Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors—New Usnic Acid Derivatives
Abstract
1. Introduction
2. Results and Discussion
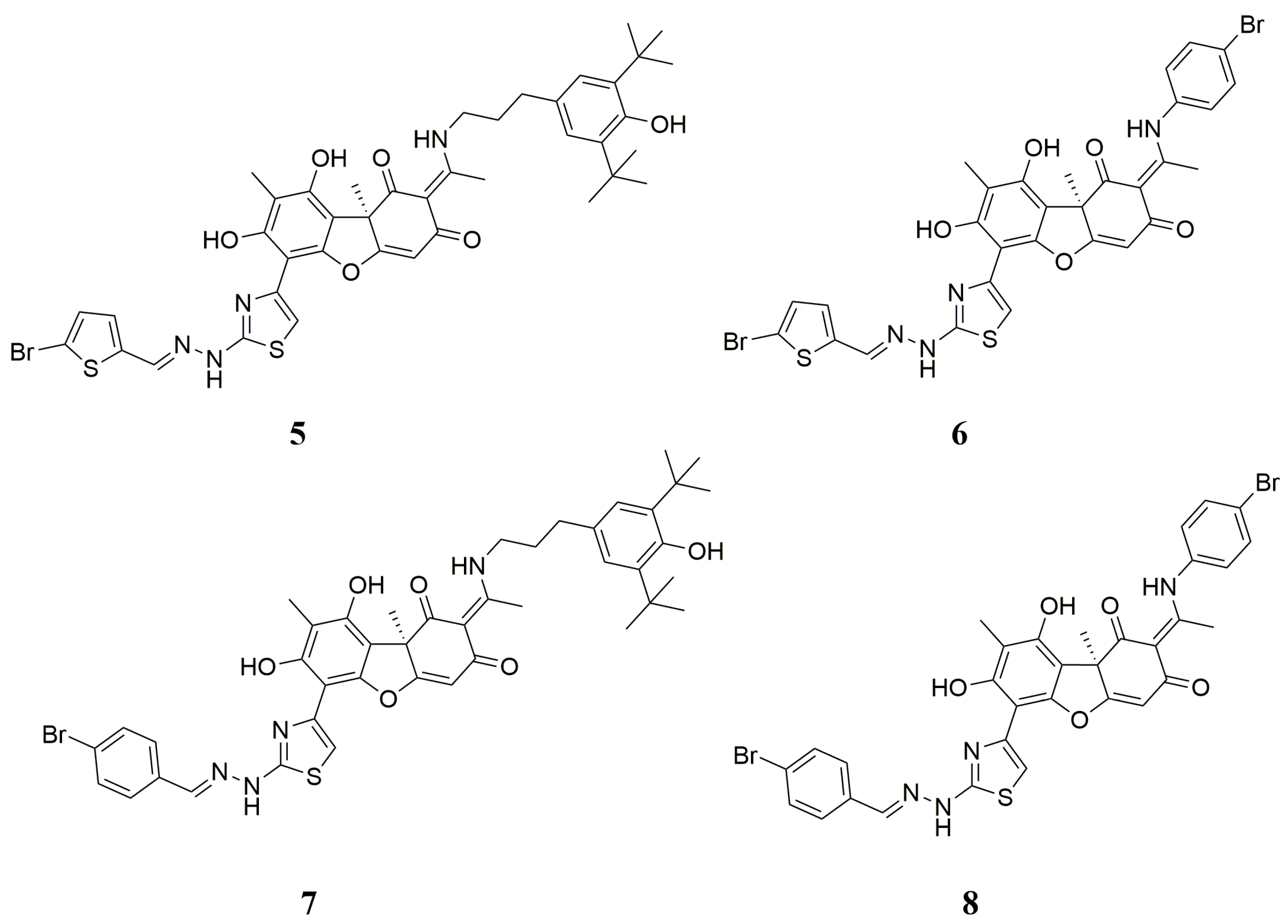
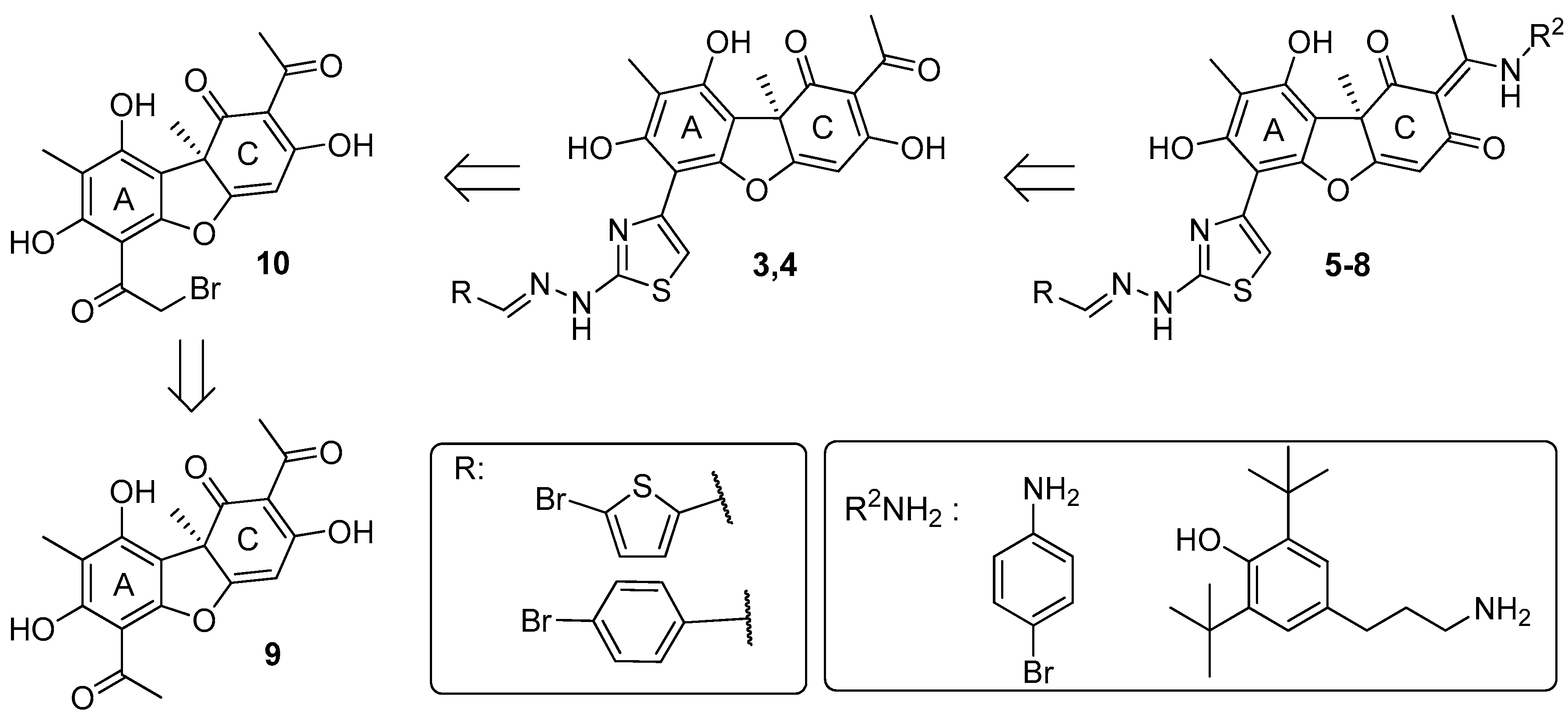
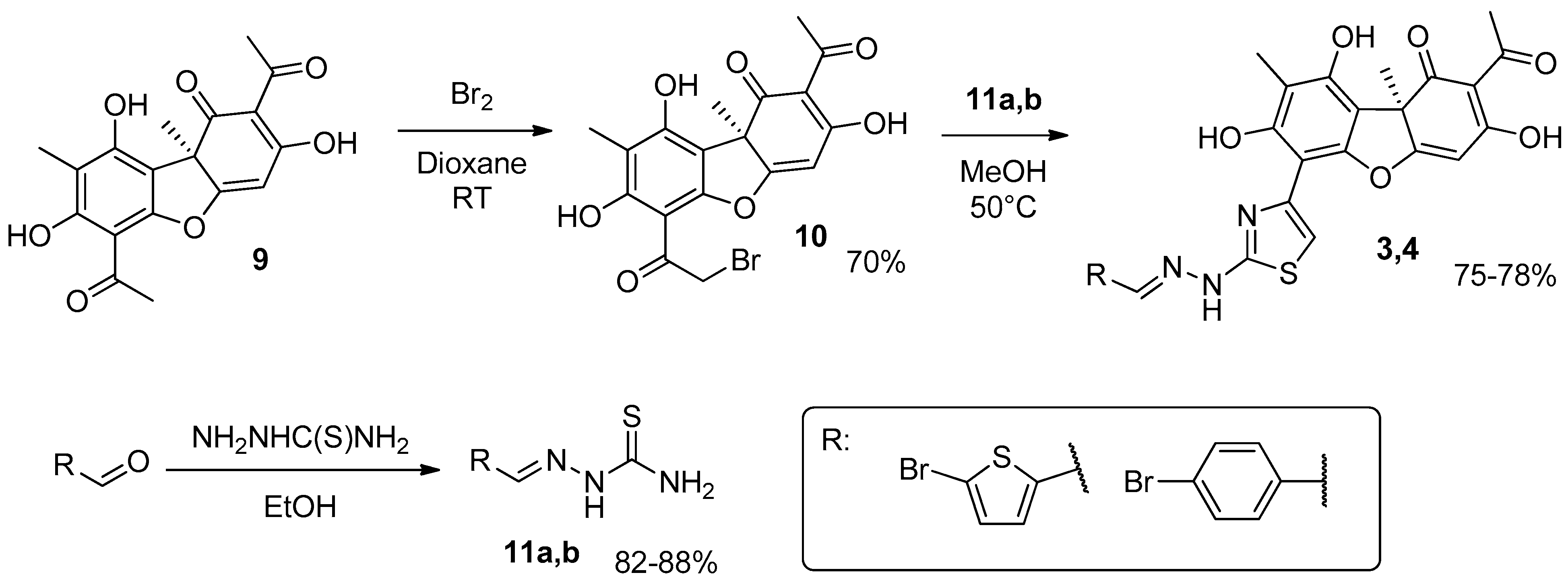
2.1. Chemistry
2.2. Biology
2.2.1. Inhibitory Properties of Usnic Acid Derivatives against Tdp1
2.2.2. The Influence of Usnic Acid Derivatives on the Survival of Various Types of Cells
2.2.3. Sensitizing Properties of Usnic Acid Derivatives In Vitro
