Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions
Abstract
1. Introduction
2. Results
2.1. Feed and Water Intake and Milk Yield
2.2. Milk Characteristics
2.3. Blood Hematology
2.4. Blood Metabolites and Hormones
2.5. Gene Expression
2.5.1. Peripheral Blood Mononuclear Cell (PBMC)
2.5.2. Hair Follicle
2.6. Physiological Indicators
3. Discussion
3.1. Feed and Water Intake and Milk Yield
3.2. Milk Characteristics
3.3. Blood Hematology
3.4. Blood Metabolites and Hormone
3.5. Evaluation of Heat Shock Protein mRNA Expression
3.6. Physiological Indicators
4. Materials and Methods
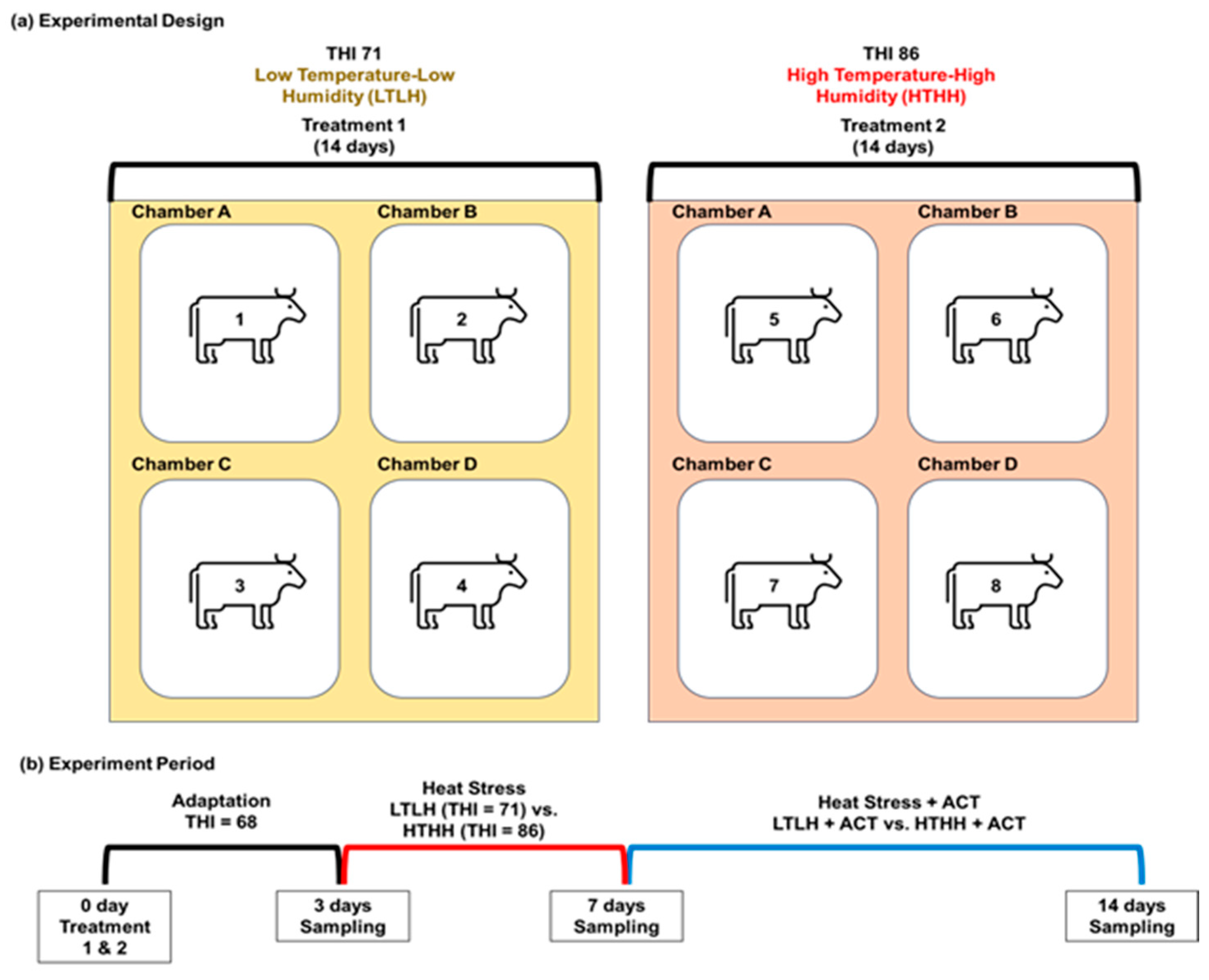
4.1. Experimental Design, and Animals
4.2. Temperature–Humidity Index
4.3. Sampling and Analysis
4.3.1. Feed and Water Intake and Milk Yield
4.3.2. Milk Characteristics
4.3.3. Blood Profiles
4.3.4. Peripheral Blood Mononuclear Cell (PBMC)
4.3.5. Hair Follicle
4.3.6. RNA Extraction from PBMC and Hair Follicle
4.3.7. Physiological Indicators
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jo, J.H.; Ghassemi Nejad, J.; Peng, D.Q.; Kim, H.R.; Kim, S.H.; Lee, H.G. Characterization of short-term heat stress in holstein dairy cows using altered indicators of metabolomics, blood parameters, milk MicroRNA-216 and characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Batistel, F.; Arroyo, J.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.; Loor, J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef]
- Jo, J.H.; Lee, J.S.; Ghassemi Nejad, J.; Kim, W.S.; Moon, J.O.; Lee, H.G. Effects of dietary supplementation of acetate and L-tryptophan conjugated bypass amino acid on productivity of pre-and post-partum dairy cows and their offspring. Animals 2021, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Priatno, W.; Jo, Y.H.; Nejad, J.G.; Lee, J.S.; Moon, J.O.; Lee, H.G. “Dietary supplementation of L-tryptophan” increases muscle development, adipose tissue catabolism and fatty acid transportation in the muscles of Hanwoo steers. J. Anim. Sci. Technol. 2020, 62, 595. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Nejad, J.G.; Moon, J.O.; Lee, H.G. Dietary supplementation of acetate-conjugated tryptophan alters feed intake, milk yield and composition, blood profile, physiological variables, and heat shock protein gene expression in heat-stressed dairy cows. J. Therm. Biol. 2021, 98, 102949. [Google Scholar] [CrossRef]
- Weiss, Y.G.; Maloyan, A.; Tazelaar, J.; Raj, N.; Deutschman, C.S. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J. Clin. Investig. 2002, 110, 801–806. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Rhoads, M.; Rhoads, R.; VanBaale, M.; Collier, R.; Sanders, S.; Weber, W.; Crooker, B.; Baumgard, L. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Ominski, K.; Kennedy, A.; Wittenberg, K.; Nia, S.M. Physiological and production responses to feeding schedule in lactating dairy cows exposed to short-term, moderate heat stress. J. Dairy Sci. 2002, 85, 730–737. [Google Scholar] [CrossRef]
- West, J.; Mullinix, B.; Bernard, J. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J. Dairy Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.; Eley, R.; Sharma, A.; Pereira, R.; Buffington, D. Shade management in subtropical environment for milk yield and composition in Holstein and Jersey cows. J. Dairy Sci. 1981, 64, 844–849. [Google Scholar] [CrossRef]
- Spiers, D.; Spain, J.; Sampson, J.; Rhoads, R. Use of physiological parameters to predict milk yield and feed intake in heat-stressed dairy cows. J. Therm. Biol. 2004, 29, 759–764. [Google Scholar] [CrossRef]
