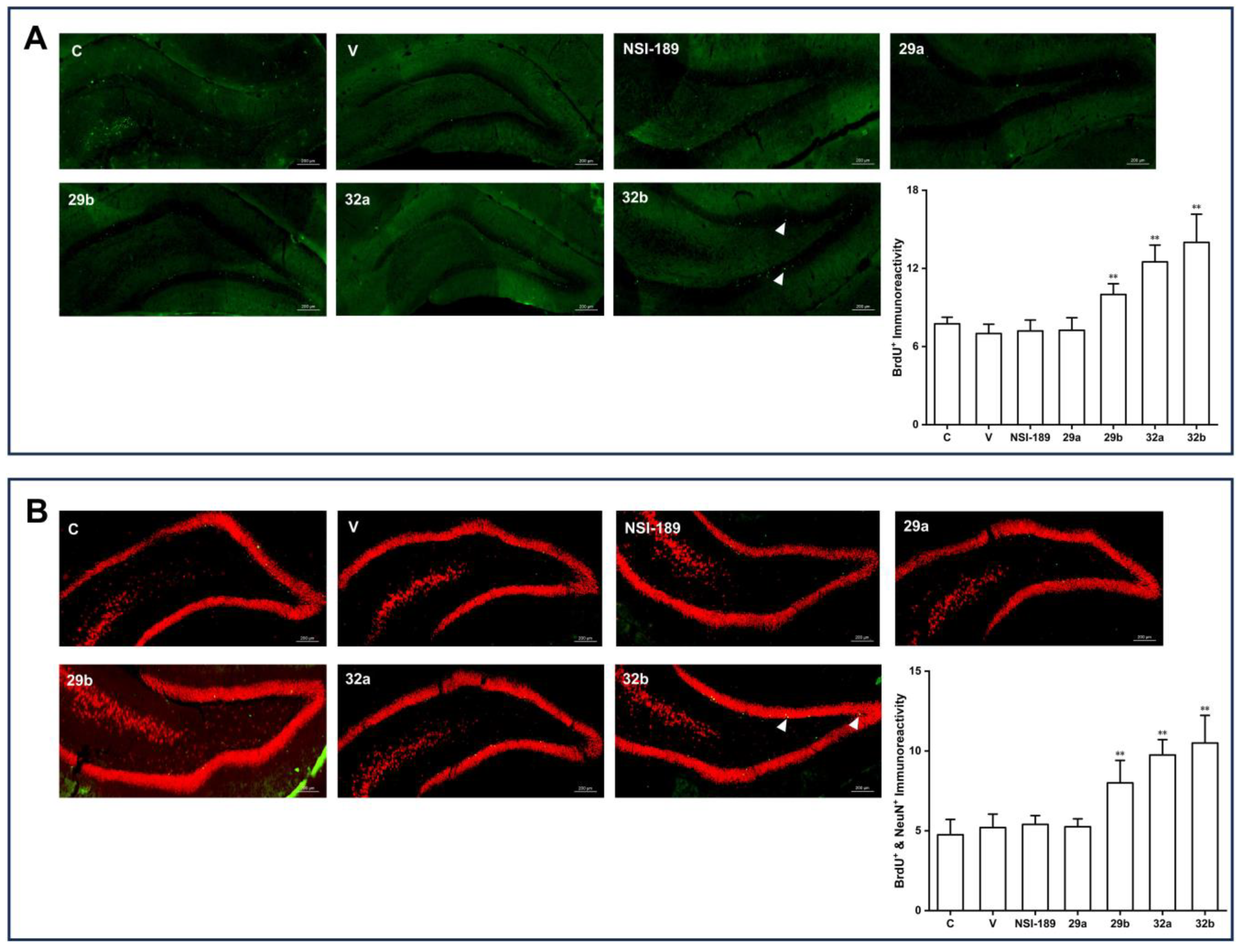
3.1. Chemistry
All reagents and solvents were purchased from commercial sources (J&K Scientific) and used without further purification unless otherwise noted. All non-aqueous reactions were carried out under argon using dry solvents, unless otherwise noted. Reactions were monitored through thin-layer chromatography, and 254 nm UV light was used for visualization. Column chromatography was performed on silica gel (100–200 or 200–300 mesh) purchased from Qingdao Haiyang.
1H and
13C nuclear magnetic resonance (NMR) spectra were recorded on Bruker ARX 400/500 MHz with tetramethylsilane (TMS) as an internal standard. High resolution mass spectra (HRMS) were obtained with Agilent 6210 LC-MS TOF or Agilent Q-TOF 6520 LC-MS (
Table S1 and Supplementary Materials).
General procedure 1: synthesis of compounds 3–4. A mixture of methyltriphenylphosphonium bromide (2.79 g, 7.8 mmol) and sodium amide (0.30 g, 7.8 mmol) in anhydrous ether (20 mL) was stirred at 25 °C under argon protection for 1 h. The mixture was cooled to −10 °C, and 1 (0.59 g, 3.9 mmol) was added. The mixture continued to be stirred at 25 °C for another 10 min. The resulting precipitate was filtered off, washed with ethyl acetate, and concentrated in vacuo. The residue was purified through column chromatography on silica gel (petroleum ether/ethyl acetate = 10:3) to afford 3 (0.19 g, 32.9%). Compound 4 (0.21 g, 32.9%) was prepared according to the same procedure as 2 (0.65 g, 3.9 mmol).
General procedure 2: synthesis of compounds 6 and 11. To a solution of substituted styrene (1.3 mmol, 1.2 eq), tetrabutylammonium bromide (0.58 g, 1.8 mmol), potassium acetate (0.19 g, 1.9 mmol), and palladium acetate (0.02 g, 0.1 mmol) in DMF (25 mL) was added, along with substituted iodobenzene (1.1 mmol, 1 eq). The mixture was stirred at 80 °C for 5 h under argon. The reaction solution was extracted using ethyl acetate. The organic layer was combined, washed with saturated NaCl solution, and concentrated in vacuo to afford 6 (or 11).
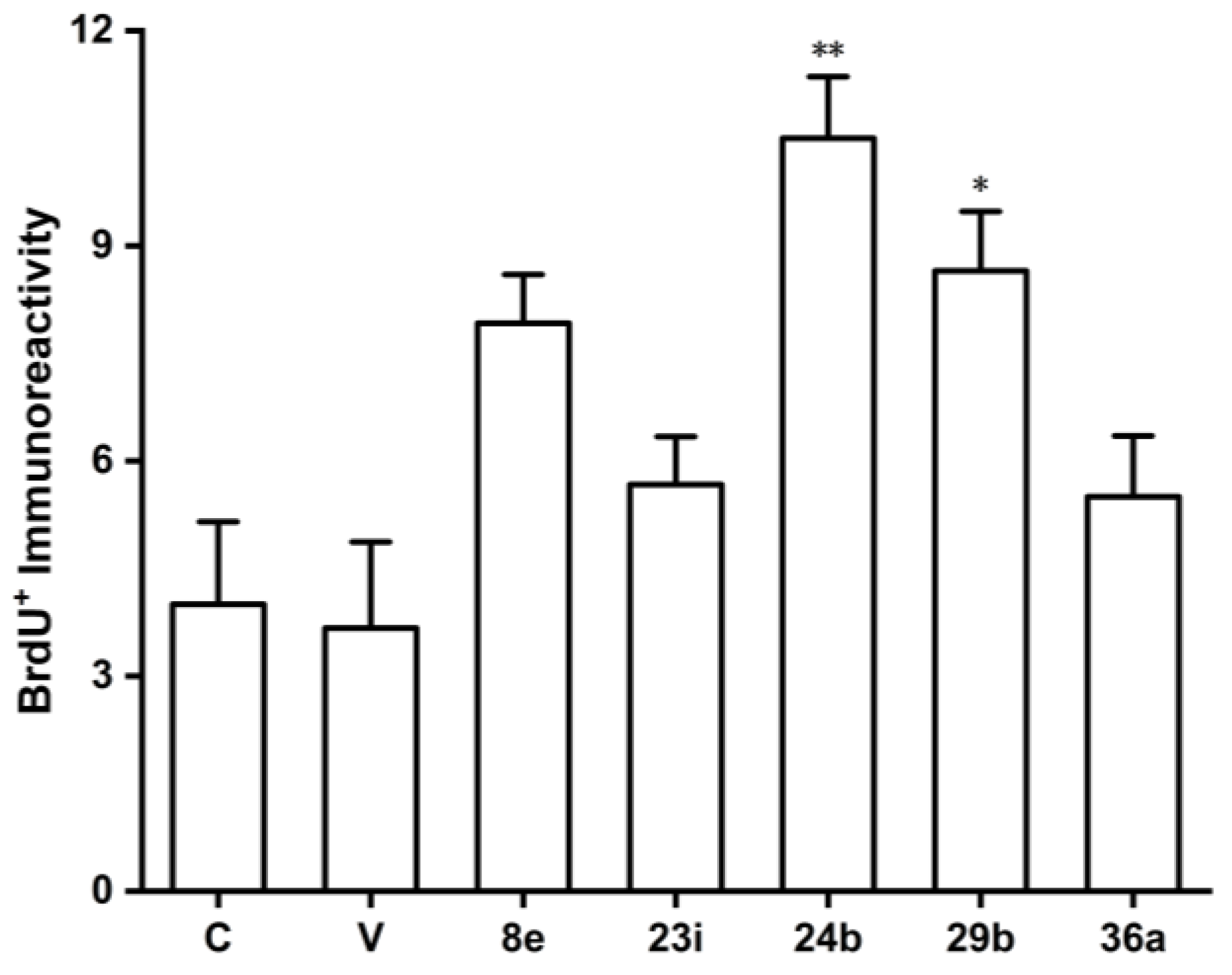
Substituted (E)-1-nitro-4-styrylbenzene (6). Compound 6 was prepared according to general procedure 2 from 5 (0.28 g, 1.1 mmol) and 3 (0.19 g, 1.3 mmol).
