A Sub-Group of Kidney-Transplant Recipients with Highly Aggressive Squamous Cell Carcinoma Expressing Phosphorylated Serine392p53
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Patients and Samples
3.2. Immunohistochemistry
3.3. Immunofluorescence
3.4. Laser-Microdissection
3.5. TP53 Gene Screening with High Resolution Melt (HRM)-Polymerase Chain Reaction (PCR) and Sequencing in p53-Expressing Tumor Cells
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Hofbauer, G.F.; Bouwes Bavinck, J.N.; Euvrard, S. Organ transplantation and skin cancer: Basic problems and new perspectives. Exp. Dermatol. 2010, 19, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.P.; Segundo, D.S.; Hollowood, K.; Marafioti, T.; Clark, T.G.; Harden, P.N.; Wood, K.J. Immune phenotype predicts risk for posttransplantation squamous cell carcinoma. J. Am. Soc. Nephrol. 2010, 21, 713–722. [Google Scholar] [CrossRef]
- Yarosh, D.B.; Boumakis, S.; Brown, A.B.; Canning, M.T.; Galvin, J.W.; Both, D.M.; Kraus, E.; O’Connor, A.; Brown, D.A. Measurement of UVB-Induced DNA damage and its consequences in models of immunosuppression. Methods 2002, 28, 55–62. [Google Scholar] [CrossRef]
- Laing, M.E.; Kay, E.; Conlon, P.; Murphy, G.M. Genetic factors associated with skin cancer in renal transplant patients. Photodermatol. Photoimmunol. Photomed. 2007, 23, 62–67. [Google Scholar] [CrossRef]
- Queille, S.; Luron, L.; Spatz, A.; Avril, M.F.; Ribrag, V.; Duvillard, P.; Hiesse, C.; Sarasin, A.; Armand, J.P.; Daya-Grosjean, L. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis 2007, 28, 724–731. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.M.; Harwood, C.A.; Brooks, L.; Fisher, S.A.; Kelly, D.A.; O’Nions, J.; Young, A.R.; Surentheran, T.; Breuer, J.; Millard, T.P.; et al. Relationship between p53 codon 72 polymorphism and susceptibility to sunburn and skin cancer. J. Investig. Dermatol. 2002, 119, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 2004, 4, 793–805. [Google Scholar] [CrossRef]