- Cowley, F.; Barber, D.; Houlihan, A.; Poppi, D. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef]
- Miao, J.; Adewole, D.; Liu, S.; Xi, P.; Yang, C.; Yin, Y. Tryptophan supplementation increases reproduction performance, milk yield, and milk composition in lactating sows and growth performance of their piglets. J. Agric. Food Chem. 2019, 67, 5096–5104. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, S.; Guzik, A.; Van Der Meulen, J.; Dekker, R.; Kogut, J.; Kerr, B.; Southern, L. Effects of supplemental L-tryptophan on serotonin, cortisol, intestinal integrity, and behavior in weanling piglets. J. Anim. Sci. 2006, 84, 963–971. [Google Scholar] [CrossRef]
- Tossou, M.C.B.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L. Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig. BioMed Res. Int. 2016, 2016, 2912418. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, J.; Zhu, X.; Jia, Z. Effects of rumen-protected tryptophan on performance, nutrient utilization and plasma tryptophan in cashmere goats. Afr. J. Biotechnol. 2011, 10, 5806–5811. [Google Scholar]
- Pearce, S.; Sanz-Fernandez, M.; Hollis, J.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef]
- Honig, H.; Ofer, L.; Elbaz, M.; Kaim, M.; Shinder, D.; Gershon, E. Seasonal and parity effects on ghrelin levels throughout the estrous cycle in dairy cows. Gen. Comp. Endocrinol. 2016, 235, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan-Elazary, A.; Ben-Meir, Y.; Gacitua, H.; Levit, H.; Fridman, A.; Shinder, D.; Jacoby, S.; Miron, J.; Halachmi, I.; Gershon, E. Cooling management effects on dry matter intake, metabolic hormones levels and welfare parameters in dairy cows during heat stress. J. Dairy Res. 2020, 87, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Sheahan, A.; Chagas, L.; Berry, D. Concentrate supplementation reduces postprandial plasma ghrelin in grazing dairy cows: A possible neuroendocrine basis for reduced pasture intake in supplemented cows. J. Dairy Sci. 2007, 90, 1354–1363. [Google Scholar] [CrossRef]
- Nonogaki, K.; Ohashi-Nozue, K.; Oka, Y. A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biochem. Biophys. Res. Commun. 2006, 341, 703–707. [Google Scholar] [CrossRef]
- Aklan, I.; Sayar-Atasoy, N.; Deng, F.; Kim, H.; Yavuz, Y.; Rysted, J.; Laule, C.; Davis, D.; Li, Y.; Atasoy, D. Dorsal raphe serotonergic neurons suppress feeding through redundant forebrain circuits. Mol. Metab. 2023, 69, 101676. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Samad, H.; Shehzad, F.; Qayyum, A. Physiological responses of cattle to heat stress. World Appl. Sci. J. 2010, 8, 38–43. [Google Scholar]
- Kim, W.S.; Nejad, J.G.; Park, K.K.; Lee, H.G. Heat Stress Effects on Physiological and Blood Parameters, and Behavior in Early Fattening Stage of Beef Steers. Animals 2023, 13, 1130. [Google Scholar] [CrossRef]
- Pihl, R.; Young, S.; Ervin, F.; Plotnick, S. Influence of tryptophan availability on selection of alcohol and water by men. J. Stud. Alcohol. 1987, 48, 260–264. [Google Scholar] [CrossRef]
- Meyer-Gerspach, A.C.; Häfliger, S.; Meili, J.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Beglinger, C.; Wölnerhanssen, B. Effect of L-tryptophan and L-leucine on gut hormone secretion, appetite feelings and gastric emptying rates in lean and non-diabetic obese participants: A randomized, double-blind, parallel-group trial. PLoS ONE 2016, 11, e0166758. [Google Scholar] [CrossRef]
- Southern, L.L.; (Louisiana State University, Baton Rouge, LA, USA). Personal Communication, 2012.