General procedure 3: synthesis of compounds 7 and 28. A well stirred mixture of compounds with a nitro group (10 mmol, 1 eq) and stannous chloride (40.0 mmol, 4 eq) in EtOH (40 mL) was heated to reflux for 2 h. After the reaction was completed, the solvent was removed, and the residue was stirred with NaOH (50 mL, 1 M) for another 0.5 h. The aqueous solution was extracted with ethyl acetate. The organic layer was combined, washed with saturated NaCl solution and then concentrated in vacuo. Purification through column chromatography on silica gel (dichloromethane = 100%) afforded products with an amino group.
Substituted (E)-4-styrylaniline (7). Compound 7 was prepared according to general procedure 3 from 6 (2.69 g, 10.0 mmol).
General procedure 4: synthesis of compounds 8, 9, 12, 18, 23, 24, 29, 32, 39, and 42.
General procedure 4(a): Acyl chloride (3.6 mmol, 3 eq) and pyridine (0.28 mL, 3.6 mmol) were added dropwise to a solution of compounds with an amino (1.2 mmol, 1 eq) and DMAP (0.02 g, 0.1 mmol) in CH2Cl2 (20 mL). The mixture was stirred at 25 °C from 1 h to 14 d. The mixture was poured into saturated NaHCO3 solution, the organic layer was separated, and the solvent was removed in vacuo. The residue was purified through column chromatography on silica gel to afford pure products.
General procedure 4(b): Pyridine (0.19 mL, 2.4 mmol) and anhydride (2.3 mmol, 3 eq) were added dropwise to a solution of compounds with an amino (0.8 mmol, 1 eq) and DMAP (0.02 g, 0.1 mmol) in CH2Cl2 (20 mL) below 0 °C. The mixture was stirred at 0 °C from 0.5 h to 1.5 h. The reaction solution was poured into saturated NaHCO3 solution, the organic layer was separated, and the solvent was removed in vacuo. The residue was purified through column chromatography on silica gel to afford pure target products.
General procedure 4(c): Pyridine (0.34 mL, 4.2 mmol) was added dropwise to a solution of compounds with an amino (1.0 mmol, 1 eq), DMAP (0.32 g, 2.6 mmol), DCC (0.54 g, 2.6 mmol), and carboxylic acid (2.6 mmol, 2.6 eq) in CH2Cl2 (20 mL) below 0 °C. The mixture was stirred at 0 °C for 1 h, then at 25 °C for 3 h. The reaction was filtered, then the filtrate was concentrated in vacuo to obtain compounds which were then purified through column chromatography.
(E)-3-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)-1,1-dimethylurea (8c). Compound
8c was prepared according to general procedure 4(a) from
N,
N-dimethylcarbamoyl chloride (0.32 mL, 3.6 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.28 g, 1.2 mmol) for 1 h. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
8c (0.26 g, 68.9%) as a product. HRMS (ESI) (M+H)
+ m/
z 311.1397, calcd for C
18H
19N
2O
3 311.1390.
1H NMR (Acetone-
d6, 500 MHz) δ: 7.73 (s, 1H), 7.56–7.54 (m, 2H), 7.43–7.41 (m, 2H), 7.15 (d,
J = 2.0 Hz, 1H), 7.03–6.98 (m, 3H), 6.82 (d,
J = 8.5 Hz, 1H), 6.00 (s, 2H), 3.00 (s, 6H).
13C NMR (Acetone-
d6, 125 MHz) δ: 156.4, 149.2, 148.0, 141.3, 133.4, 132.2, 127.7, 127.3, 127.0, 122.0, 120.3, 120.2, 109.1, 106.0, 102.1, 36.6.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)cyclobutanecarboxamide (8d). Compound
8d was prepared according to general procedure 4(c) from cyclobutanecarboxylic acid (0.83 g, 8.3 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.76 g, 3.2 mmol) for 3 h. Purification through column chromatography (dichloromethane = 100%, petroleum ether/ethyl acetate = 10:3) on silica gel afforded pure
8d (0.22 g, 21.2%) as a product. HRMS (ESI) (M+H)
+ m/
z 322.1445, calcd for C
20H
20NO
3 322.1438.
1H NMR (Acetone-
d6, 500 MHz) δ: 8.91 (s, 1H), 7.67 (d,
J = 8.5 Hz, 2H), 7.49–7.47 (m, 2H), 7.16 (d,
J = 1.5 Hz, 1H), 7.06–7.00 (m, 3H), 6.83 (d,
J = 8.0 Hz, 1H), 6.00 (s, 2H), 3.28–3.25 (m, 1H), 2.94–2.32 (m, 2H), 2.16–2.14 (m, 2H), 2.09–2.04 (m, 1H), 1.98–1.96 (m, 1H).
13C NMR (Acetone-
d6, 125 MHz) δ: 173.6, 149.2, 148.2, 139.9, 133.5, 133.3, 127.8, 127.5, 127.4, 122.2, 120.2, 120.1, 109.1, 106.1, 102.1, 41.4, 25.7, 18.7.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)-1-cyanocyclopropane-1-carboxamide (8e). Compound
8e was prepared according to general procedure 4(c) from 1-cyanocyclopropylformic acid (0.29 g, 2.6 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.24 g, 1.0 mmol) for 3 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
8e (0.25 g, 73.5%) as a product. HRMS (ESI) (M+H)
+ m/
z 333.1235, calcd for C
20H
17N
2O
3 333.1234.
1H NMR (DMSO-
d6, 400 MHz) δ: 10.06 (s, 1H), 7.64 (d,
J = 1.5 Hz, 2H), 7.50 (d,
J = 8.5 Hz, 2H), 7.23 (s, 1H), 7.09 (d,
J = 16.5 Hz, 1H), 7.04 (d,
J = 16.5 Hz, 1H), 6.99 (d,
J = 8.0 Hz, 1H), 6.87 (dd,
J = 8.0 Hz, 2.5 Hz, 1H), 6.02 (s, 2H), 1.64–1.71 (m, 4H).