- Gu, B.; Zhu, W.G. Surf the post-translational modification network of p53 regulation. Int. J. Biol. Sci. 2012, 8, 672–684. [Google Scholar] [CrossRef]
- Bruins, W.; Zwart, E.; Attardi, L.D.; Iwakuma, T.; Hoogervorst, E.M.; Beems, R.B.; Miranda, B.; van Oostrom, C.T.; van den Berg, J.; van den Aardweg, G.J.; et al. Increased sensitivity to UV radiation in mice with a p53 point mutation at Ser389. Mol. Cell. Biol. 2004, 24, 8884–8894. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Park, B.J.; Kim, D.J.; Kim, W.H.; Kim, S.; Oh, K.S.; Lim, J.Y.; Kim, J.; Park, C.; Park, S.I. Modification of serine 392 is a critical event in the regulation of p53 nuclear export and stability. FEBS Lett. 2004, 572, 92–98. [Google Scholar] [CrossRef]
- Minamoto, T.; Buschmann, T.; Habelhah, H.; Matusevich, E.; Tahara, H.; Boerresen-Dale, A.L.; Harris, C.; Sidransky, D.; Ronai, Z. Distinct pattern of p53 phosphorylation in human tumors. Oncogene 2001, 20, 3341–3347. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Sakamoto, H.; Lewis, M.S.; Anderson, C.W.; Erickson, J.W.; Appella, E.; Xie, D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry 1997, 36, 10117–10124. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, C.; Waterschoot, B.; De Backer, O.; Dumont, P. Serine 392 phosphorylation modulates p53 mitochondrial translocation and transcription-independent apoptosis. Cell Death Differ. 2018, 25, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dreno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, A.; Gutzmer, R.; Roshdy, O.; Gonzalez Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.G.; Grob, J.-J.; Bowyer, S.E.; Day, F.; Ladwa, R.; Stein, B.; Couselo, E.M.; Basset-Seguin, N.; Guminski, A.; Mortier, L. 818P Phase II confirmatory study of cemiplimab (350mg IV Q3W) in patients with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC): Study 1540 Group 6. Ann. Oncol. 2022, 33, S921. [Google Scholar] [CrossRef]