- Kerr, B.; Moran, E., Jr.; Kidd, M. Effect of supplementary tryptophan prior to marketing on carcass quality in broilers. J. Appl. Poult. Res. 2005, 14, 306–314. [Google Scholar] [CrossRef]
- Ma, H.; Yao, S.; Bai, L.; Bai, S.; Liu, G. The effects of rumen-protected tryptophan (RPT) on production performance and relevant hormones of dairy cows. PeerJ 2022, 10, e13831. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, W.; Song, W.; Sun, P.; Jia, Z. Effects of tryptophan supplementation on cashmere fiber characteristics, serum tryptophan, and related hormone concentrations in cashmere goats. Domest. Anim. Endocrinol. 2012, 43, 239–250. [Google Scholar] [CrossRef]
- West, J.; Hill, G.; Fernandez, J.; Mandebvu, P.; Mullinix, B. Effects of dietary fiber on intake, milk yield, and digestion by lactating dairy cows during cool or hot, humid weather. J. Dairy Sci. 1999, 82, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.; Rhoads, R.; VanBaale, M.; Sanders, S.; Baumgard, L. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Urrutia, N.; Bomberger, R.; Matamoros, C.; Harvatine, K. Effect of dietary supplementation of sodium acetate and calcium butyrate on milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2019, 102, 5172–5181. [Google Scholar] [CrossRef] [PubMed]
- Ríus, A. Invited Review: Adaptations of protein and amino acid metabolism to heat stress in dairy cows and other livestock species. Appl. Anim. Sci. 2019, 35, 39–48. [Google Scholar] [CrossRef]
- Martins, C.; Fonseca, D.; Alves, B.; Arcari, M.; Ferreira, G.; Welter, K.; Oliveira, C.; Rennó, F.; Santos, M. Effect of dietary crude protein degradability and corn processing on lactation performance and milk protein composition and stability. J. Dairy Sci. 2019, 102, 4165–4178. [Google Scholar] [CrossRef]
- Cortamira, N.; Seve, B.; Lebreton, Y.; Ganier, P. Effect of dietary tryptophan on muscle, liver and whole-body protein synthesis in weaned piglets: Relationship to plasma insulin. Br. J. Nutr. 1991, 66, 423–435. [Google Scholar] [CrossRef]
- Field, S.L.; Ouellet, V.; Sheftel, C.M.; Hernandez, L.L.; Laporta, J. In vitro effects of 5-Hydroxy-L-tryptophan supplementation on primary bovine mammary epithelial cell gene expression under thermoneutral or heat shock conditions. Sci. Rep. 2022, 12, 3820. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, Y.; Wu, G.; Sun, K.; Sun, Y.; Li, W.; Wang, B.; He, B.; Zhang, Q.; Dai, Z. L-tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Conejos, J.R.V.; Ghassemi Nejad, J.; Kim, J.E.; Moon, J.O.; Lee, J.S.; Lee, H.G. Supplementing with l-tryptophan increases medium protein and alters expression of genes and proteins involved in milk protein synthesis and energy metabolism in bovine mammary cells. Int. J. Mol. Sci. 2021, 22, 2751. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, M.; Sharmin, M.M.; Yonekura, S. Mild heat stress induces transcription of the β-casein gene via unfolded protein response-activated XBP1 signaling in undifferentiated mammary epithelial cells. Anim. Sci. J. 2019, 90, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.; Grummer, R.R. Response of lactating dairy cows to fat supplementation during heat stress. J. Dairy Sci. 1991, 74, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Batistel, F.; de Souza, J.; Santos, F.A.P. Corn grain-processing method interacts with calcium salts of palm fatty acids supplementation on milk production and energy balance of early-lactation cows grazing tropical pasture. J. Dairy Sci. 2017, 100, 5343–5357. [Google Scholar] [CrossRef] [PubMed]
- Mazzullo, G.; Rifici, C.; Caccamo, G.; Rizzo, M.; Piccione, G. Effect of different environmental conditions on some haematological parameters in cow. Ann. Anim. Sci. 2014, 14, 947–954. [Google Scholar] [CrossRef]