13C NMR (DMSO-
d6, 100 MHz) δ: 163.7, 147.8, 146.9, 137.3, 133.3, 131.7, 127.4, 126.4, 126.1, 121.4, 121.1, 120.0, 108.3, 105.3, 101.1, 17.0, 14.8.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)-2-phenylacetamide (8g). Compound
8g was prepared according to general procedure 4(a) from phenylacetyl chloride (0.46 mL, 3.5 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.28 g, 1.2 mmol) for 1 h. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
8g (0.28 g, 66.6%) as a product. HRMS (ESI) (M+H)
+ m/
z 358.1445, calcd for C
23H
20NO
3 358.1438.
1H NMR (Acetone-
d6, 500 MHz) δ: 9.29 (s, 1H), 7.64–7.66 (m, 2H), 7.47–7.49 (m, 2H), 7.38–7.39 (m, 2H), 7.30–7.33 (m, 2H), 7.23–7.26 (m, 1H), 7.16 (d,
J =1.5 Hz, 1H), 7.08 (d,
J = 16.5 Hz, 1H), 7.04 (d,
J = 16.5 Hz, 1H), 7.00 (dd,
J = 8.0 Hz, 1.5 Hz, 1H), 6.82 (d,
J = 8.0 Hz, 1H), 6.00 (s, 2H), 3.70 (s, 2H).
13C NMR (Acetone-
d6, 125 MHz) δ: 169.7, 149.2, 148.2, 139.7, 136.9, 133.2, 130.1, 129.2, 128.0, 127.6, 127.5, 127.4, 122.2, 120.2, 120.2, 109.1, 106.1, 102.1, 44.8.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)picolinamide (8h). Compound
8h was prepared according to general procedure 4(c) from 2-picolinic acid (0.32 g, 2.6 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.24 g, 1.0 mmol) for 1 h. Purification through column chromatography (dichloromethane = 100%, petroleum ether/ethyl acetate = 10:3) on silica gel afforded pure
8h (0.16 g, 45.7%) as a product. HRMS (ESI) (M+H)
+ m/
z 345.1240, calcd for C
21H
17N
2O
3 345.1234.
1H NMR (DMSO-
d6, 500 MHz) δ: 10.55 (s, 1H), 8.64 (d,
J = 1.5 Hz, 1H), 8.06 (d,
J = 7.5 Hz, 1H), 7.96–7.99 (m, 1H), 7.82 (d,
J = 8.5 Hz, 2H), 7.57–7.59 (m, 1H), 7.45 (d,
J = 8.5 Hz, 2H), 7.15 (s, 1H), 7.03 (d,
J = 16.0 Hz, 1H), 6.98 (d,
J = 17.0 Hz, 1H), 6.91 (dd,
J = 8.0 Hz, 1.0 Hz, 1H), 6.81 (d,
J = 7.5 Hz, 1H), 5.93 (s, 2H).
13C NMR (DMSO-
d6, 125 MHz) δ: 162.3, 149.8, 148.4, 147.8, 146.8, 138.1, 137.5, 133.0, 131.8, 127.2, 126.9, 126.6, 126.2, 122.3, 121.4, 120.3, 108.4, 105.2, 101.0.
(E)-1-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)-N,N-dimethylmethanesulfonamide (8j). Compound
8j was prepared according to general procedure 4(a) from dimethylsulfamoyl chloride (0.27 mL, 2.5 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.20 g, 0.8 mmol) for 14 d. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
8j (0.02 g, 6.9%) as a product. HRMS (ESI) (M+H)
+ m/
z 347.1055, calcd for C
17H
19N
2O
4S 347.1060.
1H NMR (Acetone-
d6, 500 MHz) δ: 8.77 (s, 1H), 7.51–7.54 (m, 2H), 7.33–7.35 (m, 2H), 7.18 (d,
J = 1.5 Hz, 1H), 7.11 (d,
J = 16.5 Hz, 1H), 7.06 (d,
J = 16.5 Hz, 1H), 7.01 (dd,
J = 8.0 Hz, 2.0 Hz, 1H), 6.84 (d,
J = 8.0 Hz, 1H), 6.01 (s, 2H), 2.79 (s, 6H).
13C NMR (Acetone-
d6, 125 MHz) δ: 149.2, 148.2, 138.9, 134.2, 133.1, 128.3, 127.9, 127.1, 122.3, 120.9, 109.1, 106.1, 102.1, 38.4.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)cyclopropanesulfonamide (8k). Compound
8k was prepared according to general procedure 4(a) from cyclopropylsulfonyl chloride (0.25 mL, 2.5 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.20 g, 0.8 mmol) for 6 d. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
8k (0.24 g, 84.9%) as a product. HRMS (ESI) (M+H)
+ m/
z 344.0959, calcd for C
18H
18NO
4S 344.0951.
1H NMR (DMSO-
d6, 500 MHz) δ: 9.74 (s, 1H), 7.50 (d,
J = 8.5 Hz, 2H), 7.24 (dd,
J = 8.0 Hz, 1.5 Hz, 1H), 7.23 (d,
J = 8.5 Hz, 2H), 7.08 (d,
J = 16.5 Hz, 1H), 7.05 (d,
J = 16.5 Hz, 1H), 7.00 (dd,
J = 8.0 Hz, 1.5 Hz, 1H), 6.89 (d,
J = 8.0 Hz, 1H), 6.02 (s, 2H), 2.60–2.64 (m, 1H), 0.92–0.94 (m, 4H).
13C NMR (DMSO-
d6, 125 MHz) δ: 147.8, 146.8, 137.4, 133.0, 131.7, 127.3, 127.0, 126.0, 121.4, 120.4, 108.3, 105.2, 101.0, 29.6, 4.9.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)benzenesulfonamide (8l). Compound
8l was prepared according to general procedure 4(a) from benzenesulfonyl chloride (0.46 mL, 3.6 mmol) and
7 (R
1 R
2 = OCH
2O, R
3 = H) (0.29 g, 1.2 mmol) for 1 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
8l (0.20 g, 43.3%) as a product. HRMS (ESI) (M+H)
+ m/
z 380.0950, calcd for C
21H
18NO
4S 380.0951.
1H NMR (DMSO-
d6, 500 MHz) δ: 10.30 (s, 1H), 7.77 (d,
J = 7.0 Hz, 2H), 7.52–7.76 (m, 3H), 7.40 (d,
J = 8.5 Hz, 2H), 7.20 (s, 1H), 7.08 (d,
J = 8.5 Hz, 2H), 7.02 (d,
J = 16.0 Hz, 1H), 6.97 (d,
J = 16.0 Hz, 1H), 6.96 (d,
J = 8.0 Hz, 1H), 6.88 (d,
J = 8.0 Hz, 1H), 6.01 (s, 2H).
13C NMR (Acetone-
d6, 125 MHz) δ: 149.2, 148.2, 140.9, 137.7, 135.1, 133.6, 132.9, 129.9, 128.7, 127.9, 126.8, 122.3, 122.0, 109.1, 106.1, 102.1.
(E)-N-(4-(3,5-dimethoxystyryl)phenyl)isobutyramide (9b). Compound 9b was prepared according to general procedure 4(b) from isobutyric anhydride (0.38 mL, 2.3 mmol) and 7 (R1, R3 = OCH3, R2 =H) (0.20 g, 0.8 mmol) for 1.5 h. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure 9b (0.10 g, 41.4%) as a product. HRMS (ESI) (M+H)+ m/z 326.1746, calcd for C20H24NO3 326.1751. 1H NMR (DMSO-d6, 500 MHz) δ: 9.90 (s, 1H), 7.65 (d, J = 8.5 Hz, 2H), 7.52 (d, J = 8.5 Hz, 2H), 7.21 (d, J = 16.5 Hz, 1H), 7.07 (d, J = 16.5 Hz, 1H), 6.76 (d, J = 2.0 Hz, 2H), 6.41 (t, J = 2.0 Hz, 1H), 3.78 (s, 6H), 2.58–2.64 (m, 1H), 1.12 (s, 3H), 1.11 (s, 3H). 13C NMR (DMSO-d6, 125 MHz) δ: 175.2, 160.6, 139.3, 139.0, 131.6, 128.6, 126.9, 126.9, 119.2, 104.2, 99.6, 55.1, 35.0, 19.5.