- Migden, M.; Schmults, C.; Khushanlani, N.; Guminski, A.; Chang, A.; Lewis, K.; Ansstas, G.; Bowyer, S.; Hughes, B.; Schadendorf, D. 814P Phase II study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (CSCC): Final analysis from EMPOWER-CSCC-1 groups 1, 2 and 3. Ann. Oncol. 2022, 33, S918–S919. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Palmer, J.D.; Schneider, C.J.; Hockstein, N.; Hanlon, A.L.; Silberg, J.; Strasser, J.; Mauer, E.A.; Dzeda, M.; Witt, R.; Raben, A. Combination of post-operative radiotherapy and cetuximab for high-risk cutaneous squamous cell cancer of the head and neck: A propensity score analysis. Oral Oncol. 2018, 78, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kreinbrink, P.J.; Mierzwa, M.L.; Huth, B.; Redmond, K.P.; Wise-Draper, T.M.; Casper, K.; Li, J.; Takiar, V. Adjuvant radiation and cetuximab improves local control in head and neck cutaneous squamous cell carcinoma: Phase II study. Head Neck 2021, 43, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Amit, M.; Nagarajan, P.; Rubin, M.L.; Yuan, Y.; Bell, D.; El-Naggar, A.K.; Johnson, J.M.; Morrison, W.H.; Rosenthal, D.I.; et al. Pilot Phase II Trial of Neoadjuvant Immunotherapy in Locoregionally Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2021, 27, 4557–4565. [Google Scholar] [CrossRef] [PubMed]
- Jarkowski, A., 3rd; Hare, R.; Loud, P.; Skitzki, J.J.; Kane, J.M., 3rd; May, K.S.; Zeitouni, N.C.; Nestico, J.; Vona, K.L.; Groman, A.; et al. Systemic Therapy in Advanced Cutaneous Squamous Cell Carcinoma (CSCC): The Roswell Park Experience and a Review of the Literature. Am. J. Clin. Oncol. 2016, 39, 545–548. [Google Scholar] [CrossRef]
- Trodello, C.; Pepper, J.P.; Wong, M.; Wysong, A. Cisplatin and Cetuximab Treatment for Metastatic Cutaneous Squamous Cell Carcinoma: A Systematic Review. Dermatol. Surg. 2017, 43, 40–49. [Google Scholar] [CrossRef]