- Ondruska, L.; Rafay, J.; Okab, A.; Ayoub, M.; Al-Haidary, A.; Samara, E.; Parkanyi, V.; Chrastinova, L.; Jurcik, R.; Massanyi, P. Influence of elevated ambient temperature upon some physiological measurements of New Zealand White rabbits. Vet. Med. 2011, 56, 180–186. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop. Anim. Health Prod. 2004, 36, 685–692. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Umesh, D.; Sharma, M.C. Seasonal changes in certain blood antioxidants in cattle and buffaloes. Indian J. Anim. Sci. 2014, 84, 173–176. [Google Scholar] [CrossRef]
- Bruneaux, M.; Visse, M.; Gross, R.; Pukk, L.; Saks, L.; Vasemägi, A. Parasite infection and decreased thermal tolerance: Impact of proliferative kidney disease on a wild salmonid fish in the context of climate change. Funct. Ecol. 2017, 31, 216–226. [Google Scholar] [CrossRef]
- Azeez, O.M.; Olaifa, F.H.; Adah, A.S.; Basiru, A.; Akorede, G.J.; Ambali, H.M.; Suleiman, K.Y.; Sanusi, F.; Bolaji, M. Effect of heat stress on vital and hematobiochemical parameters of healthy dogs. Vet. World 2022, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Dallak, M. Camel’s milk protects against cadmium chloride-induced hypocromic microcytic anemia and oxidative stress in red blood cells of white albino rats. Am. J. Pharmacol. Toxicol. 2009, 4, 134–141. [Google Scholar] [CrossRef]
- Moffett, J.R.; Namboodiri, M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003, 81, 247–265. [Google Scholar] [CrossRef]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef]
- Hussein, H.A.; Thurmann, J.P.; Staufenbiel, R. 24-h variations of blood serum metabolites in high yielding dairy cows and calves. BMC. Vet. Res. 2020, 16, 327. [Google Scholar] [CrossRef]
- Aleena, J.; Sejian, V.; Krishnan, G.; Bagath, M.; Pragna, P.; Bhatta, R. Heat stress impact on blood biochemical response and plasma aldosterone level in three different indigenous goat breeds. J. Anim. Behav. Biometeorol. 2020, 8, 266–275. [Google Scholar] [CrossRef]
- Helal, A.; Hashem, A.; Abdel-Fattah, M.; El-Shaer, H. Effect of heat stress on coat characteristics and physiological responses of Balady and Damascus goats in Sinai, Egypt. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 60–69. [Google Scholar]
- Attia, N.E.S. Physiological, hematological and biochemical alterations in heat stressed goats. Benha Med. J. 2016, 31, 56–62. [Google Scholar] [CrossRef]
- Blomstrand, E.; Perrett, D.; Parry-Billings, M.; Newsholme, E. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol. Scand. 1989, 136, 473–482. [Google Scholar] [CrossRef]
- Yamamoto, T.; Castell, L.M.; Botella, J.; Powell, H.; Hall, G.M.; Young, A.; Newsholme, E.A. Changes in the albumin binding of tryptophan during postoperative recovery: A possible link with central fatigue? Brain Res. Bull. 1997, 43, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Daujat-Chavanieu, M.; Kot, M. Albumin is a secret factor involved in multidirectional interactions among the serotoninergic, immune and endocrine systems that supervises the mechanism of CYP1A and CYP3A regulation in the liver. Pharmacol. Therapeut. 2020, 215, 107616. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan–kynurenine and kynurenine–nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Paulmann, N.; Grohmann, M.; Voigt, J.P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevšek, M.; Fink, H.; Rupnik, M. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Kamemura, N.; Oda, M.; SAkURAI, J.; NAkAYA, Y.; Harada, N.; Suenaga, M.; Matsunaga, Y.; Ishidoh, K.; Katunuma, N. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J. Nutr. Sci. Vitaminol. 2012, 58, 415–422. [Google Scholar] [CrossRef]