(E)-4-(3,5-dimethoxystyryl)-2-fluoroaniline (11). Compound 11 (1.89 g, 49.2%) was prepared according to general procedure 2 from 4 (1.60 g, 9.7 mmol) and 10 (2.00 g, 8.4 mmol).
(E)-N-(4-(3,5-dimethoxystyryl)-2-fluorophenyl)benzamide (12f). Compound 12f was prepared according to general procedure 4(b) from benzoic anhydride (0.68 g, 3.0 mmol) and 11 (0.28 g, 1.0 mmol) for 1 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure 12f (0.15 g, 40.4%) as a product. HRMS (ESI) (M+H)+ m/z 378.1503, calcd for C23H21FNO3 378.1500. 1H NMR (DMSO-d6, 500 MHz) δ: 10.17 (s, 1H), 8.03 (d, J = 7.5 Hz, 2H), 7.69 (t, J = 8.0 Hz, 1H), 7.53–7.63 (m, 4H), 7.48 (dd, J = 8.0 Hz, 1.5 Hz, 1H), 7.31 (d, J = 16.5 Hz, 1H), 7.26 (d, J = 16.5 Hz, 1H), 6.82 (d, J = 2.0 Hz, 2H), 6.47 (t, J = 2.0 Hz, 1H), 3.80 (s, 6H). 13C NMR (DMSO-d6, 125 MHz) δ: 165.4, 160.7, 156.7, 154.8, 138.8, 136.1, 134.0, 131.8, 129.4, 128.4, 127.8, 127.4, 126.8, 125.1, 122.6,113.1, 104.6, 100.1, 55.2.
General procedure 5: synthesis of compounds 15 and 20. To a solution of LiOH•H2O (12.08 g, 288.0 mmol, 16 eq) in MeOH (15 mL) was added substituted acetophenone (18.0 mmol, 1 eq). The mixture was stirred at 25 °C for 1 h, then substituted benzaldehyde (18.0 mmol, 1 eq) was added. The mixture was stirred for another 3 h and then concentrated under reduced pressure. To the residue was added distilled water (30 mL), and the aqueous solution was acidified with hydrochloric acid (10%) to pH 3–4. The resulting precipitate was collected through filtration and washed with cold MeOH to afford pure products.
(E)-1-(benzo[d][
1,
3]
dioxol-5-yl)-3-(4-nitrophenyl)prop-2-en-1-one (15). Compound
15 (5.25 g, 97.9%) was prepared according to general procedure 5 from
13 (2.95 g, 18.0 mmol) and
14 (2.72 g, 18.0 mmol).
General procedure 6: synthesis of compounds 16 and 21. To a solution of anhydrous nickel chloride (2.63 g, 20.0 mmol) and chalcone (10.0 mmol) in EtOH (75 mL) was added NaBH4 (2.3 g, 60.0 mmol). The mixture was stirred at 25 °C for 5 min under argon. When the reaction was completed, the mixture was filtered, poured into distilled water (20 mL), then extracted with CH2Cl2. The organic layer was washed with saturated NaCl solution and concentrated in vacuo to afford the products.
3-(4-aminophenyl)-1-(benzo[d][
1,
3]
dioxol-5-yl)propan-1-ol (16). Compound
16 was prepared according to general procedure 6 from
15 (2.97 g, 10.0 mmol).
General procedure 7: synthesis of compounds 17 and 22. A well-stirred mixture of p-toluenesulfonic acid (0.35 g) and 16 or 21 in toluene (50 mL) was heated to reflux for 3 h, then cooled to 25 °C. The mixture was poured into a saturated NaHCO3 solution to stir for 0.5 h and then concentrated in vacuo. The residue was purified through column chromatography on silica gel to afford 17 or 22.
(E)-4-(3-(benzo[d][1,3]dioxol-5-yl)allyl)aniline (17). Compound
17 was prepared according to general procedure 7 from
16. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
17 (0.26 g, 14.3%) as a product.
(E)-N-(4-(3-(benzo[d][
1,
3]
dioxol-5-yl)allyl)phenyl)isobutyramide (18b). Compound
18b was prepared according to general procedure 4(b) from isobutyric anhydride (0.33 mL, 2.0 mmol) and
17 (0.17 g, 0.7 mmol). Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
18b (0.12 g, 57.8%) as a product. HRMS (ESI) (M+H)
+ m/
z 324.1586, calcd for C
20H
22NO
3 324.1594.
1H NMR (DMSO-
d6, 500 MHz) δ: 9.75 (s, 1H), 7.54 (d,
J = 8.5 Hz, 2H), 7.14 (d,
J = 8.5 Hz, 2H), 7.06 (s, 1H), 6.80–6.84 (m, 2H), 6.35 (d,
J = 16.0 Hz, 1H), 6.26 (dt,
J = 16.0 Hz, 7.0 Hz, 1H), 5.98 (s, 2H), 3.41 (d,
J = 7.0 Hz, 2H), 2.54–2.60 (m, 1H), 1.10 (s, 3H), 1.08 (s, 3H).
13C NMR (DMSO-
d6, 125 MHz) δ: 175.0, 147.7, 146.4, 137.5, 134.6, 131.6, 130.0, 128.5, 127.7, 120.5, 119.3, 108.2, 105.2, 100.8, 37.9, 34.8, 19.5.
Substituted (E)-1-(4-nitrophenyl)-3-phenylprop-2-en-1-one (20). Compound 20 (99.2%) was prepared according to general procedure 5 from 19 (3.00 g, 18.0 mmol) and 1 (2.70 g, 18.0 mmol) or 2 (2.99 g, 18.0 mmol).
Substituted 1-(4-aminophenyl)-3-phenylpropan-1-ol (21). Compound 21 was prepared according to general procedure 6 from 20.
Substituted (E)-4-(3-phenylprop-1-en-1-yl)aniline (22). Compound 22 was prepared according to general procedure 7 from 21. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure 22 as a product.
(E)-N-(4-(3-(benzo[d][
1,
3]
dioxol-5-yl)prop-1-en-1-yl)phenyl)isobutyramide (23b). Compound
23b was prepared according to general procedure 4(b) from isobutyric anhydride (0.33 mL, 2.0 mmol) and
22 (R
1 R
2 = OCH
2O, R
3 = H) (0.17 g, 0.7 mmol) for 0.5 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
23b (0.10 g, 47.5%) as a product. HRMS (ESI) (M+H)
+ m/
z 324.1586, calcd for C
20H
22NO
3 324.1594.
1H NMR (DMSO-
d6, 500 MHz) δ: 9.81 (s, 1H), 7.56 (d,
J = 8.0 Hz, 2H), 7.31 (d,
J = 8.0 Hz, 2H), 6.80–6.83 (m, 2H), 6.69 (d,
J = 8.0 Hz, 1H), 6.37 (d,
J = 15.5 Hz, 1H), 6.27 (dt,
J = 15.5 Hz, 6.5 Hz, 1H), 5.96 (s, 2H), 3.40 (d,
J = 6.5 Hz, 2H), 2.55–2.60 (m, 1H), 1.10 (s, 3H), 1.08 (s, 3H).
13C NMR (DMSO-
d6, 125 MHz) δ: 175.0, 147.3, 145.4, 138.4, 134.0, 131.8, 129.8, 128.0, 126.2, 121.2, 119.1, 108.9, 108.1, 100.6, 38.2, 34.9, 19.5.
(E)-N-(4-(3-(benzo[d][
1,
3]
dioxol-5-yl)prop-1-en-1-yl)phenyl)methanesulfonamide (23i). Compound
23i was prepared according to general procedure 4(a) from methanesulfonyl chloride (0.23 mL, 2.9 mmol) and
22 (R
1 R
2 = OCH
2O, R
3 = H) (0.25 g, 1.0 mmol) for 0.5 h. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
23i (0.11 g, 32.7%) as a product. HRMS (ESI) (M+NH
4)
+ m/
z 349.1210, calcd for C
17H
21N
2O
4S 349.1217.