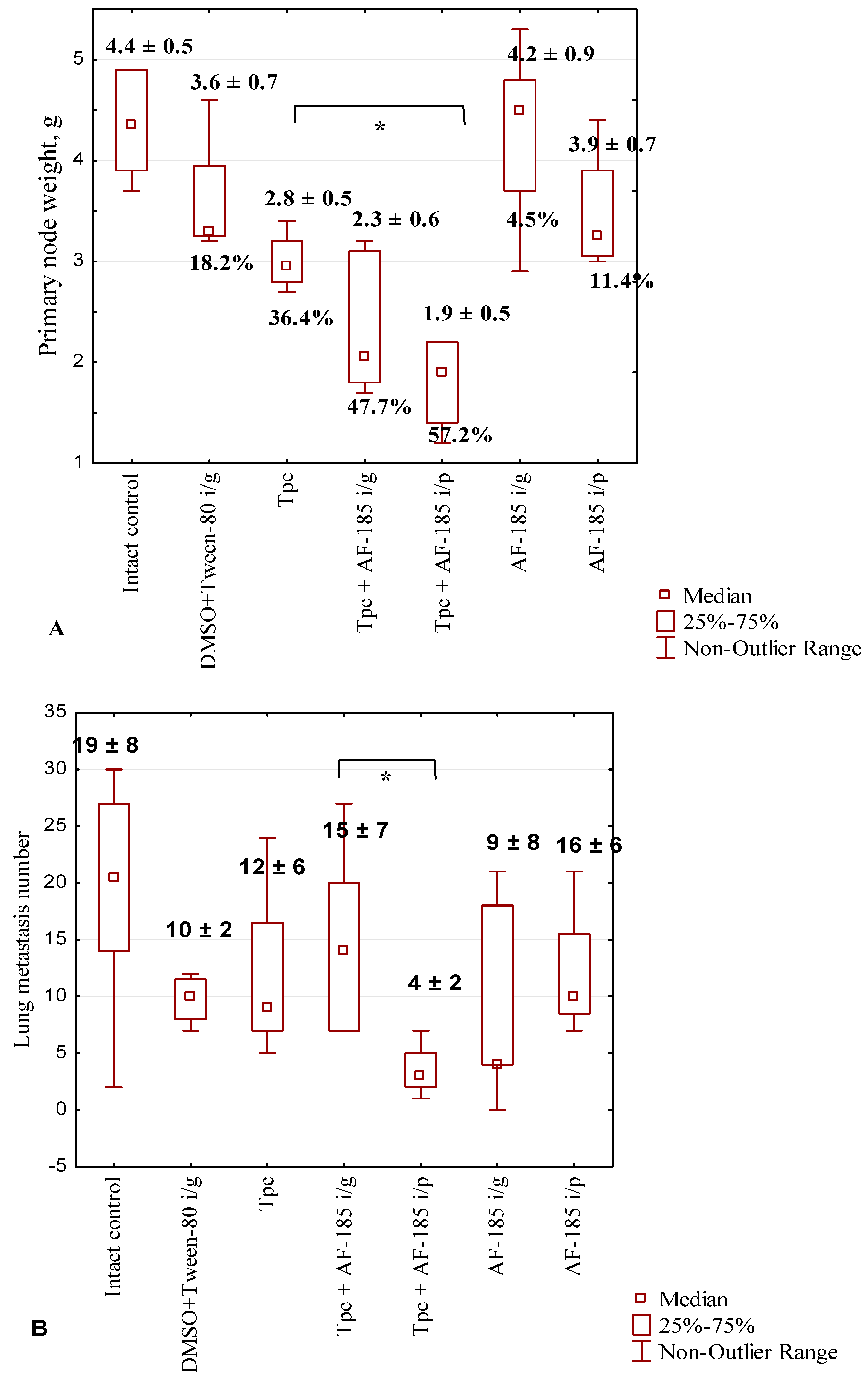
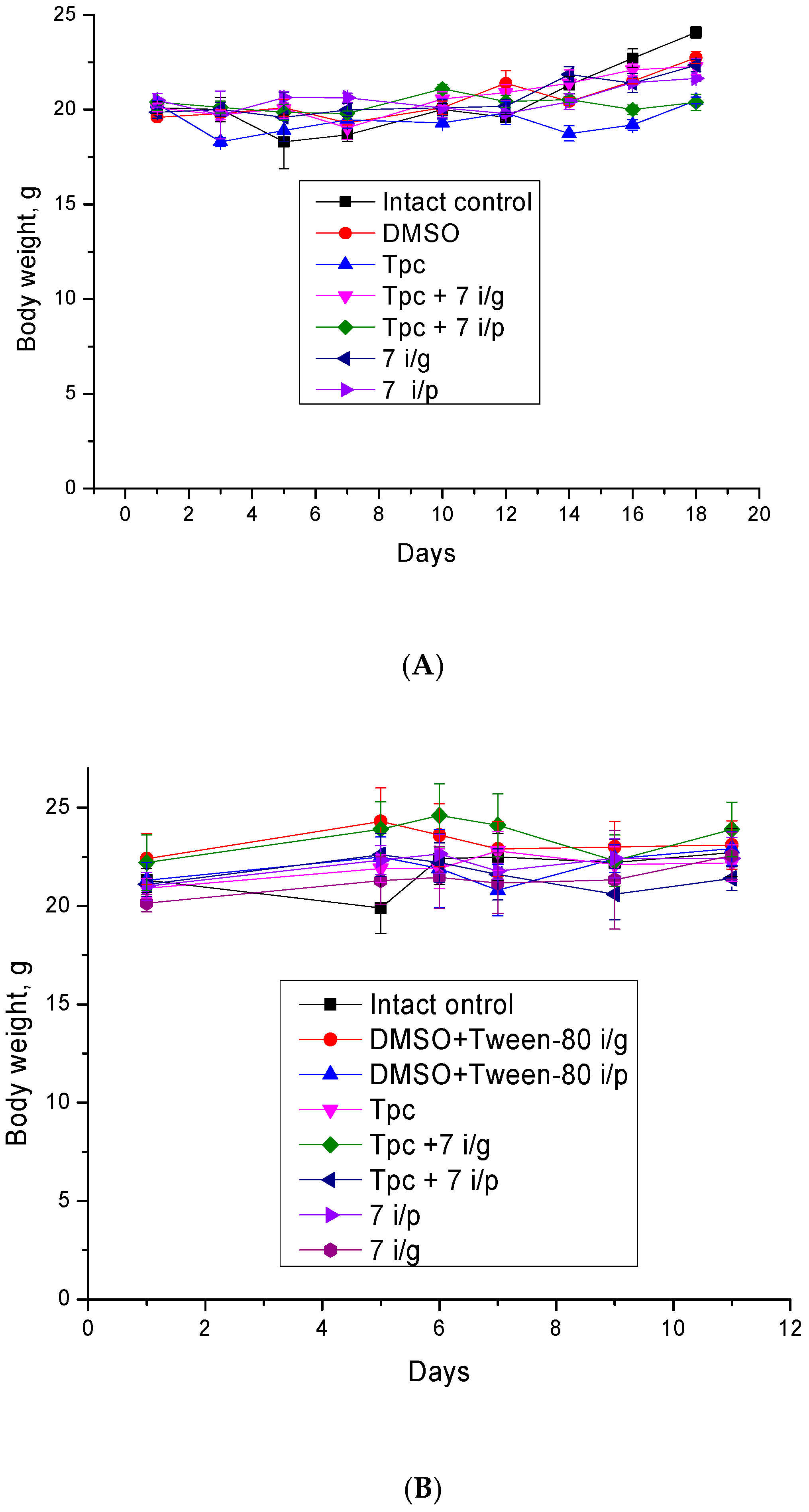
2.2.4. Anticancer and Antimetastatic Effects of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Lewis Lung Carcinoma Model
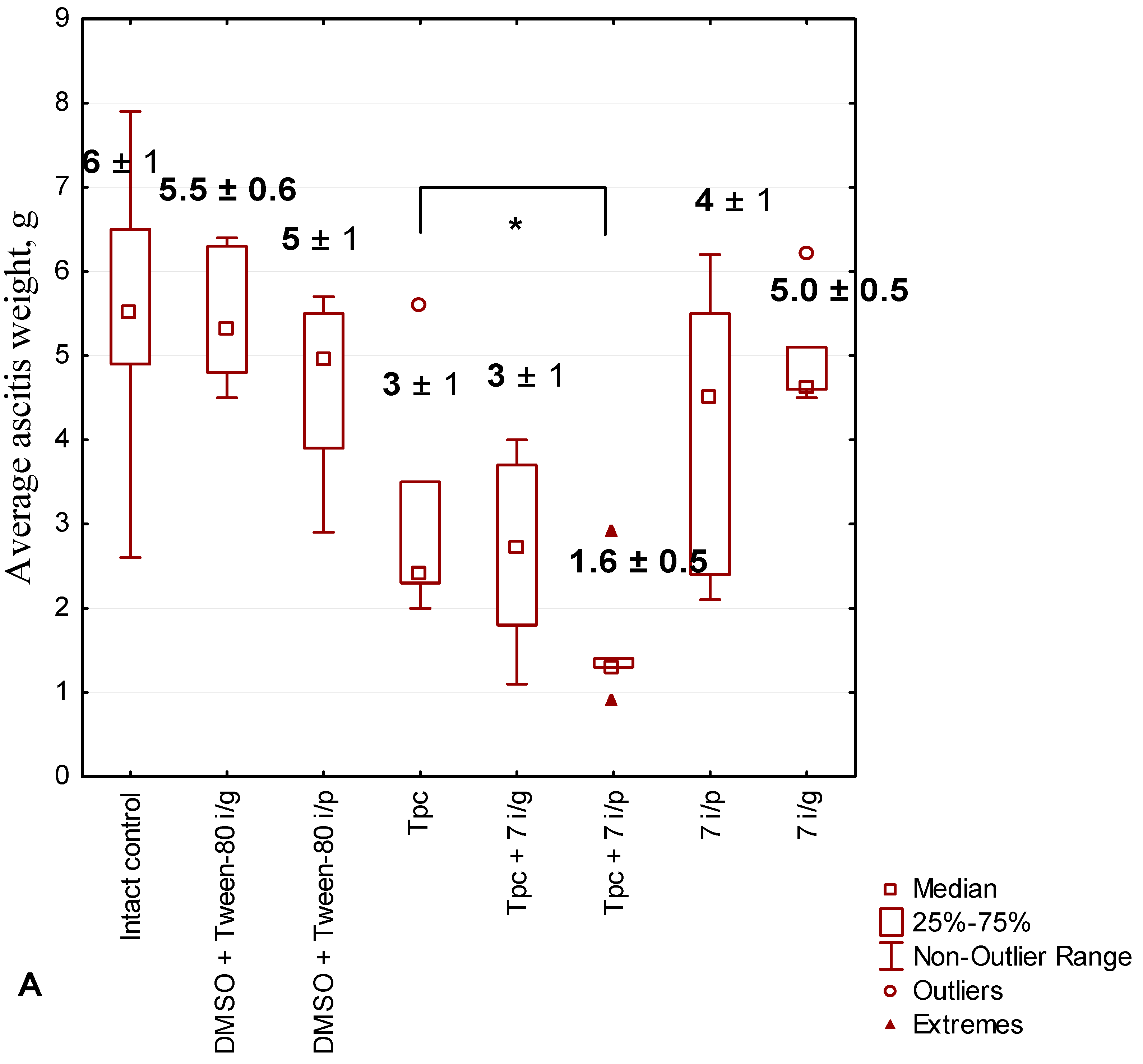
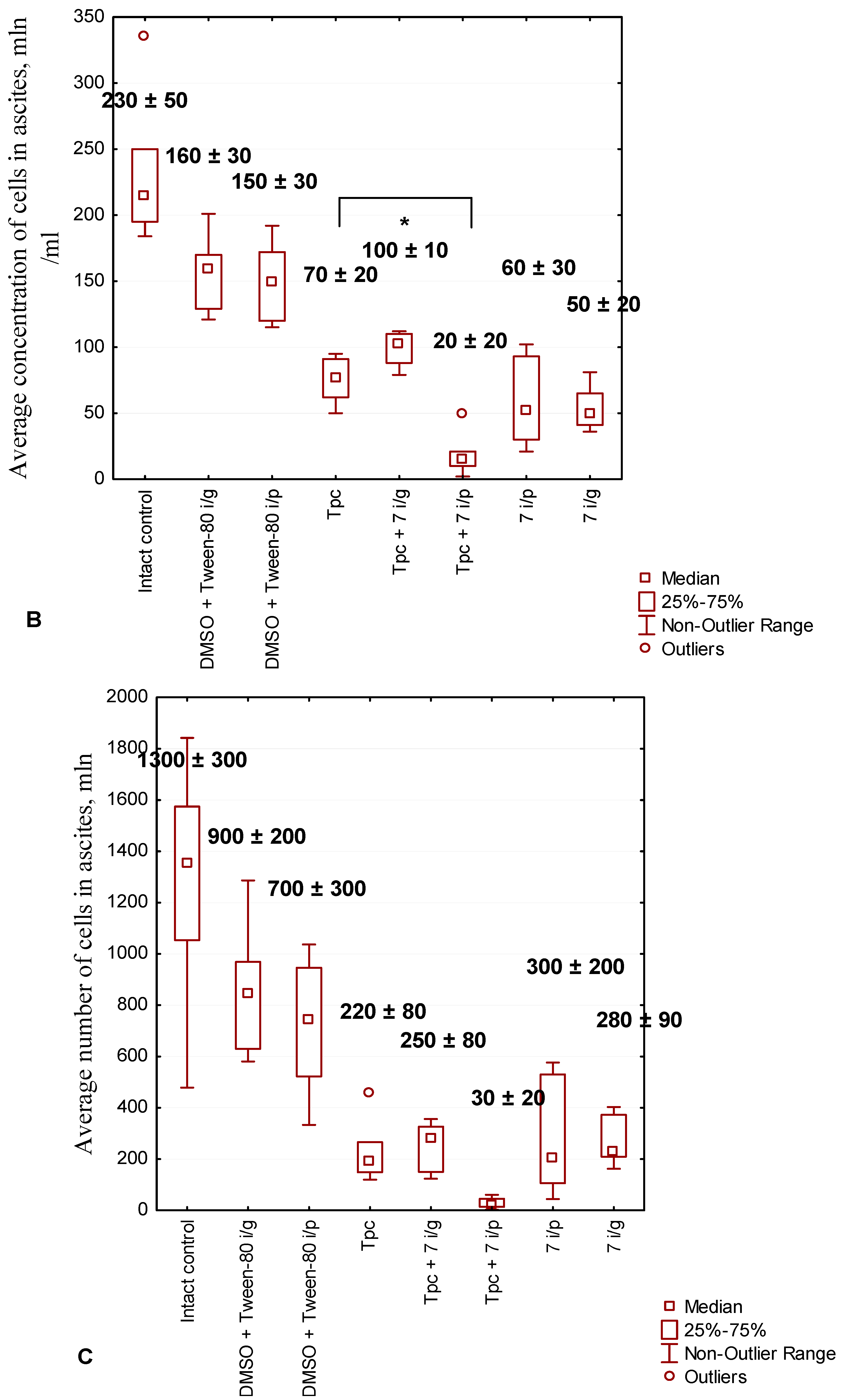
2.2.5. Anticancer Effect of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Krebs-2 Ascitic Carcinoma Model
2.2.6. The Toxic Effect of Drugs and Their Combination