- Chi, T.C.; Ho, Y.J.; Chen, W.P.; Chi, T.L.; Lee, S.S.; Cheng, J.T.; Su, M.J. Serotonin enhances β-endorphin secretion to lower plasma glucose in streptozotocin-induced diabetic rats. Life Sci. 2007, 80, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Tudhope, S.J.; Wang, C.C.; Petrie, J.L.; Potts, L.; Malcomson, F.; Kieswich, J.; Yaqoob, M.M.; Arden, C.; Hampson, L.J.; Agius, L. A novel mechanism for regulating hepatic glycogen synthesis involving serotonin and cyclin-dependent kinase-5. Diabetes 2012, 61, 49–60. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.; Masclee, A. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef]
- Bruschetta, G.; Di Pietro, P.; Sanzarello, L.; Giacoppo, E.; Ferlazzo, A. Plasma serotonin levels in Italian Fresian dairy cows. Vet. Res. Commun. 2010, 34 (Suppl. S1), 17–20. [Google Scholar] [CrossRef][Green Version]
- Caroprese, M.; Albenzio, M.; Marino, R.; Santillo, A.; Sevi, A. Immune response and milk production of dairy cows fed graded levels of rumen-protected glutamine. Res. Vet. Sci. 2012, 93, 202–209. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.; Pragna, P.; Lees, A.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Kim, B.W.; Lee, B.H.; Sung, K.I. Coat and hair color: Hair cortisol and serotonin levels in lactating Holstein cows under heat stress conditions. Anim. Sci. J. 2017, 88, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Millischer, V.; Heinzl, M.; Faka, A.; Resl, M.; Trepci, A.; Klammer, C.; Egger, M.; Dieplinger, B.; Clodi, M.; Schwieler, L. Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: A prospective placebo-controlled cross-over trial. J. Neuroinflamm. 2021, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, S.; Salama, A.; Albanell, E.; Such, X.; Caja, G. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J. Dairy Sci. 2013, 96, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’H, N.; Melchior, D.; Sève, B. Dietary tryptophan helps to preserve tryptophan homeostasis in pigs suffering from lung inflammation. J. Anim. Sci. 2008, 86, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.; Naudé, P.J.; Kema, I.P.; Nollen, E.A.; Deyn, P.P.D. Tryptophan metabolism in inflammaging: From biomarker to therapeutic target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Collier, R.; Collier, J.; Rhoads, R.; Baumgard, L. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Collier, M.P.; Alderson, T.R.; de Villiers, C.P.; Nicholls, D.; Gastall, H.Y.; Allison, T.M.; Degiacomi, M.T.; Jiang, H.; Mlynek, G.; Fürst, D.O. HspB1 phosphorylation regulates its intramolecular dynamics and mechanosensitive molecular chaperone interaction with filamin C. Sci. Adv. 2019, 5, eaav8421. [Google Scholar] [CrossRef]
- Livernois, A.; Mallard, B.; Cartwright, S.; Cánovas, A. Heat stress and immune response phenotype affect DNA methylation in blood mononuclear cells from Holstein dairy cows. Sci. Rep. 2021, 11, 11371. [Google Scholar] [CrossRef] [PubMed]
- Boel, N.M.E.; Edkins, A.L. Regulation of the extracellular matrix by heat shock proteins and molecular chaperones. In Heat Shock Proteins in the Immune System; Binder, R., Srivastava, P., Eds.; Springer: Cham, Germany, 2018; pp. 97–121. [Google Scholar]
- Han, Z.Y.; Mu, T.; Yang, Z. Methionine protects against hyperthermia-induced cell injury in cultured bovine mammary epithelial cells. Cell Stress Chaperones 2015, 20, 109–120. [Google Scholar] [CrossRef]