1H NMR (DMSO-
d6, 500 MHz) δ: 9.72 (s, 1H), 7.37 (d,
J = 8.5 Hz, 2H), 7.15 (d,
J = 8.5 Hz, 2H), 6.81–6.84 (m, 2H), 6.71 (dd,
J = 7.5 Hz, 0.5 Hz, 1H), 6.40 (d,
J = 16.0 Hz, 1H), 6.31 (dt,
J = 16.0 Hz, 7.0 Hz, 1H), 5.97 (s, 2H), 3.42 (d,
J = 7.0 Hz, 2H), 2.96 (s, 3H).
13C NMR (DMSO-
d6, 125 MHz) δ: 147.3, 145.5, 137.2, 133.9, 132.9, 129.6, 128.8, 126.9, 121.2, 119.9, 108.9, 108.2, 100.7, 39.1, 38.2.
(E)-N-(4-(3-(3,5-dimethoxyphenyl)prop-1-en-1-yl)phenyl)isobutyramide (24b). Compound 24b was prepared according to general procedure 4(b) from isobutyric anhydride (0.76 mL, 4.7 mmol) and 22 (R1, R3 = OCH3, R2 = H) (0.42 g, 1.6 mmol) for 1.5 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure 24b (0.29 g, 54.8%) as a product. HRMS (ESI) (M+H)+ m/z 340.1926, calcd for C21H26NO3 340.1907. 1H NMR (DMSO-d6, 500 MHz) δ: 9.83 (s, 1H), 7.59 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 6.42 (d, J = 2.0 Hz, 2H), 6.38 (s, 1H), 6.35 (t, J = 2.0 Hz, 1H), 6.30 (dt, J = 16.0 Hz, 6.5 Hz, 1H), 3.72 (s, 6H), 3.41–3.44 (m, 2H), 2.56–2.64 (m, 1H), 1.11 (s, 3H), 1.10 (s, 3H). 13C NMR (DMSO-d6, 125 MHz) δ: 175.2, 160.6, 142.6, 138.5, 131.9, 130.2, 127.5, 126.3, 119.2, 106.5, 97.9, 55.0, 38.9, 35.0, 19.5.
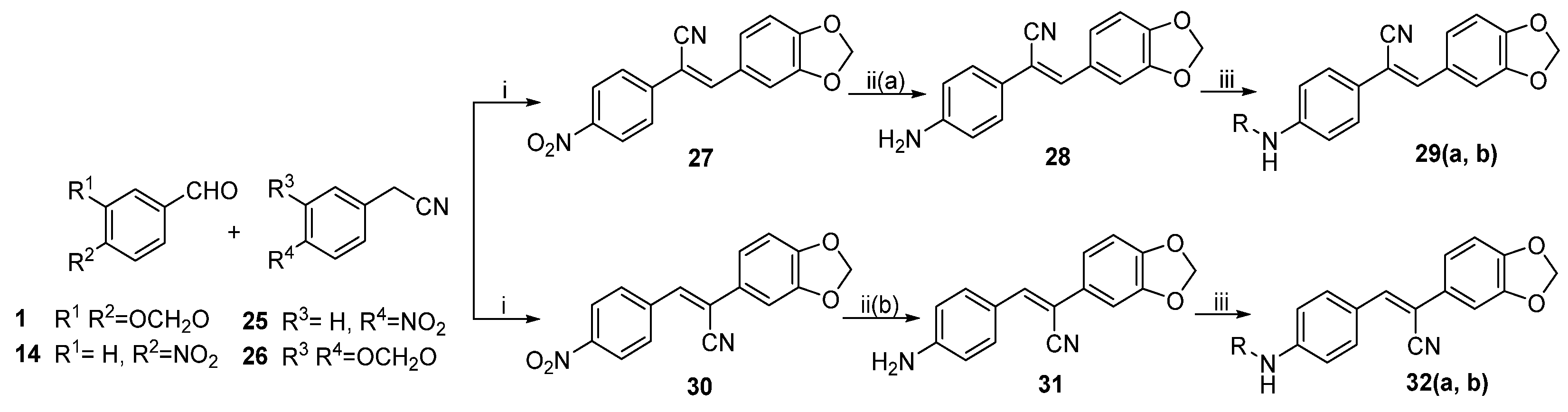
General procedure 8: synthesis of compounds 27 and 30. To a mixture of substituted phenylacetonitrile (5.5 mmol, 1.1 eq) and 1 (0.75 g, 5.0 mmol) or 14 (0.76 g, 5.0 mmol) in anhydrous methanol (23 mL), sodium methoxide (4 mL, 5 mmol/L) was added dropwise. Under argon protection, the mixture was stirred at reflux temperature for 3 h and then cooled to 25 °C. The mixture was poured into cold distilled water. The resulting precipitate was collected through filtration and washed with cold distilled methanol to afford 27 or 30.
(Z)-3-(benzo[d][
1,
3]
dioxol-5-yl)-2-(4-nitrophenyl)acrylonitrile (27). Compound
27 (0.40 g, 27.2%) was prepared according to general procedure 8 from
1 (0.75 g, 5.0 mmol) and
25 (0.89 g, 5.5 mmol).
(Z)-2-(4-aminophenyl)-3-(benzo[d][
1,
3]
dioxol-5-yl)acrylonitrile (28). Compound
28 (0.13 g, 37.6%) was prepared according to general procedure 3 from
27 (0.40 g, 1.35 mmol).
(Z)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)-1-cyanovinyl)phenyl)acetamide (29a). Compound
29a was prepared according to general procedure 4(b) from acetic anhydride (0.54 mL, 5.7 mmol) and
28 (0.50 g, 1.9 mmol) for 1.5 h. Purification through column chromatography (dichloromethane/methanol = 10:0.1) on silica gel afforded pure
29a (0.36 g, 61.9%) as a product. HRMS (ESI) (M+H)
+ m/
z 307.1070, calcd for C
18H
15N
2O
3 307.1077.
1H NMR (DMSO-
d6, 400 MHz) δ: 10.15 (s, 1H), 7.83 (s, 1H), 7.73–7.67 (m, 2H), 7.67–7.62 (m, 2H), 7.57 (d,
J = 1.8 Hz, 1H), 7.43 (dd,
J = 1.8, 8.5 Hz, 1H), 7.09 (d,
J = 8.2 Hz, 1H), 6.14 (s, 2H), 2.07 (s, 3H).
13C NMR (DMSO-
d6, 101 MHz) δ: 169.02, 149.63, 148.25, 141.23, 140.47, 128.87, 128.50, 126.52, 126.01, 119.64, 118.76, 109.26, 107.97, 107.84, 102.34, 24.54.