2.2.7. Effects of the Test Substances on the Peripheral Blood in Mice with Krebs-2 Carcinoma
3. Materials and Methods
3.1. Chemistry
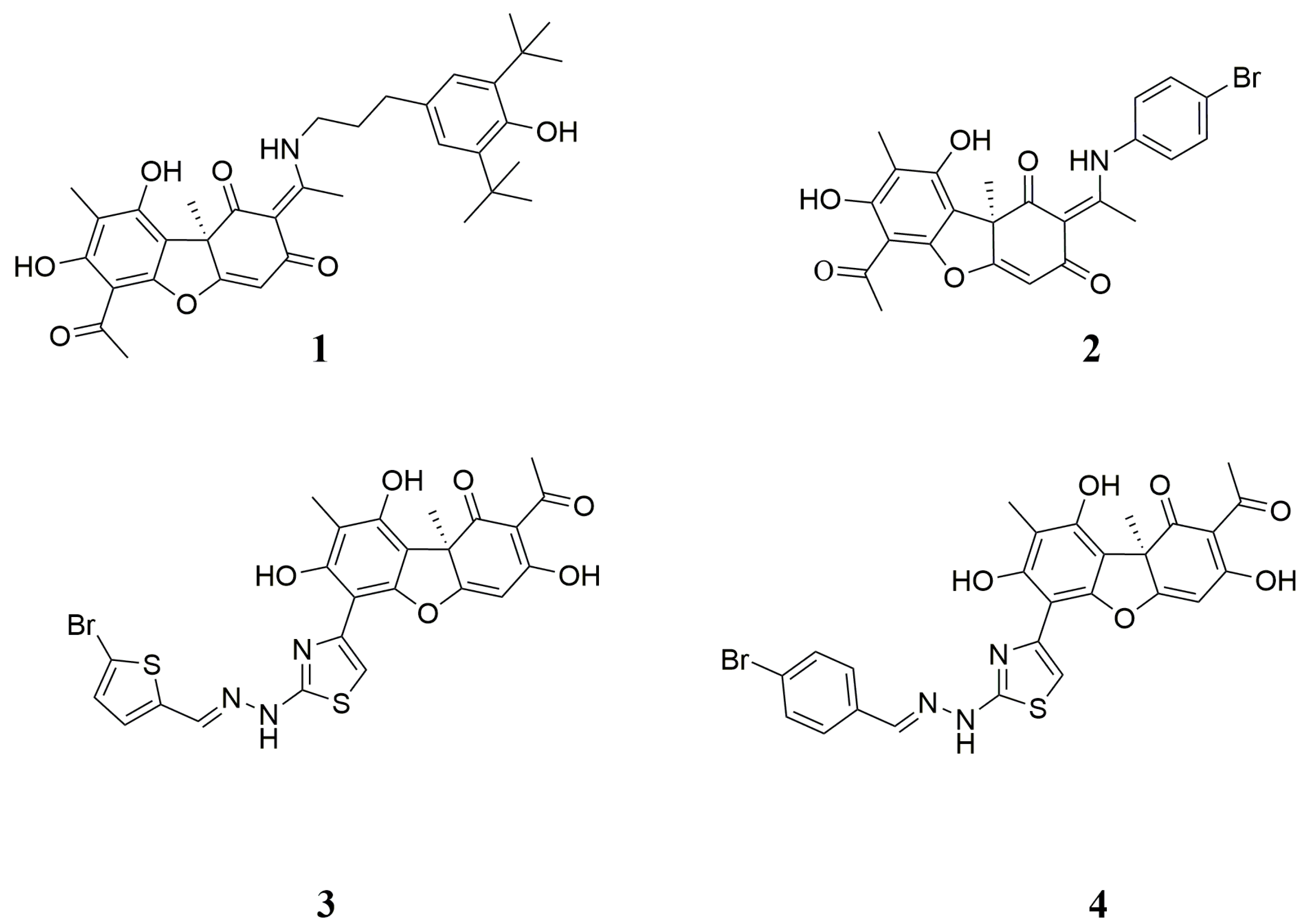
Synthesis of Hybrid Compounds 5–8

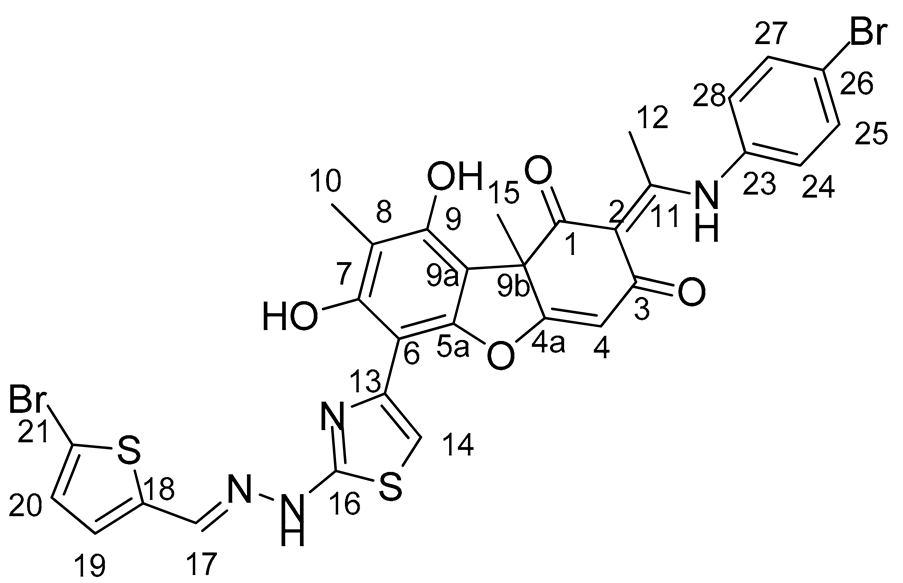
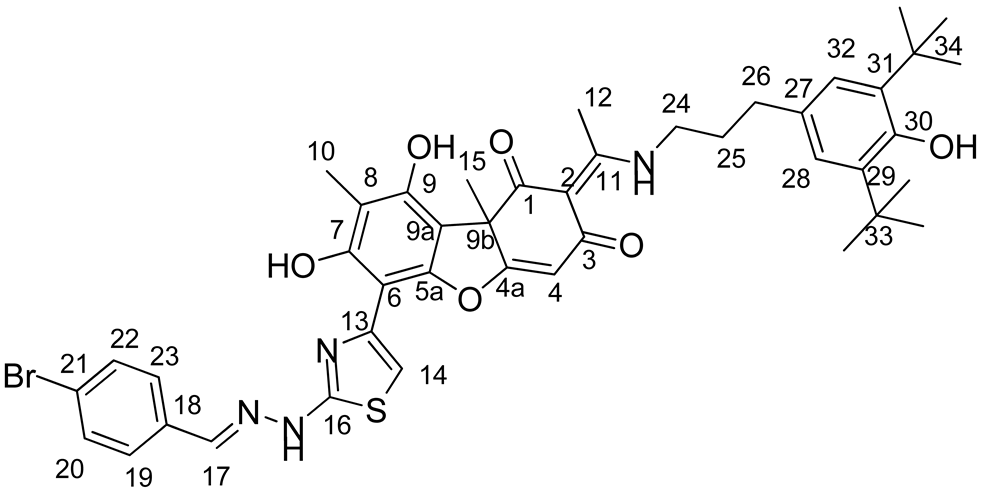
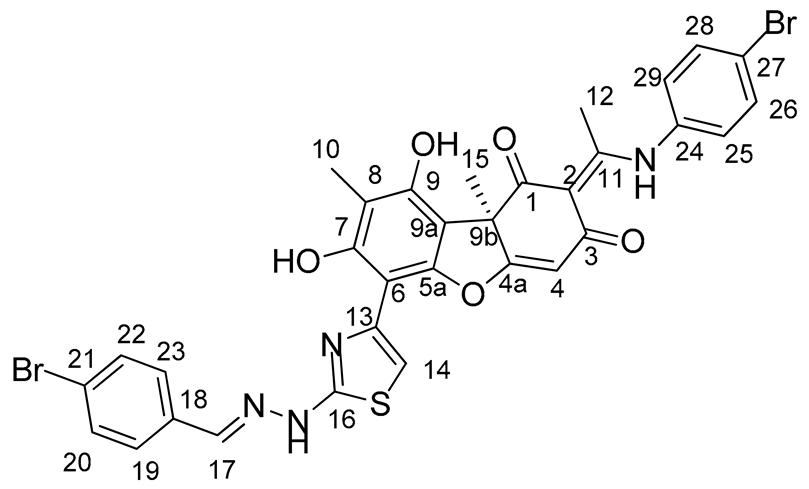
3.2. Biological Assays
3.2.1. Tdp1 Activity
3.2.2. Cytotoxicity Assays
3.2.3. Laboratory Animals and Tumor Models
3.2.4. Antitumor and Antimetastatic Effects of Compound 7 Used as Monotherapy or Combined with Topotecan in the LLC Model
- -
- Group 1—Intact control—mice of this group were inoculated with a tumor, receiving 0.2 mL of saline intraperitoneally;
- -
- Group 2—DMSO-Tween-80—mice were injected once intragastrically with a probe 200 μL of a solution containing saline, Tween-80 (10%) and DMSO (15%), a solvent for 7;
- -
- Group 3—Tpc (aqueous solution)—mice were injected once intraperitoneally with 200 µL of Tpc at a single dose of 1 mg/kg;
- -
- Group 4—Tpc + 7 i/g—mice received Tpc once as described for group 3 and 7 in the form of 200 µL of suspension (Tween-80 (10%) and DMSO (15%) in saline) once intragastrically at a dose of 50 mg/kg;
- -
- Group 5—7 i/g—mice were injected with 7 as described for group 4;
- -
- Group 6—Tpc + 7 i/p—mice were injected with topotecan once as described for groups 3 and 7 in the form of 200 µL of suspension (Tween-80 (10%) and DMSO (15%) in saline) once intraperitoneally at a dose of 50 mg/kg;
- -
- Group 7—7 i/p—mice were injected with 7 as for group 6.