- Collier, R.; Stiening, C.; Pollard, B.; VanBaale, M.; Baumgard, L.; Gentry, P.; Coussens, P. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006, 84 (Suppl. S13), E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. Prospects for gene introgression or gene editing as a strategy for reduction of the impact of heat stress on production and reproduction in cattle. Theriogenology 2020, 154, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, A.; Ghasura, R.; Mir, N.; Bumla, N.; Sankar, G.; Wani, S. Biochemical and physiological changes during thermal stress in bovines: A review. Iran. J. Appl. Anim. Sci. 2013, 3, 423–430. [Google Scholar] [CrossRef]
- Al-Qaisi, M.; Horst, E.; Kvidera, S.; Mayorga, E.; Timms, L.; Baumgard, L. Developing a heat stress model in dairy cows using an electric heat blanket. J. Dairy Sci. 2019, 102, 684–689. [Google Scholar] [CrossRef]
- Sutoh, M.; Kasuya, E.; Yayou, K. Effects of intravenous tryptophan infusion on thermoregulation in steers exposed to acute heat stress. Anim. Sci. J. 2018, 89, 777–783. [Google Scholar] [CrossRef]
- Adeola, O.; Ball, R. Hypothalamic neurotransmitter concentrations and meat quality in stressed pigs offered excess dietary tryptophan and tyrosine. J. Anim. Sci. 1992, 70, 1888–1894. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Jo, J.H.; Nejad, J.G.; Lee, J.S.; Lee, H.G. Evaluation of heat stress effects in different geographical areas on milk and rumen characteristics in holstein dairy cows using robot milking and rumen sensors: A survey in South Korea. Animals 2022, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Hansen, P. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Nejad, J.G.; Peng, D.Q.; Jung, U.S.; Kim, M.J.; Jo, Y.H.; Jo, J.H.; Lee, J.S.; Lee, H.G. Identification of heat shock protein gene expression in hair follicles as a novel indicator of heat stress in beef calves. Animal 2020, 14, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H. Characterization of Heat Stress and Recovery on Productive Performance and Physiological Indicators, Blood and Milk Characteristic, and Gene Expression in Early Lactating Holstein Cows; American Society of Animal Science: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| DMI, kg/d | 40.74 | 40.10 | 40.51 | 39.73 | 30.11 | 36.41 | 0.843 | 0.0087 | 0.0008 | 0.0018 |
| WI, kg/d | 89.87 | 99.67 | 99.38 | 76.40 | 113.76 | 80.59 | 2.110 | <0.0001 | <0.0001 | <0.0001 |
| F:W | 0.45 | 0.40 | 0.42 | 0.52 | 0.28 | 0.45 | 0.013 | 0.0019 | 0.0025 | 0.0297 |
| Milk yield, kg/d | 39.63 | 40.63 | 41.15 | 36.06 | 30.89 | 31.15 | 0.773 | 0.0083 | 0.0262 | 0.0880 |
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| Milk protein, % | 2.81 | 2.82 | 2.81 | 2.89 | 2.81 | 3.04 | 0.025 | 0.1227 | 0.0523 | 0.0379 |
| Milk fat, % | 4.46 | 4.13 | 4.65 | 4.66 | 3.68 | 3.72 | 0.317 | 0.2012 | 0.7293 | 0.7735 |
| Lactose, % | 5.05 | 4.98 | 4.99 | 4.98 | 4.96 | 4.90 | 0.027 | 0.5961 | 0.821 | 0.6476 |
| SNF, % | 8.34 | 8.41 | 8.29 | 8.42 | 8.44 | 8.48 | 0.033 | 0.3701 | 0.6384 | 0.3553 |
| Milk protein, kg/d | 1.02 | 1.16 | 1.12 | 1.04 | 0.90 | 1.08 | 0.039 | 0.3158 | 0.5113 | 0.2742 |
| Milk fat, kg/d | 1.68 | 1.66 | 1.92 | 1.99 | 1.12 | 1.30 | 0.116 | 0.0418 | 0.4235 | 0.8834 |
| Lactose, kg/d | 1.89 | 2.03 | 2.06 | 1.80 | 1.53 | 1.75 | 0.081 | 0.1433 | 0.5315 | 0.6347 |
| SNF, kg/d | 3.11 | 3.42 | 3.40 | 3.05 | 2.60 | 3.02 | 0.127 | 0.1780 | 0.5292 | 0.4954 |
| 3.5% FCM | 43.37 | 44.46 | 48.90 | 47.81 | 31.49 | 36.50 | 2.264 | 0.0306 | 0.3684 | 0.9561 |
| ECM | 41.27 | 43.12 | 46.34 | 45.03 | 31.09 | 36.24 | 1.977 | 0.0368 | 0.3653 | 0.8335 |
| SC, 103/mL | 90.00 | 75.50 | 47.00 | 72.75 | 39.00 | 95.00 | 20.228 | 0.4930 | 0.2688 | 0.9215 |
| MUN, mg/dL | 15.96 | 15.45 | 13.15 | 13.38 | 13.73 | 16.33 | 0.369 | 0.7089 | 0.8681 | 0.0136 |