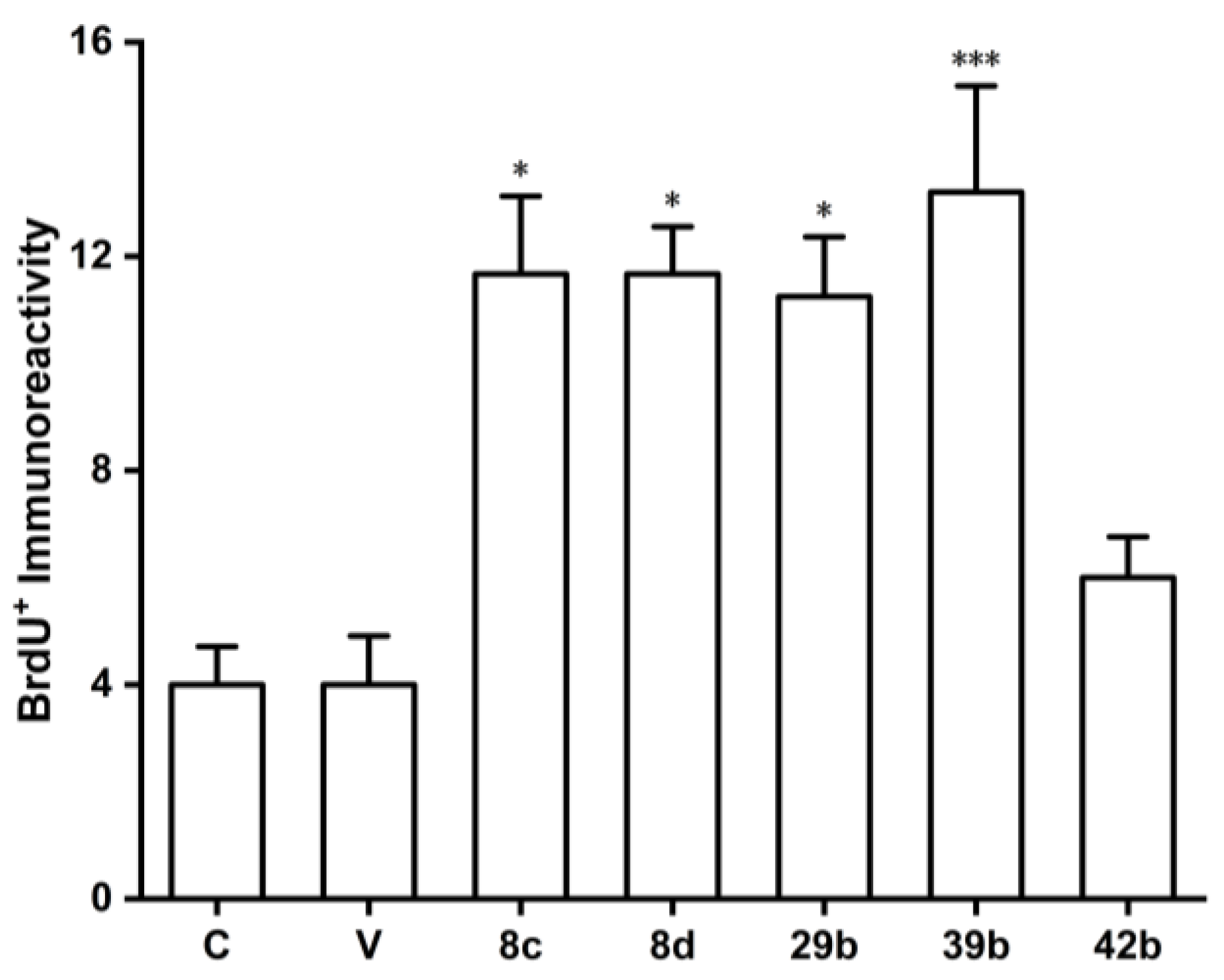
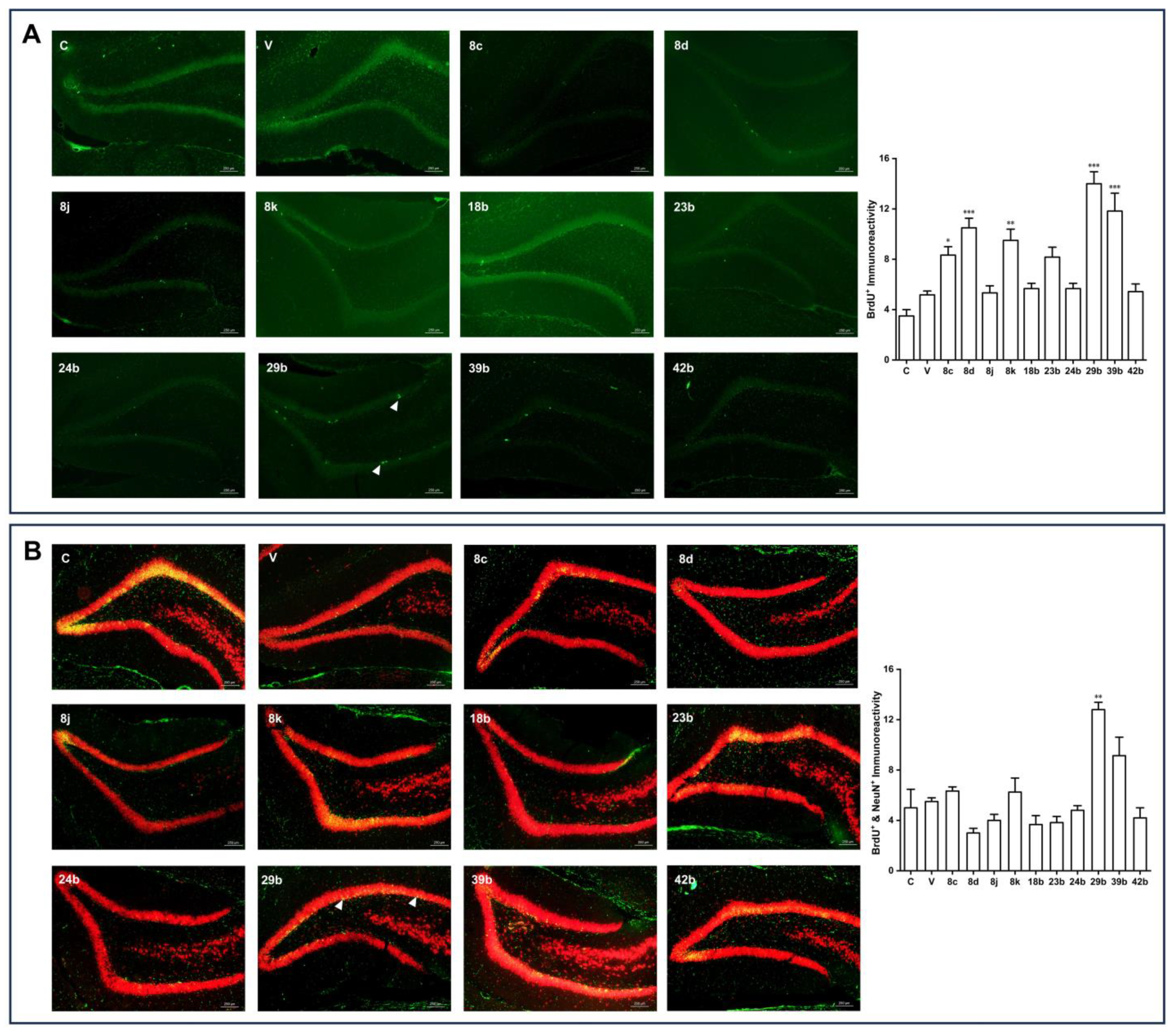
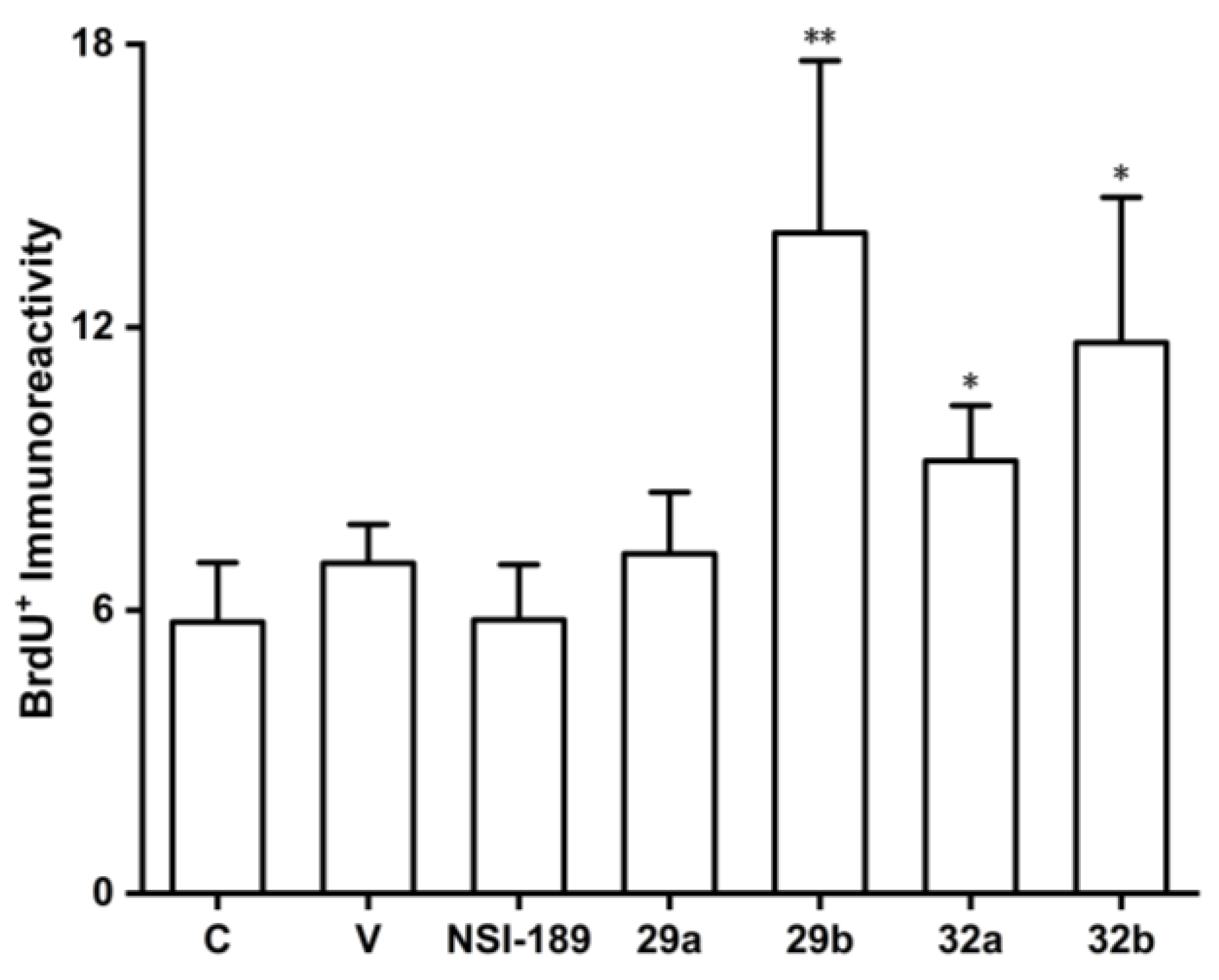
(Z)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)-1-cyanovinyl)phenyl)isobutyramide (29b). Compound
29b was prepared according to general procedure 4(b) from isobutyric anhydride (0.64 mL, 3.9 mmol) and
28 (0.34 g, 1.3 mmol) for 1 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
29b (0.12 g, 28.8%) as a product. HRMS (ESI) (M+H)
+ m/
z 335.1397, calcd for C
20H
19N
2O
3 335.1390.