3.2.5. Antitumor Effect of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Krebs-2 Ascitic Carcinoma Model
- -
- Group 1—Intact control—mice of this group were inoculated with a tumor, receiving 0.2 mL of saline intraperitoneally;
- -
- Group 2—DMSO-Tween-80—mice were injected once intragastrically with a probe 200 μL of a solution containing saline, Tween-80 (10%), and DMSO (15%);
- -
- Group 3—DMSO-Tween-80—mice were injected once intraperitoneally with a solvent for 7;
- -
- Group 4—Tpc (aqueous solution)—mice were injected once intraperitoneally with 200 µL of Tpc at a single dose of 1 mg/kg;
- -
- Group 5—Tpc + 7 i/g—mice received Tpc once as described for groups 3 and 7 in the form of 200 µL of suspension in Tween-80-DMSO once intragastrically at a dose of 50 mg/kg;
- -
- Group 6—Tpc + 7 i/p—mice were injected with topotecan once as described for groups 3 and 7 in the form of 200 µL of suspension Tween-80-DMSO once intraperitoneally at a dose of 50 mg/kg;
- -
- Group 7—7 i/g—mice were injected with 7 as described for group 5;
- -
- Group 8—7 i/p—mice were injected with 7 as described for group 6.
3.2.6. The Toxic Effect of Drugs and Their Combination
3.2.7. The Effects of the Substances on the Differential Blood Count
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comeaux, E.Q.; van Waardenburg, R.C. Tyrosyl-DNA phosphodiesterase I resolves both naturally and chemically induced DNA adducts and its potential as a therapeutic target. Drug Metab. Rev. 2014, 46, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Brettrager, E.J.; Segura, I.A.; van Waardenburg, R.C.A.M. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes 2019, 10, 897. [Google Scholar] [CrossRef]
- Alagoz, M.; Gilbert, D.C.; El-Khamisy, S.; Chalmers, A.J. DNA repair and resistance to topoisomerase I inhibitors: Mechanisms, biomarkers and therapeutic targets. Curr. Med. Chem. 2012, 19, 3874–3885. [Google Scholar] [CrossRef]
- Pommier, Y.; Huang, S.Y.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Cuya, S.M.; Bjornsti, M.A.; van Waardenburg, R. DNA topoisomerase-targeting chemotherapeutics: What’s new? Cancer Chemother. Pharmacol. 2017, 80, 1–14. [Google Scholar] [CrossRef]
- He, X.; Van Waardenburg, R.C.; Babaoglu, K.; Price, A.C.; Nitiss, K.C.; Nitiss, J.L.; Bjornsti, M.A.; White, S.W. Mutation of a conserved active site residue converts tyrosyl-DNA phosphodiesterase I into a DNA topoisomerase I-dependent poison. J. Mol. Biol. 2007, 372, 1070–1081. [Google Scholar] [CrossRef]
- Alagoz, M.; Wells, O.S.; El-Khamisy, S.F. TDP1 deficiency sensitizes human cells to base damage via distinct topoisomerase I and PARP mechanisms with potential applications for cancer therapy. Nucleic Acids Res. 2014, 42, 3089–3103. [Google Scholar] [CrossRef]
- Barthelmes, H.U.; Habermeyer, M.; Christensen, M.O.; Mielke, C.; Interthal, H.; Pouliot, J.J.; Boege, F.; Marko, D. TDP1 overexpression in human cells counteracts DNA damage mediated by topoisomerases I and II. J. Biol. Chem. 2004, 279, 55618–55625. [Google Scholar] [CrossRef]
- Inamdar, K.V.; Pouliot, J.J.; Zhou, T.; Lees-Miller, S.P.; Rasouli-Nia, A.; Povirk, L.F. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J. Biol. Chem. 2002, 277, 27162–27168. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.Y.; Das, B.B.; Dexheimer, T.S.; Takeda, S.; Pommier, Y. Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J. Biol. Chem. 2012, 287, 12848–12857. [Google Scholar] [CrossRef]
- Nitiss, K.C.; Malik, M.; He, X.; White, S.W.; Nitiss, J.L. Tyrosyl-DNA phosphodiesterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc. Natl. Acad. Sci. USA 2006, 103, 8953–8958. [Google Scholar] [CrossRef]
- Beretta, G.L.; Cossa, G.; Gatti, L.; Zunino, F.; Perego, P. Tyrosyl-DNA phosphodiesterase 1 targeting for modulation of camptothecin-based treatment. Curr. Med. Chem. 2010, 17, 1500–1508. [Google Scholar] [CrossRef]
- Cuya, S.M.; Comeaux, E.Q.; Wanzeck, K.; Yoon, K.J.; van Waardenburg, R.C. Dysregulated human Tyrosyl-DNA phosphodiesterase I acts as cellular toxin. Oncotarget 2016, 7, 86660–86674. [Google Scholar] [CrossRef]
- Katyal, S.; El-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007, 26, 4720–4731. [Google Scholar] [CrossRef]
- Gao, R.; Das, B.B.; Chatterjee, R.; Abaan, O.D.; Agama, K.; Matuo, R.; Vinson, C.; Meltzer, P.S.; Pommier, Y. Epigenetic and genetic inactivation of tyrosyl-DNA-phosphodiesterase 1 (TDP1) in human lung cancer cells from the NCI-60 panel. DNA Repair 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Fam, H.K.; Walton, C.; Mitra, S.A.; Chowdhury, M.; Osborne, N.; Choi, K.; Sun, G.; Wong, P.C.; O’Sullivan, M.J.; Turashvili, G.; et al. TDP1 and PARP1 deficiency are cytotoxic to rhabdomyosarcoma cells. Mol. Cancer Res. 2013, 11, 1179–1192. [Google Scholar] [CrossRef]
- Huang, H.C.; Liu, J.; Baglo, Y.; Rizvi, I.; Anbil, S.; Pigula, M.; Hasan, T. Mechanism-informed Repurposing of Minocycline Overcomes Resistance to Topoisomerase Inhibition for Peritoneal Carcinomatosis. Mol. Cancer Ther. 2018, 17, 508–520. [Google Scholar] [CrossRef]