- Preneau, S.; Rio, E.; Brocard, A.; Peuvrel, L.; Nguyen, J.M.; Quereux, G.; Dreno, B. Efficacy of cetuximab in the treatment of squamous cell carcinoma. J. Dermatol. Treat. 2014, 25, 424–427. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; van Akkooi, A.; Bataille, V.; Bastholt, L.; Dreno, B.; Dummer, R.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma: Part 2. Treatment-Update 2023. Eur. J. Cancer 2023, 193, 113252. [Google Scholar] [CrossRef]
- Petzold, A.; Steeb, T.; Wessely, A.; Schatton, T.; Berking, C.; Heppt, M.V. Comparative efficacy analysis identifies immune checkpoint blockade as a new survival benchmark in advanced cutaneous squamous cell carcinoma. Eur. J. Cancer 2022, 170, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.F.; Pereira, J.L.; Pais, A.A. Is standard multivariate analysis sufficient in clinical and epidemiological studies? J. Biomed. Inform. 2013, 46, 75–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
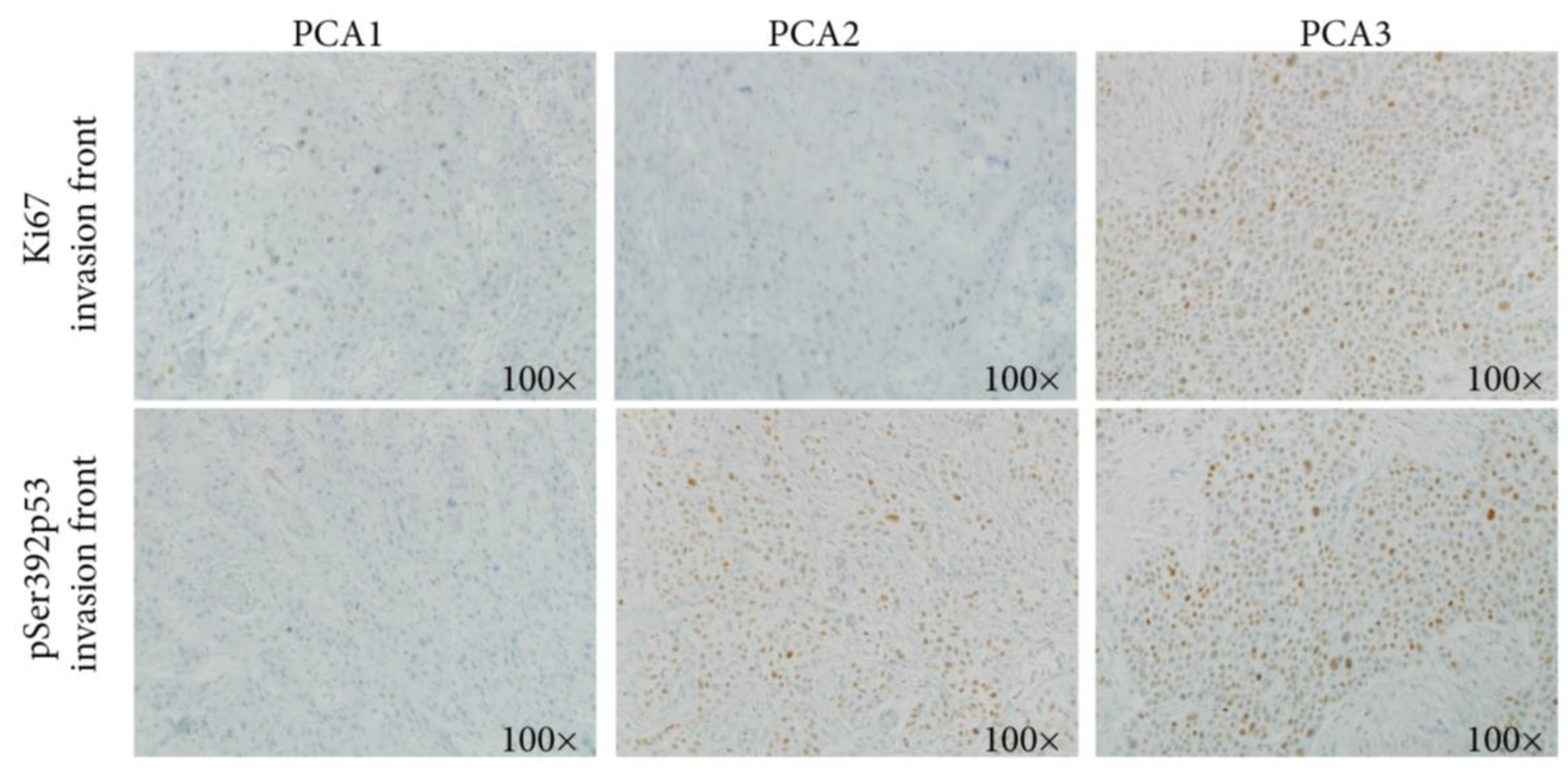
| Variables | N (%) or Median [Q1–Q3] | Transplantation (n = 25) | No Transplantation (n = 22) | p * |
|---|---|---|---|---|
| Age at diagnosis (y), n = 47 | 71.0 ± 18.0 | 65.0 ± 15.0 | 77.0 ± 18.0 | 0.006 |
| Gender (male), n = 47 | 30 (64) | 16 (64) | 14 (64) | 0.98 |
| Transplantation | ||||
| Yes | 25 (53) | - | - | - |
| Number | - | 2.0 [1.0–2.0] | - | - |
| Time since first transplant (y) | - | 19.0 [5.5–26.0] | - | - |
| Number of carcinomas, n = 47 | 3.0 ± 17.0 | 15.0 ± 18.0 | 1.0 ± 0.75 | <0.001 |
| Thickness (mm), n = 42 | 4.0 ± 7.1 | 4.5 ± 12.0 | 3.2 ± 3.5 | 0.32 |
| Clark’s level ≥ 4 (yes), n = 47 | 40 (85) | 22 (88) | 18 (82) | 0.19 |
| Level of differentiation, n = 47 | 0.22 | |||
| Good | 41 (87) | 22 (88) | 19 (86) | |
| Fair | 4 (8.5) | 1 (4) | 3 (14) | |
| Poor | 2 (4.5) | 2 (8) | 0 (0) | |
| Peri-neural invasion (yes), n = 45 | 5 (11) | 5 (20) | 0 (0) | 0.02 |
| Metastases | ||||