1H NMR (DMSO-
d6, 500 MHz) δ: 10.02 (s, 1H), 7.82 (s, 1H), 7.73 (d,
J = 8.5 Hz, 2H), 7.64 (d,
J = 8.5 Hz, 2H), 7.56 (s, 1H), 7.42 (d,
J = 8.0 Hz, 1H), 7.06 (d,
J = 8.0 Hz, 1H), 6.12 (s, 2H), 2.58–2.64 (m, 1H), 1.12 (s, 3H), 1.10 (s, 3H).
13C NMR (DMSO-
d6, 125 MHz) δ: 175.5, 149.1, 147.8, 140.6, 140.1, 128.3, 128.0, 125.9, 125.5, 119.3, 118.3, 108.7, 107.4, 107.3, 101.8, 35.0, 19.4.
(Z)-2-(benzo[d][
1,
3]
dioxol-5-yl)-3-(4-nitrophenyl)acrylonitrile (30). Compound
30 (0.52 g, 17.8%) was prepared according to general procedure 8 from
14 (1.662 g, 11 mmol) and
26 (1.612 g, 10 mmol).
(Z)-3-(4-aminophenyl)-2-(benzo[d][
1,
3]
dioxol-5-yl)acrylonitrile (31). To a suspension of iron powder (0.40 g, 7.1 mmol) and ammonium chloride (0.24 g, 4.4 mmol) in water (20 mL),
30 in methanol (20 mL) was added dropwise. The mixture was stirred at 80 °C for 2 h. After the reaction was completed, the mixture was extracted with ethyl acetate (50 mL) and alkalified with NaOH (2M) to adjust the pH to 9–10. The organic layer was washed sequentially with saturated NH
4Cl, saturated NaHCO
3, water, and saturated NaCl solution. Recrystallization from ethyl acetate and petroleum ether afforded compound
31 (0.39 g, 82.2%).
(Z)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)-2-cyanovinyl)phenyl)acetamide (32a). Compound
32a was prepared according to general procedure 4(b) from acetic anhydride (0.30 mL, 3.1 mmol) and
31 (0.28 g, 1.0 mmol) for 1.5 h. Recrystallization from dichloromethane and petroleum ether afforded pure
32a (0.10 g, 31.7%) as a product. HRMS (ESI) (M+H)
+ m/
z 307.1079, calcd for C
18H
15N
2O
3 307.1077.
1H NMR (DMSO-
d6, 400 MHz) δ: 10.23 (s, 1H), 7.86 (d,
J = 8.6 Hz, 2H), 7.83 (s, 1H), 7.72 (d,
J = 8.5 Hz, 2H), 7.39 (d,
J = 2.0 Hz, 1H), 7.19 (dd,
J = 2.0, 8.1 Hz, 1H), 7.03 (d,
J = 8.2 Hz, 1H), 6.11 (s, 2H), 2.09 (s, 3H).
13C NMR (DMSO-
d6, 101 MHz) δ: 169.22, 148.77, 148.51, 141.66, 141.22, 130.42, 128.82, 128.77, 120.78, 119.24, 118.80, 109.07, 108.07, 105.71, 102.19, 24.60.
(Z)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)-2-cyanovinyl)phenyl)isobutyramide (32b). Compound
32b was prepared according to general procedure 4(b) from isobutyric anhydride (1.74 mL, 10.7 mmol) and
31 (0.95 g, 3.6 mmol) for 1 h. Purification through column chromatography (dichloromethane = 100%) on silica gel afforded pure
32b (0.325 g, 27.2%) as a product. HRMS (ESI) (M+H)
+ m/
z 335.1390, calcd for C
20H
19N
2O
3 335.1390.
1H NMR (DMSO-
d6, 400 MHz) δ: 10.14 (s, 1H), 7.99–7.81 (m, 3H), 7.76 (d,
J = 8.8 Hz, 2H), 7.39 (d,
J = 2.0 Hz, 1H), 7.19 (dd,
J = 2.0, 8.2 Hz, 1H), 7.03 (d,
J = 8.2 Hz, 1H), 6.11 (s, 2H), 2.70–2.56 (m, 1H), 1.12 (d,
J = 6.8 Hz, 6H).
13C NMR (DMSO-
d6, 101 MHz) δ: 176.12, 148.76, 148.50, 141.82, 141.24, 130.38, 128.78, 120.77, 119.39, 118.81, 109.07, 108.05, 105.70, 102.19, 35.51, 19.90.
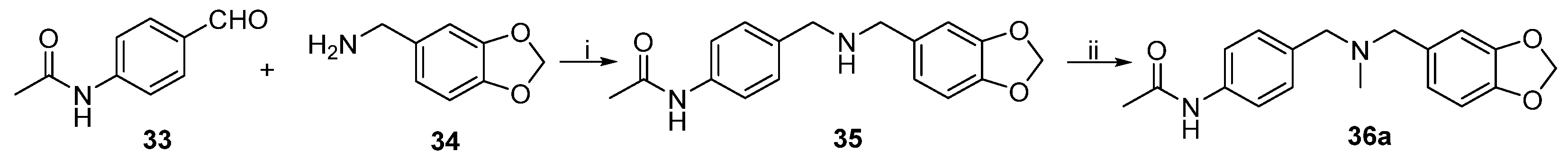
N-(4-(((benzo[d][
1,
3]
dioxol-5-ylmethyl)amino)methyl)phenyl)acetamide (35). A mixture of
33 (0.20 g, 1.2 mmol),
34 (0.18 mL, 1.4 mmol), NaBH
4 (0.06 g, 1.6 mmol), and molecular sieve 4A (5.00 g) in anhydrous CHCl
3 (20 mL) was stirred at 25 °C for 10 h. The mixture was quenched with distilled water (20 mL), then extracted with ethyl acetate. The organic layer was washed with saturated NaCl solution, then concentrated in vacuo. The residue was purified through column chromatography on silica gel (dichloromethane/methanol/ammonia = 10:0.3:0.03) to afford
35 (0.21 g, 58.7%) as a product.