| Acetone, mM | 0.03 | 0.04 | 0.05 | 0.10 | 0.05 | 0.00 | 0.012 | 0.6792 | 0.5572 | 0.3675 |
| BHB, mM | 0.06 | 0.08 | 0.08 | 0.08 | 0.07 | 0.05 | 0.005 | 0.3501 | 0.4846 | 0.4846 |
| Cas.B | 2.08 | 2.18 | 2.07 | 2.21 | 2.24 | 2.30 | 0.028 | 0.0936 | 0.7647 | 0.2222 |
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| WBC, 103 cells/μL | 8.46 | 7.73 | 7.98 | 9.85 | 8.40 | 10.30 | 0.417 | 0.2162 | 0.3022 | 0.4286 |
| RBC, 106 cells/μL | 5.52 | 4.95 | 5.34 | 6.03 | 5.96 | 6.49 | 0.147 | 0.0039 | 0.1296 | 0.8111 |
| HGB, g/dL | 9.90 | 9.48 | 9.55 | 10.25 | 10.25 | 10.75 | 0.191 | 0.1112 | 0.5345 | 0.6454 |
| HCT, % | 26.53 | 24.00 | 25.75 | 27.40 | 26.83 | 29.10 | 0.619 | 0.0785 | 0.1755 | 0.8563 |
| MCV, fL | 48.08 | 48.65 | 48.18 | 45.50 | 45.05 | 44.93 | 0.554 | 0.0453 | 0.8153 | 0.8915 |
| MCH, pg | 17.90 | 19.53 | 17.88 | 17.03 | 17.23 | 16.65 | 0.351 | 0.0696 | 0.1805 | 0.5098 |
| MCHC, g/dL | 37.33 | 40.10 | 37.05 | 37.45 | 38.23 | 37.08 | 0.505 | 0.2846 | 0.1017 | 0.4461 |
| PLT, 103 cells/μL | 666.00 | 606.00 | 549.25 | 637.75 | 659.50 | 524.50 | 29.396 | 0.9484 | 0.2070 | 0.6001 |
| MPV, fL | 6.83 | 9.00 | 6.43 | 5.90 | 6.13 | 6.38 | 0.376 | 0.1081 | 0.1849 | 0.1110 |
| PDW, % | 58.33 | 71.55 | 60.53 | 51.48 | 51.13 | 57.68 | 3.077 | 0.2282 | 0.7668 | 0.2520 |
| PCT, % | 0.45 | 0.56 | 0.34 | 0.37 | 0.40 | 0.33 | 0.031 | 0.3418 | 0.0600 | 0.3299 |
| NEUT, % | 39.88 | 33.68 | 37.48 | 40.48 | 39.00 | 55.48 | 2.839 | 0.2053 | 0.1418 | 0.3500 |
| LYM, % | 47.63 | 55.20 | 47.30 | 48.40 | 48.50 | 35.00 | 2.798 | 0.3367 | 0.1292 | 0.6828 |
| MONO, % | 6.45 | 4.40 | 7.60 | 4.88 | 5.13 | 4.83 | 0.494 | 0.5352 | 0.2417 | 0.1611 |
| EOS, % | 4.63 | 5.45 | 5.15 | 4.50 | 5.95 | 3.23 | 0.360 | 0.3027 | 0.0880 | 0.1657 |
| LUC, % | 0.58 | 0.23 | 1.25 | 0.90 | 0.48 | 0.68 | 0.133 | 0.4240 | 0.0656 | 0.2040 |
| BASO, % | 0.88 | 1.05 | 1.20 | 0.88 | 0.93 | 0.78 | 0.052 | 0.0594 | 1.0000 | 0.2109 |
| NEUT, 103 cells/μL | 3.29 | 2.53 | 3.00 | 4.11 | 3.27 | 6.07 | 0.428 | 0.1271 | 0.1050 | 0.2407 |
| LYM, 103 cells/μL | 4.14 | 4.35 | 3.81 | 4.67 | 4.09 | 3.30 | 0.273 | 0.8313 | 0.3536 | 0.8656 |
| MONO, 103 cells/μL | 0.51 | 0.34 | 0.56 | 0.47 | 0.42 | 0.48 | 0.027 | 0.2152 | 0.0317 | 0.2009 |
| EOS, 103 cells/μL | 0.41 | 0.41 | 0.42 | 0.44 | 0.52 | 0.29 | 0.035 | 0.8839 | 0.2146 | 0.2050 |
| LUC, 103 cells/μL | 0.05 | 0.02 | 0.10 | 0.08 | 0.03 | 0.08 | 0.011 | 0.3207 | 0.0325 | 0.4886 |
| BASO, 103 cells/μL | 0.08 | 0.08 | 0.10 | 0.09 | 0.08 | 0.08 | 0.006 | 0.7909 | 0.7342 | 0.4990 |
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| Albumin, g/dL | 3.25 | 3.12 | 3.12 | 3.26 | 3.47 | 3.16 | 0.029 | 0.0001 | 0.0049 | 0.0067 |
| BUN, mg/dL | 19.00 | 18.75 | 15.25 | 14.25 | 16.25 | 20.25 | 0.758 | 0.7054 | 0.8916 | 0.0521 |
| CA, mg/dL | 9.30 | 9.13 | 9.13 | 9.20 | 9.38 | 9.70 | 0.107 | 0.3252 | 0.5502 | 0.5502 |
| CHO, mg/dL | 230.25 | 227.75 | 241.50 | 217.50 | 239.50 | 221.75 | 11.003 | 0.9377 | 0.9463 | 0.5974 |
| Globulin, g/dL | 4.00 | 3.78 | 3.78 | 3.50 | 3.82 | 3.47 | 0.083 | 0.8103 | 0.4248 | 0.4248 |
| Glucose, mg/dL | 64.00 | 58.75 | 56.50 | 62.75 | 68.75 | 67.00 | 0.964 | 0.0001 | 0.0307 | 0.7728 |
| GOT, U/L | 73.25 | 61.50 | 57.75 | 76.25 | 67.75 | 76.50 | 3.547 | 0.2058 | 0.7763 | 0.4799 |
| r-GT, U/L | 31.00 | 29.00 | 27.50 | 26.75 | 27.25 | 24.75 | 1.716 | 0.8737 | 0.6650 | 0.9136 |
| MG, mg/dL | 2.30 | 2.53 | 2.45 | 2.20 | 2.30 | 2.30 | 0.046 | 0.1045 | 0.7366 | 0.7366 |
| NEFA, uEq/L | 311.00 | 263.50 | 207.75 | 175.50 | 231.75 | 323.00 | 24.379 | 0.9243 | 0.4593 | 0.9283 |
| IP, mg/dL | 5.40 | 6.13 | 6.45 | 5.68 | 5.25 | 5.78 | 0.181 | 0.2172 | 0.3394 | 0.8201 |
| Total protein, g/dL | 7.25 | 6.90 | 6.89 | 6.76 | 7.29 | 6.63 | 0.102 | 0.9089 | 0.2010 | 0.2075 |
