Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata
Abstract
1. Introduction
2. Results
2.1. AaAtg8 Plays a Role in Iron Uptake during A. alternata Infection
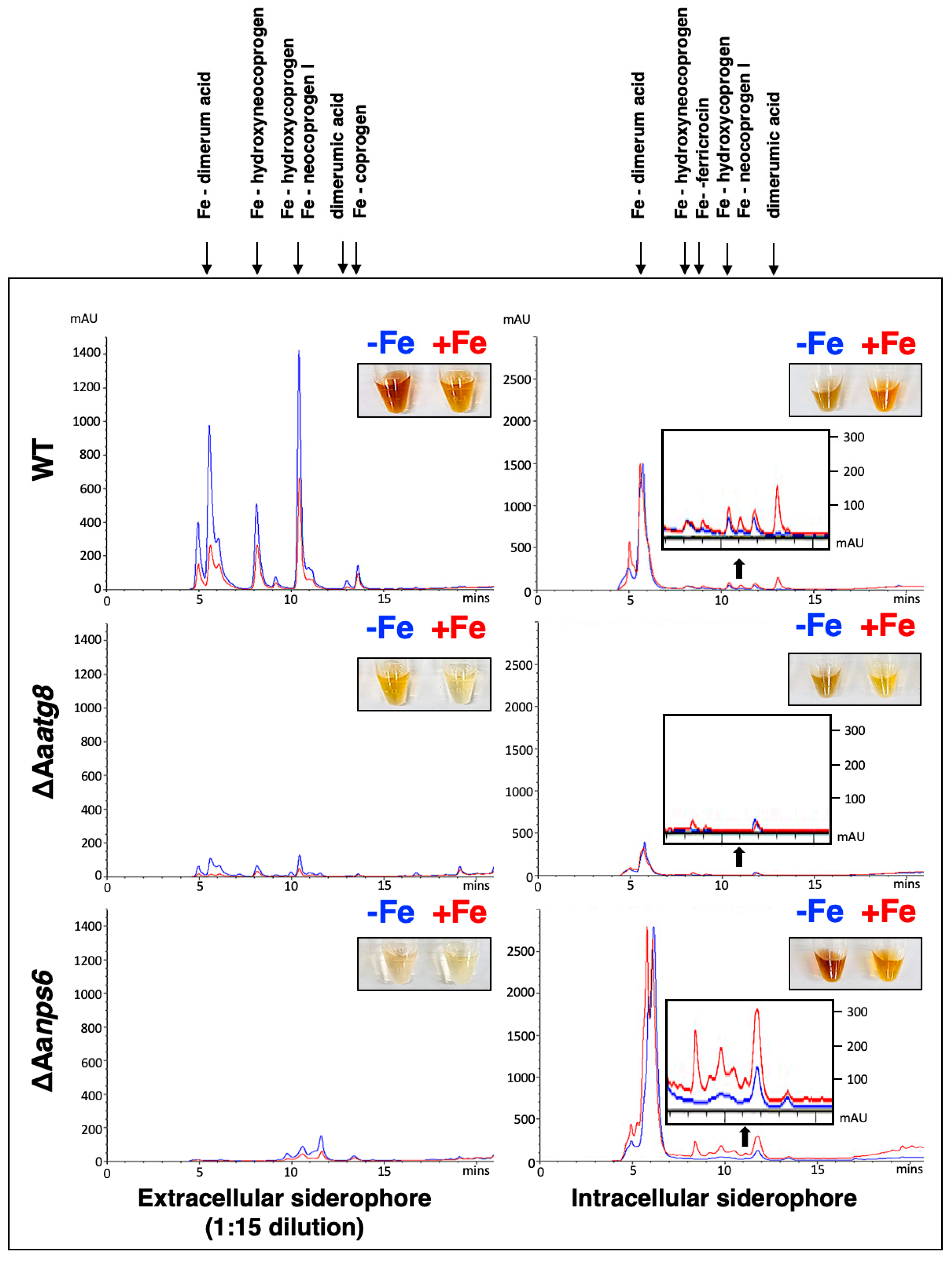
2.2. AaAtg8-Mediated Autophagy Regulates the Production of Intra- and Extracellular Siderophores