N-(4-(((benzo[d][
1,
3]
dioxol-5-ylmethyl)(methyl)amino)methyl)phenyl)acetamide (36a). Aqueous formaldehyde solution (0.14 mL, 37%) and formic acid (0.13 mL, 98%) were added dropwise to a solution of
35 (0.21 g, 0.7 mmol) in anhydrous CHCl
3 (30 mL). The mixture was stirred at reflux temperature for 3 h. The mixture was quenched by distilled water (20 mL), acidified through the addition of HCl (30%) to pH 5, then extracted with CHCl
3 (20 mL). The organic layer was alkalified with NaOH solution to adjust the pH to 9, then concentrated in vacuo. The residue was purified through column chromatography on silica gel (dichloromethane/methanol/ammonia = 10:0.5:0.03) to afford
36a (0.17 g, 44.3%) as a product. HRMS (ESI) (M+H)
+ m/
z 313.1542, calcd for C
18H
21N
2O
3 313.1547.
1H NMR (CDCl
3, 500 MHz) δ: 8.35 (s, 1H), 7.47 (d,
J = 8.0 Hz, 2H), 7.24 (d,
J = 8.0 Hz, 2H), 6.86 (d,
J = 1.0 Hz, 1H), 6.71–6.75 (m, 2H), 5.89 (s, 2H), 3.42 (s, 2H), 3.38 (s, 2H), 2.11 (s, 6H).
13C NMR (CDCl
3, 125 MHz) δ: 168.9, 147.4, 146.3, 136.8, 134.9, 133.0, 129.2, 121.7, 119.9, 109.1, 107.6, 100.6, 61.2, 60.9, 41.8, 24.1.
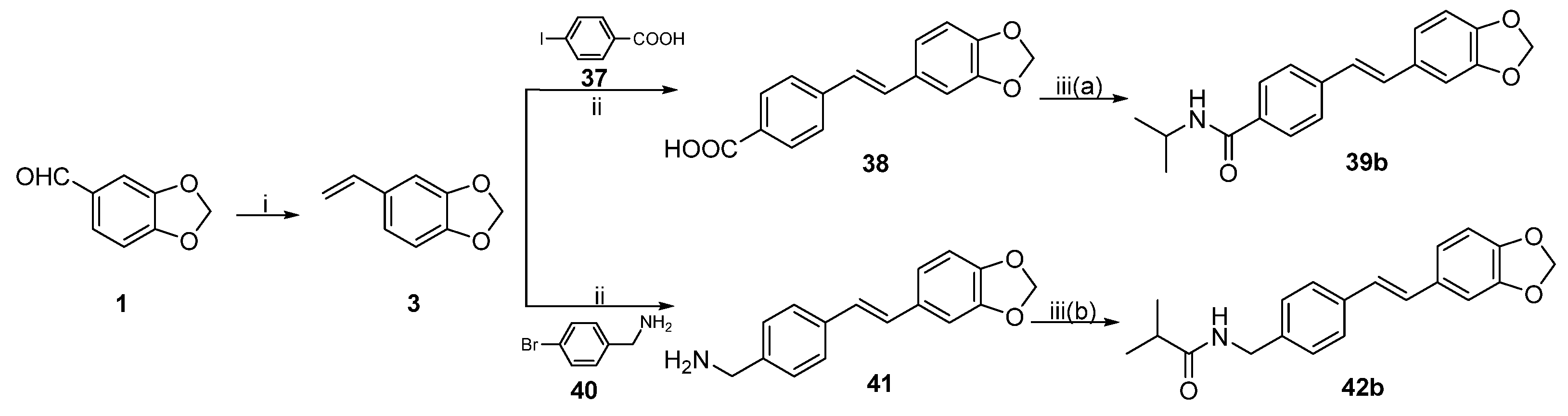
General procedure 9: synthesis of compounds 38 and 41. To a solution of 3 (0.74 g, 5.0 mmol), P(o-MePh)3 (0.61 g, 2.0 mmol), triethylamine (0.21 mL, 1.5 mmol), and palladium acetate (0.22 g, 1.0 mmol) in CH3CN (20 mL) was added the derivative of the aromatic halogen (1 eq). Under argon protection, the mixture was stirred at 60 °C for 1 h, then warmed to 90 °C for 24 h. After the reaction was completed, the mixture was extracted with CH2Cl2 (50 mL), then washed with water and saturated NaCl solution. The organic layer was concentrated in vacuo. Recrystallization from ethyl acetate and petroleum ether afforded 38 or 41.
(E)-4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)benzoic acid (38). 37 (1.24 g, 5 mmol) was used to yield the compound
38 (1.15 g, 86.0%) according to the general procedure 9.
(E)-4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)-N-isopropylbenzamide (39b). To a solution of compound
38 in CH
2Cl
2 (40 mL), triethylamine (1.10 mL, 8.0 mmol) and pivaloyl chloride (0.98 mL, 8.0 mmol) were added dropwise. The mixture was stirred at 0 °C for 0.5 h, and isopropylamine (2.00 mL, 24 mmol) and DMAP (0.98 g, 8 mmol) were added. After the reaction was completed, the mixture was washed with saturated NH
4Cl, saturated NaHCO
3, distilled water, and saturated NaCl solution, then concentrated in vacuo. Recrystallization from ethyl acetate provided the compound
39b (1.46 g, 59.1%). HRMS (ESI) (M+H)
+ m/
z 310.1442, calcd for C
19H
20NO
3 310.1438.
1H NMR (DMSO-
d6, 500 MHz) δ: 8.19 (d,
J = 7.8 Hz, 1H), 7.98–7.78 (m, 2H), 7.62 (d,
J = 8.4 Hz, 2H), 7.38–7.23 (m, 2H), 7.16 (d,
J = 16.3 Hz, 1H), 7.07 (dd,
J = 1.7, 8.1 Hz, 1H), 6.94 (d,
J = 8.0 Hz, 1H), 6.05 (s, 2H), 4.16–4.06 (m, 1H), 1.18 (d,
J = 6.6 Hz, 6H).
13C NMR (DMSO-
d6, 126 MHz) δ: 165.39, 148.39, 147.74, 140.29, 133.68, 131.82, 130.29, 128.14, 126.30, 126.27, 122.55, 108.92, 105.88, 101.64, 41.41, 22.84.
(E)-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)phenyl)methanamine (41). Compound
40 (0.93 g, 5.0 mmol) was used to obtain
41 (0.99 g, 78.1%) according to general procedure 9.
(E)-N-(4-(2-(benzo[d][
1,
3]
dioxol-5-yl)vinyl)benzyl)isobutyramide (42b). Compound
42 was prepared according to general procedure 4(b) from isobutyric anhydride (0.24 mL, 1.5 mmol) and
41 (0.13 g, 0.5 mmol). Recrystallization from dichloromethane and petroleum ether afforded
42b (0.14 g, 86.6%). HRMS (ESI) (M+H)
+ m/
z 324.1609, calcd for C
20H
22NO
3 324.1594.
1H NMR (DMSO-
d6, 400 MHz) δ: 8.28 (t,
J = 6.0 Hz, 1H), 7.51 (d,
J = 7.8 Hz, 2H), 7.32–7.21 (m, 3H), 7.12 (d,
J = 9.5 Hz, 2H), 7.03 (dd,
J = 1.7, 8.1 Hz, 1H), 6.90 (dd,
J = 1.3, 8.0 Hz, 1H), 6.04 (s, 2H), 4.28 (d,
J = 6.0 Hz, 2H), 2.48–2.40 (m, 1H), 1.07 (d,
J = 6.9 Hz, 6H).
13C NMR (DMSO-
d6, 101 MHz) δ: 176.60, 148.36, 147.40, 139.45, 136.25, 132.11, 128.26, 127.89, 126.86, 126.64, 122.04, 108.84, 105.76, 101.55, 42.16, 34.54, 20.07.