| Lymph node (yes), n = 42 | 4 (10) | 4 (16) | 0 (0) | 0.09 |
| Visceral (yes), n = 41 | 3 (7) | 3 (12) | 0 (0) | 0.15 |
| Biomarker expression (%) | ||||
| Ki67 center, n = 42 | 0.0 ± 10.0 | 2.5 ± 45.0 | 0.0 ± 6.0 | 0.21 |
| Ki67 invasion, 42 | 20.0 ± 47.5 | 12.5 ± 65.0 | 20.0 ± 22.5 | 0.95 |
| p53 center, n = 45 | 12.5 ± 47.5 | 30.0 ± 50.0 | 5.0 ± 20.0 | 0.08 |
| p53 invasion, n = 42 | 70.0 ± 65.0 | 75.0 ± 62.5 | 50.0 ± 65.0 | 0.69 |
| pSer392 p53 center, n = 41 | 0.0 ± 20.0 | 0.0 ± 30.0 | 0.0 ± 6.0 | 0.16 |
| pSer392p53 invasion, n = 44 | 27.5 ± 70.0 | 37.5 ± 80.0 | 10.0 ± 70.0 | 0.45 |
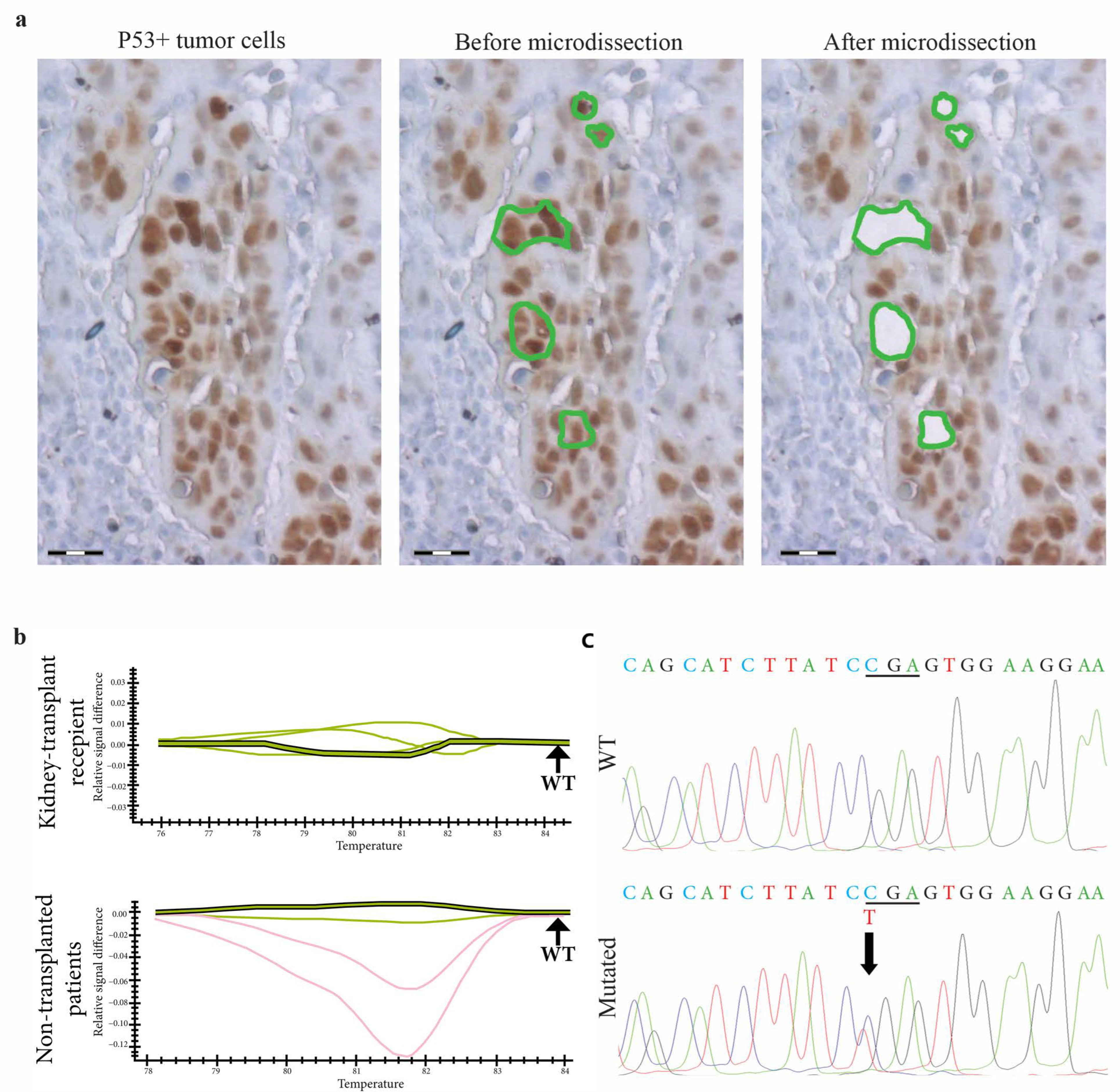
| Kidney-Transplant Recipients | Non-Transplanted Patients | |||||
|---|---|---|---|---|---|---|
| Sample | PCR-HRM | Sanger Sequencing | Sample | PCR-HRM | Sanger Sequencing | |
| 1 | Exon 5B | p.V157F c.469 G > T | 1 | Exon 5B | p.V157F c.469 G > T | |
| 2 | Exon 7B | p.R248W c.742 C > T | 2 | Exon 6A | p.R196 * c.586 C > T | |
| 3 | Exon 9B | WT | 3 | Exon 6B | p.E221V c.662 A > T | |
| 4 | Exon 7A | WT | 4 | Exon 8A | p.R282W c.844 C > T | |
| 5 | Exon 6A | WT | 5 | Exon 5B | WT | |
| 6 | Exon 4A | WT | 6 | Exon 6B | WT | |
| 7 | Exon 8A | WT | 7 | Exon 6B | WT | |
| 8 | Exon 6B | WT | 8 | Exon 6B | WT | |
| 9 | Exon 6A | WT | 9 | Exon 7A | WT | |
| 10 | Exon 6A | WT | 10 | WT | - | |
| 11 | Exon 5C | WT | 11 | WT | - | |
| 12 | Exon 9A | WT | 12 | WT | - | |
| 13 | WT | - | 13 | Insufficient material | ||
| 14 | WT | - | 14 | Insufficient material | ||
| 15 | WT | - | 15 | Insufficient material | ||
| 16 | WT | - | 16 | Insufficient material | ||
| 17 | WT | - | 17 | WT | - | |
| 18 | WT | - | 18 | WT | - | |
| 19 | WT | - | 19 | WT | - | |
| 20 | WT | - | 20 | WT | - | |
| 21 | WT | - | 21 | WT | - | |
| 22 | WT | - | 22 | WT | - | |
| 23 | WT | - | ||||
| 24 | WT | - | ||||
| 25 | WT | - | ||||