- Nivens, M.C.; Felder, T.; Galloway, A.H.; Pena, M.M.; Pouliot, J.J.; Spencer, H.T. Engineered resistance to camptothecin and antifolates by retroviral coexpression of tyrosyl DNA phosphodiesterase-I and thymidylate synthase. Cancer Chemother. Pharmacol. 2004, 53, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, C.; Gilbert, D.C.; Chalmers, A.; Haley, V.; Gollins, S.; Ward, S.E.; El-Khamisy, S.F. Clinical and cellular roles for TDP1 and TOP1 in modulating colorectal cancer response to irinotecan. Mol. Cancer Ther. 2015, 14, 575–585. [Google Scholar] [CrossRef]
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A Eukaryotic Enzyme That Can Disjoin Dead-End Covalent Complexes between DNA and Type I Topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, L.; Lu, W. Evolution in medicinal chemistry of E-ring-modified Camptothecin analogs as anticancer agents. Eur. J. Med. Chem. 2013, 63, 746–757. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anticancer Agents Med. Chem. 2008, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: Rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Pommier, Y.; Marchand, C. Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) inhibitors. Expert Opin. Ther. Pat. 2011, 21, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.L.; Luzina, O.A.; Chepanova, A.A.; Dyrkheeva, N.S.; Salakhutdinov, N.F.; Lavrik, O.I. Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1. Int. J. Mol. Sci. 2023, 24, 5781. [Google Scholar] [CrossRef]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.; Gushchina, I.; Dyrkheeva, N.; Švedas, V.; Salakhutdinov, N.; Lavrik, O. Tyrosyl-DNA Phosphodiesterase 1 Inhibitors: Usnic Acid Enamines Enhance the Cytotoxic Effect of Camptothecin. J. Nat. Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef]
- Dyrkheeva, N.S.; Filimonov, A.S.; Luzina, O.A.; Zakharenko, A.L.; Ilina, E.S.; Malakhova, A.A.; Medvedev, S.P.; Reynisson, J.; Volcho, K.P.; Zakian, S.M.; et al. New Hybrid Compounds Combining Fragments of Usnic Acid and Monoterpenoids for Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibition. Biomolecules 2021, 11, 973. [Google Scholar] [CrossRef]
- Nikolin, V.P.; Popova, N.A.; Kaledin, V.I.; Luzina, O.A.; Zakharenko, A.L.; Salakhutdinov, N.F.; Lavrik, O.I. The influence of an enamine usnic acid derivative (a tyrosyl-DNA phosphodiesterase 1 inhibitor) on the therapeutic effect of topotecan against transplanted tumors in vivo. Clin. Exp. Metastasis 2021, 38, 431–440. [Google Scholar] [CrossRef]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kuprushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Abo-Khatwa, A.N.; Al-Robai, A.A.; Al-Jawhari, D.A. Lichen Acids as Uncouplers of Oxidative Phosphorylation of Mouse-Liver Mitochondria. Nat. Toxins 1996, 4, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, Y.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef]
- Huneck, S.; Akinniyi, J.A.; Cameron, A.F.; Connolly, J.D.; Mulholland, A.G. The absolute configurations of (+)-usnic and (+)-isousnic acid. X-ray analyses of the (−)-α-phenylethylamine derivative of (+)-usnic acid and of (−)-pseudoplacodiolic acid, a new dibenzofuran, from the lichen Rhizoplaca chrysoleuca. Tetrahedron Lett. 1981, 22, 351–352. [Google Scholar] [CrossRef]
- Kutney, J.P.; Sanchez, I.H. Studies in the usnic acid series. I. The condensation of (+)-usnic acid with aliphatic and aromatic amines. Can. J. Chem. 1976, 54, 2795–2803. [Google Scholar] [CrossRef]
- Bazin, M.-A.; Le Lamer, A.-C.; Delcros, J.-G.; Rouaud, I.; Uriac, P.; Boustie, J.; Corbel, J.-C.; Tomasi, S. Synthesis and cytotoxic activities of usnic acid derivatives. Bioorg. Med. Chem. 2008, 16, 6860–6866. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Bruno, M.; Trucchi, B. Dibenzofuran Derivatives with Antibacterial and Wound-Healing Activity. Patent WO2013189950A1, 18 June 2013. [Google Scholar]
- Bruno, M.; Trucchi, B.; Monti, D.; Romeo, S.; Kaiser, M.; Verotta, L. Synthesis of a potent new antimalarial through natural products conjugation. ChemMedChem 2013, 8, 221–225. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.T.; Wu, M.; Wang, W.; Agama, K.; Pommier, Y.; An, L.K. Synthesis of 11-aminoalkoxy substituted benzophenanthridine derivatives as tyrosyl-DNA phosphodiesterase 1 inhibitors and their anticancer activity. Bioorg. Chem. 2022, 123, 105789. [Google Scholar] [CrossRef]
- Leung, E.; Patel, J.; Hollywood, J.A.; Zafar, A.; Tomek, P.; Barker, D.; Pilkington, L.I.; van Rensburg, M.; Langley, R.J.; Helsby, N.A.; et al. Validating TDP1 as an Inhibition Target for the Development of Chemosensitizers for Camptothecin-Based Chemotherapy. Drugs Oncol. Ther. 2021, 9, 541–556. [Google Scholar] [CrossRef]