| Cortisol, ng/mL | 80.70 | 107.05 | 89.42 | 95.97 | 192.22 | 138.08 | 8.641 | 0.0001 | 0.0012 | 0.0687 |
| Haptoglobin, ng/mL | 396.45 | 437.81 | 408.94 | 401.57 | 430.97 | 389.80 | 1.570 | 0.1586 | 0.0765 | 0.7460 |
| Ghrelin, pg/mL | 126.63 | 118.18 | 116.31 | 128.93 | 106.62 | 108.81 | 2.012 | 0.0001 | 0.8116 | 0.0094 |
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| PBMC | ||||||||||
| HSP70 | 0.50 | 0.60 | 0.68 | 0.65 | 0.52 | 0.86 | 0.028 | 0.5812 | 0.0011 | 0.0238 |
| HSP90 | 0.74 | 0.90 | 0.98 | 0.86 | 0.73 | 0.83 | 0.032 | 0.1854 | 0.2828 | 0.9641 |
| Hair follicle | ||||||||||
| HSP90 | 1.82 | 1.43 | 0.90 | 1.08 | 1.84 | 1.22 | 0.180 | 0.7165 | 0.2687 | 0.9305 |
| LTLH | HTHH | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adaptation | HS | HS + ACT | Adaptation | HS | HS + ACT | HS | ACT | HS × ACT | ||
| Heart rate, beat/min | 67.00 | 79.00 | 77.00 | 67.25 | 89.25 | 85.00 | 1.826 | 0.0001 | 0.0242 | 0.7454 |
| Rectal temperature, °C | 38.18 | 38.33 | 38.13 | 38.23 | 39.18 | 38.73 | 0.094 | <0.0001 | <0.0001 | 0.6740 |
| Items | TMR | Concentrates | ACT |
|---|---|---|---|
| Composition, % (DM basis) | |||
| Dry matter | 61.91 | 88.84 | |
| Crude Protein | 6.06 | 18.06 | |
| Crude Fat | 1.11 | 2.75 | |
| Crude Fiber | 8.06 | 6.44 | |
| Crude Ash | 3.15 | 6.17 | |
| Calcium | 0.37 | 0.61 | |
| Phosphorus | 0.17 | 0.50 | |
| NDF | 21.53 | 21.61 | |
| ADF | 11.30 | 10.71 | |
| NDFn | 19.36 | 18.79 | |
| NDIP | 2.17 | 2.82 | |
| ADIP | 0.52 | 0.73 | |
| tdNFC | 71.67 | 55.27 | |
| tdCP | 5.46 | 17.77 | |
| tdFA | 0.11 | 1.75 | |
| Amino acids, % (DM basis) | |||
| Tryptophan | 0.06 | 0.19 | 0.16 |
| Threonine | 0.22 | 0.60 | |
| Serine | 0.26 | 0.77 | |
| Proline | 0.33 | 0.94 | |
| Valine | 0.27 | 0.70 | |
| Isoleucine | 0.17 | 0.51 | |
| Leucine | 0.37 | 1.26 | |
| Tyrosine | 0.10 | 0.39 | |
| Methionine | 0.06 | 0.18 | |
| Cystine | 0.13 | 0.41 | |
| Lysine | 0.25 | 0.52 | |
| Glycine | 0.24 | 0.71 | |
| Alanine | 0.30 | 0.84 | |
| Arginine | 0.33 | 1.01 | |
| Glutamic acid | 0.84 | 2.83 | |
| Aspartic acid | 0.49 | 1.34 | |
| Histidine | 0.10 | 0.33 | |
| Phenylalanine | 0.22 | 0.68 | |
| Gene | Accession Number 1 | Sequence (5′ to 3′) | Length (bp) |
|---|---|---|---|
| HSP70 | U09861 | F: TACGTGGCCTTCACCGATAC R: GTCGTTGATGACGCGGAAAG | 171 |
| HSP90 | NM_001012670 | F: GGAGGATCACTTGGCTGTCA R: GGGATTAGCTCCTCGCAGTT | 177 |
| RPS15A | NM_001037443.2 | F: CCGTGCTCCAAAGTCATCGT R: GGGAGCAGGTTATTCTGCCA | 200 |
| B2M | NM_173893.3 | F: GACACCCACCAGAAGATGGA R: CAGGTCTGACTGCTCCGATT | 125 |
| GAPDH | NM_001034034.2 | F: GGCAAGGTCATCCCTGAG R: GCAGGTCAGATCCACAACAG | 166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.-H.; Jalil, G.N.; Kim, W.-S.; Moon, J.-O.; Lee, S.-D.; Kwon, C.-H.; Lee, H.-G. Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions. Int. J. Mol. Sci. 2024, 25, 1217. https://doi.org/10.3390/ijms25021217
Jo J-H, Jalil GN, Kim W-S, Moon J-O, Lee S-D, Kwon C-H, Lee H-G. Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions. International Journal of Molecular Sciences. 2024; 25(2):1217. https://doi.org/10.3390/ijms25021217
Chicago/Turabian StyleJo, Jang-Hoon, Ghassemi Nejad Jalil, Won-Seob Kim, Jun-Ok Moon, Sung-Dae Lee, Chan-Ho Kwon, and Hong-Gu Lee. 2024. "Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions" International Journal of Molecular Sciences 25, no. 2: 1217. https://doi.org/10.3390/ijms25021217
APA StyleJo, J.-H., Jalil, G. N., Kim, W.-S., Moon, J.-O., Lee, S.-D., Kwon, C.-H., & Lee, H.-G. (2024). Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions. International Journal of Molecular Sciences, 25(2), 1217. https://doi.org/10.3390/ijms25021217