| Variables | Age | N° Carcinomas | Thickness | Clark’s Level | Ki67 Center | Ki67 Invasion | P53 Center | P53 Invasion | pSer392p53 Center | pSer392p53 Invasion |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | |||||||||
| N° carcinomas | −0.47 ** | 1.00 | ||||||||
| Thickness | −0.31 | 0.26 | 1.00 | |||||||
| Clark’s level | −0.29 | 0.31 | 0.42 * | 1.00 | ||||||
| Ki67 center | −0.26 | 0.29 | 0.36 * | 0.44 * | 1.00 | |||||
| Ki67 invasion | −0.10 | 0.16 | 0.32 | 0.36 * | 0.85 *** | 1.00 | ||||
| p53 center | −0.07 | 0.28 | 0.03 | 0.17 | 0.30 | 0.27 | 1.00 | |||
| p53 invasion | 0.06 | 0.19 | −0.19 | −0.001 | 0.02 | 0.14 | 0.69 *** | 1.00 | ||
| pSer392p53 center | −0.23 | 0.34 | 0.23 | 0.25 | 0.39 * | 0.42 * | 0.74 *** | 0.59 *** | 1.00 | |
| pSer392p53 invasion | −0.05 | 0.30 | 0.03 | 0.13 | 0.26 | 0.41 * | 0.53 ** | 0.83 *** | 0.69 *** | 1.00 |
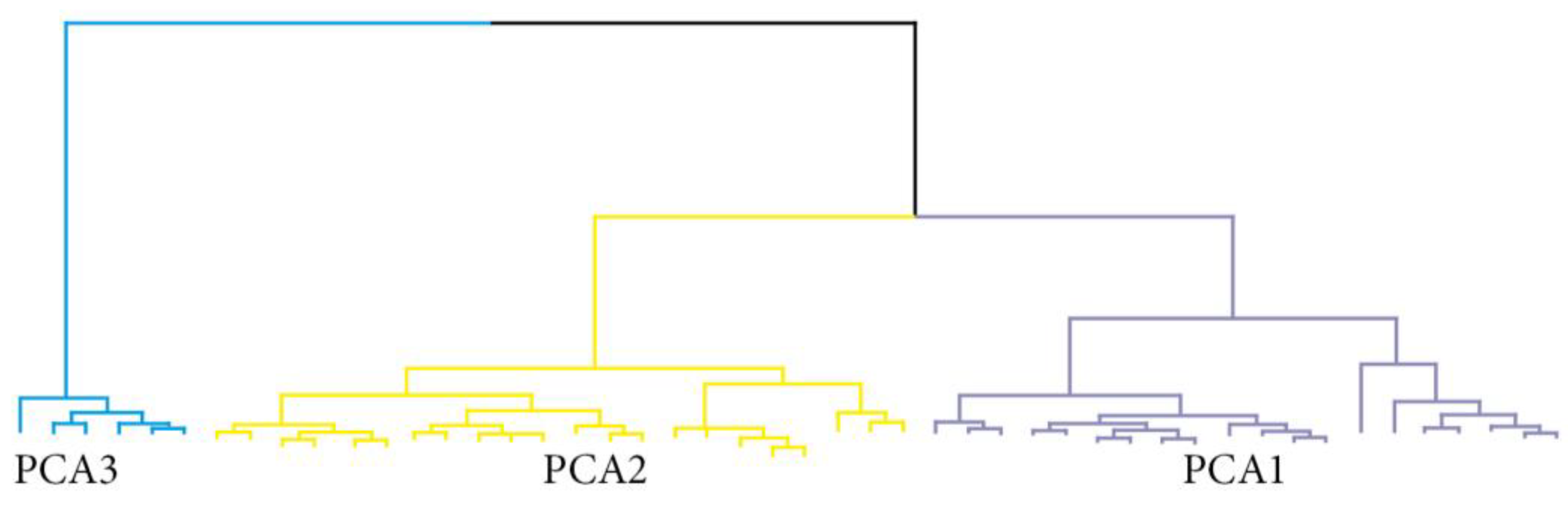
| Variables | N (%) or Median ± IQR | p * | ||
|---|---|---|---|---|
| PCA1 (n = 20) | PCA2 (n = 21) | PCA3 (n = 6) | ||
| Age at diagnosis (y) | 65.0 ± 15.5 | 75.0 ± 12.0 | 61.5 ± 6.0 | 0.009 |
| Gender (male) | 13 (65) | 12 (54.5) | 5 (83) | 0.49 |
| Transplantation | ||||
| Yes | 10.0 (50) | 9 (41) | 6 (100) | 0.03 |
| N° of transplants | 0.5 ± 2.0 | 0.0 ± 1.0 | 2.0 ± 1.0 | 0.01 |
| Time since first transplant (y) | 0.0 ± 14.5 | 0.0 ± 2.0 | 23.0 ± 8.0 | 0.007 |
| N° of carcinomas | 3.0 ± 13.5 | 2.0 ± 6.0 | 24 ± 17.0 | 0.02 |
| Thickness (mm) | 4.0 ± 9.5 | 3.1 ± 4.0 | 7.5 ± 9.5 | 0.15 |
| Clark’s level ≥ 4 (yes) | 15 (75) | 19 (86) | 6 (100) | 0.20 |
| Level of differentiation | 0.89 | |||
| Good | 18 (90) | 18 (86) | 5 (83) | |
| Fair | 1 (5) | 2 (9.5) | 1 (7) | |
| Poor | 1 (5) | 1 (4.5) | 0 (0) | |
| Peri-neural invasion (yes) | 4 (20) | 0 (0) | 1 (17) | 0.07 |
| Metastases | ||||
| Lymph node (yes) | 2 (10) | 0 (0) | 2 (33) | 0.05 |
| Visceral (yes) | 2 (10) | 1 (4.5) | 0 (0) | 0.59 |
| Biomarker expression (%) | ||||
| Ki67 center | 0.0 ± 9.0 | 0.0 ± 5.0 | 70.0 ± 22.5 | 0.0001 |