- Hu, D.X.; Tang, W.L.; Zhang, Y.; Yang, H.; Wang, W.; Agama, K.; Pommier, Y.; An, L.K. Synthesis of Methoxy-, Methylenedioxy-, Hydroxy-, and Halo-Substituted Benzophenanthridinone Derivatives as DNA Topoisomerase IB (TOP1) and Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors and Their Biological Activity for Drug-Resistant Cancer. J. Med. Chem. 2021, 64, 7617–7629. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S.; Janik, P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Zhu, H.; Kauffman, M.E.; Trush, M.A.; Jia, Z.Q.; Li, Y.R. A simple bioluminescence imaging method for studying cancer cell growth and metastasis after subcutaneous injection of Lewis lung carcinoma cells in syngeneic C57BL/6 mice. React. Oxyg. Species 2018, 5, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Berdel, W.E. Ether lipids and analogs in experimental cancer therapy. A brief review of the Munich experience. Lipids 1987, 22, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.N.; Lu, L.; Broxmeyer, H.E. New therapeutic strategies in the treatment of murine diseases induced by virus and solid tumors: Biology and implications for the potential treatment of human leukemia, AIDS, and solid tumors. Crit. Rev. Oncol. Hematol. 1990, 10, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Yu, M.W.; Zhang, G.L.; Yu, J.; Cao, K.X.; Sun, X.; Yang, G.W.; Wang, X.M. Comparison of mouse models of Lewis lung carcinoma subcutaneously transplanted at different sites. Acta Lab. Anim. Sci. Sin. 2017, 25, 386–390. [Google Scholar]
- Klein, G.; Klein, E. The transformation of a solid transplantable mouse carcinoma into an “ascites tumor”. Cancer Res. 1951, 11, 466–469. [Google Scholar]
- Yushok, W.D.; Mallalieu, L.J.; Batt, W.G. Properties of Krebs 2 ascites carcinoma cells: Weight, size, specific gravity, and protein content. J. Frankl. Inst. 1956, 262, 507–509. [Google Scholar] [CrossRef]
- Parsons, D.F.; Marko, M.; Braun, S.J.; Wansor, K.J. Ascites tumor invasion of mouse peritoneum studied by high-voltage electron microscope stereoscopy. Cancer Res. 1982, 42, 4574–4583. [Google Scholar]
- Pryme, I.F.; Pusztai, A.J.; Bardocz, S. A diet containing the lectin phytohaemagglutinin (PHA) slows down the proliferation of Krebs II cell tumours in mice. Cancer Lett. 1994, 76, 133–137. [Google Scholar] [CrossRef]
- Julia, A.M.; Canal, P.; Berg, D.; Bachaud, J.M.; Daly, N.J.; Bugat, R. Concomitant evaluation of efficiency, acute and delayed toxicities of combined treatment of radiation and CDDP on an in vivo model. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, T.E.; Zakharenko, A.L.; Ilina, E.S.; Chepanova, A.A.; Zakharova, O.D.; Dyrkheeva, N.S.; Popova, N.A.; Nikolin, V.P.; Filimonov, A.S.; Luzina, O.A.; et al. Effect of Usnic Acid-Derived Tyrosyl-DNA Phosphodiesterase 1 Inhibitor Used as Monotherapy or in Combination with Olaparib on Transplanted Tumors In Vivo. Mol. Biol. 2023, 57, 220–231. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

| Compound | IC50 | A-549 | HCT-116 | HEK293A | HeLa | MCF-7 | MRC-5 | T98G |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.14 ± 0.02 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6 | 1.0 ± 0.4 | >100 | 62 ± 8 | >100 | 47 ± 12 | 60 ± 20 | 90 ± 20 | >100 |
| 7 | 0.12 ± 0.01 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 8 | 5 ± 1 | 30 ± 10 | 88 ± 26 | >100 | 30 ± 20 | 44 ± 13 | ||
| 4 | 0.03 ± 0.01 | 3.9 ± 0.2 | 7.0 ± 0.6 | 12 ± 5 | 9.3 ± 2.2 | 7.0 ± 0.7 | 4.0 ± 1.0 |
| Cell Line | MRC-5 | HeLa | HEK293A | HCT-116 | A-549 | MCF-7 | T98G | |
|---|---|---|---|---|---|---|---|---|
| Compound | ||||||||
| Tpc | >10 | 9.9 | 0.033 | 0.81 | 1.5 | 1.4 | 2.5 | |
| Tpc + 5 | >10 | nd ** | 0.042 | 0.51 | 0.44 | 1.7 | 1.7 | |
| Tpc + 6 | >10 | 7.3 | 0.046 | 1.3 | 0.66 | 0.77 | 1.6 | |
| Tpc + 7 | >10 | 4.6 * | 0.033 | 0.21 * | 0.25 * | 0.63 * | 1.3 | |
| Tpc + 8 | >10 | 5.2 | 0.052 | 0.72 | 1.3 | 0.95 | 2.0 | |
| Blood Components | Healthy Mice | Intact Control | DMSO + Tween-80 i/g | DMSO + Tween-80 i/p | Tpc | Tpc + 7 i/g | Tpc + 7 i/p | 7 i/p | 7 i/g |
|---|---|---|---|---|---|---|---|---|---|
| Leukocytes | 7.5 ± 1.3 | 13.6 ± 1.2 | 12.8 ± 1.5 | 12.3 ± 1.1 | 11.2 ± 0.7 | 11.7 ± 1.1 | 8.9 ± 0.9 | 10.1 ± 0.9 | 10.7 ± 0.8 |
| Erythrocytes | 8.5 ±0.4 | 6 ± 0.6 | 6.2 ± 0.4 | 6.4 ± 0.6 | 7.6 ± 0.4 | 7.5 ± 0.4 | 8.3 ± 0.5 | 7 ± 0.6 | 7.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornienko, T.E.; Chepanova, A.A.; Zakharenko, A.L.; Filimonov, A.S.; Luzina, O.A.; Dyrkheeva, N.S.; Nikolin, V.P.; Popova, N.A.; Salakhutdinov, N.F.; Lavrik, O.I. Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors—New Usnic Acid Derivatives. Int. J. Mol. Sci. 2024, 25, 1210. https://doi.org/10.3390/ijms25021210
Kornienko TE, Chepanova AA, Zakharenko AL, Filimonov AS, Luzina OA, Dyrkheeva NS, Nikolin VP, Popova NA, Salakhutdinov NF, Lavrik OI. Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors—New Usnic Acid Derivatives. International Journal of Molecular Sciences. 2024; 25(2):1210. https://doi.org/10.3390/ijms25021210
Chicago/Turabian StyleKornienko, Tatyana E., Arina A. Chepanova, Alexandra L. Zakharenko, Aleksandr S. Filimonov, Olga A. Luzina, Nadezhda S. Dyrkheeva, Valeriy P. Nikolin, Nelly A. Popova, Nariman F. Salakhutdinov, and Olga I. Lavrik. 2024. "Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors—New Usnic Acid Derivatives" International Journal of Molecular Sciences 25, no. 2: 1210. https://doi.org/10.3390/ijms25021210
APA StyleKornienko, T. E., Chepanova, A. A., Zakharenko, A. L., Filimonov, A. S., Luzina, O. A., Dyrkheeva, N. S., Nikolin, V. P., Popova, N. A., Salakhutdinov, N. F., & Lavrik, O. I. (2024). Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors—New Usnic Acid Derivatives. International Journal of Molecular Sciences, 25(2), 1210. https://doi.org/10.3390/ijms25021210