| Ki67 invasion | 20.0 ± 30.0 | 10.0 ± 27.0 | 75.0 ± 10.0 | 0.0004 |
| p53 center | 0.0 ± 0.0 | 25.0 ± 40.0 | 60.0 ± 27.5 | <0.0001 |
| p53 invasion | 20.0 ± 26.0 | 90.0 ± 10.0 | 90.0 ± 7.5 | 0.04 |
| pSer392-p53 center | 0.0 ± 0.0 | 5.0 ± 30.0 | 50.0 ± 22.5 | <0.0001 |
| pSer392-p53 invasion | 0.0 ± 9.0 | 70.0 ± 57.5 | 75.0 ± 17.5 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan, D.; Gardair, C.; Pamoukdjian, F.; Peraldi Gardin, M.-N.; Nakouri, I.; Leboeuf, C.; Janin, A.; Lebbé, C.; Battistella, M.; Bousquet, G. A Sub-Group of Kidney-Transplant Recipients with Highly Aggressive Squamous Cell Carcinoma Expressing Phosphorylated Serine392p53. Int. J. Mol. Sci. 2024, 25, 1147. https://doi.org/10.3390/ijms25021147
Hamdan D, Gardair C, Pamoukdjian F, Peraldi Gardin M-N, Nakouri I, Leboeuf C, Janin A, Lebbé C, Battistella M, Bousquet G. A Sub-Group of Kidney-Transplant Recipients with Highly Aggressive Squamous Cell Carcinoma Expressing Phosphorylated Serine392p53. International Journal of Molecular Sciences. 2024; 25(2):1147. https://doi.org/10.3390/ijms25021147
Chicago/Turabian StyleHamdan, Diaddin, Charlotte Gardair, Frédéric Pamoukdjian, Marie-Noëlle Peraldi Gardin, Inès Nakouri, Christophe Leboeuf, Anne Janin, Céleste Lebbé, Maxime Battistella, and Guilhem Bousquet. 2024. "A Sub-Group of Kidney-Transplant Recipients with Highly Aggressive Squamous Cell Carcinoma Expressing Phosphorylated Serine392p53" International Journal of Molecular Sciences 25, no. 2: 1147. https://doi.org/10.3390/ijms25021147
APA StyleHamdan, D., Gardair, C., Pamoukdjian, F., Peraldi Gardin, M.-N., Nakouri, I., Leboeuf, C., Janin, A., Lebbé, C., Battistella, M., & Bousquet, G. (2024). A Sub-Group of Kidney-Transplant Recipients with Highly Aggressive Squamous Cell Carcinoma Expressing Phosphorylated Serine392p53. International Journal of Molecular Sciences, 25(2), 1147. https://doi.org/10.3390/ijms25021147