2.3. AaAtg8 Influences the Expression of Genes Related to Siderophore Production and Iron Acquisition
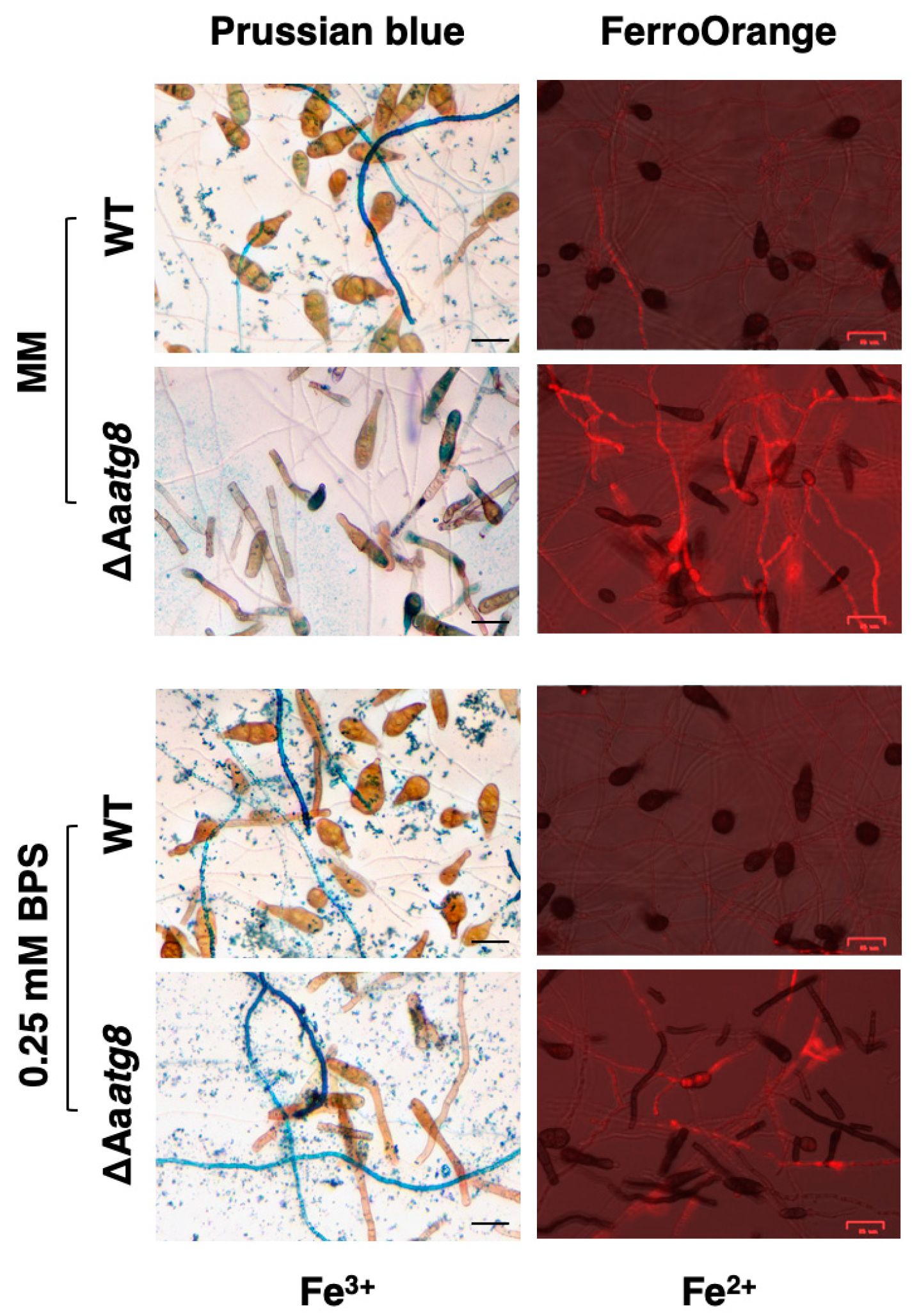
2.4. AaAtg8 Is Required to Maintain Cellular Iron Homeostasis
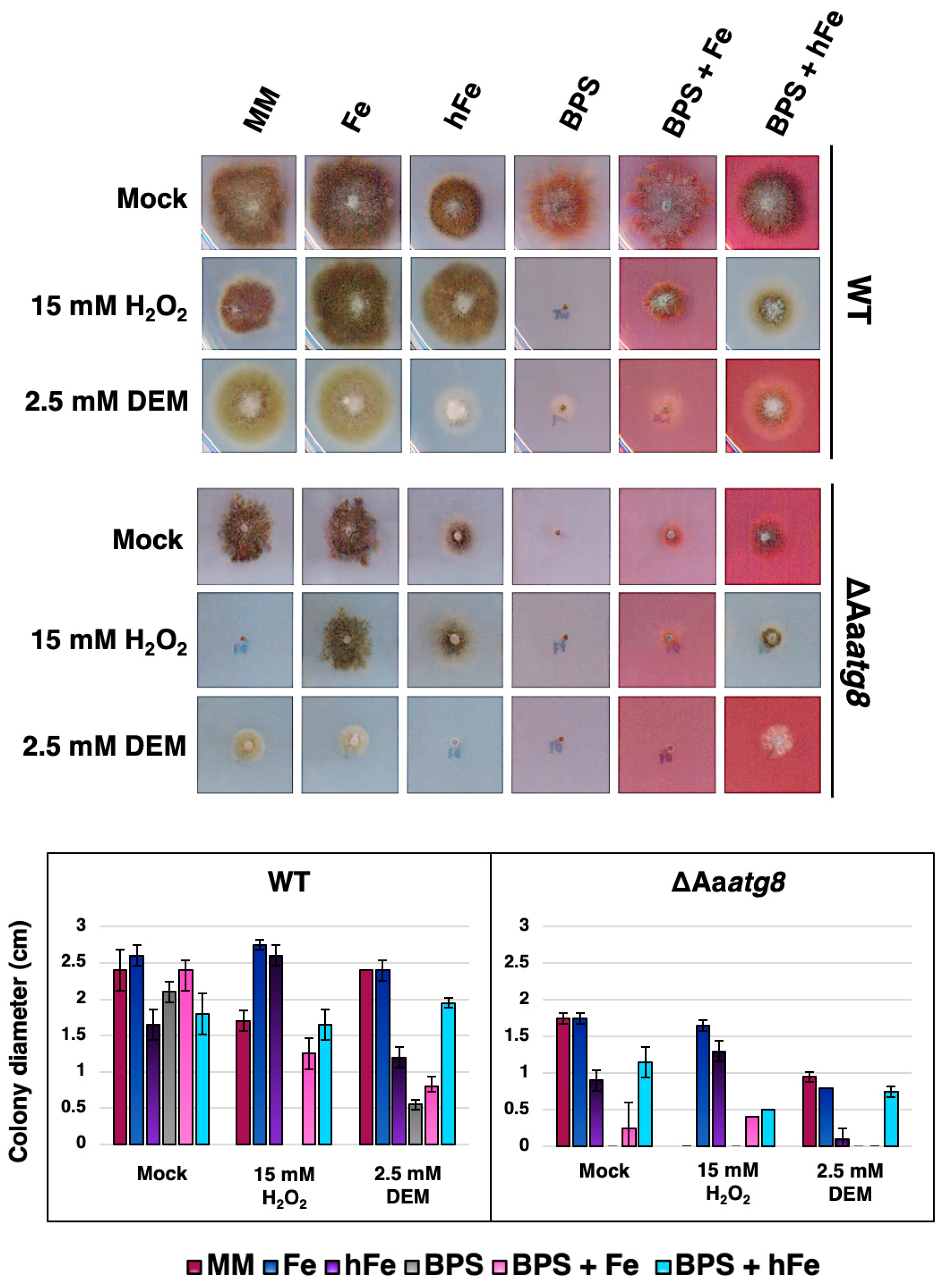
2.5. Autophagy Regulates Iron Utilization and Promotes Oxidative Stress Resistance
2.6. Excess Iron Triggers Autophagy and Lipid Peroxidation in A. alternata
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Sensitivity Assays
4.3. Virulence Tests
4.4. Prussian Blue Staining
4.5. Analysis of Siderophores
4.6. Quantitative RT-PCR and Gene Expression Analyses
4.7. Fluorescence Microscopy
4.8. sGFP-AaAtg8 Proteolysis Assay
4.9. MDA Measurement
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenshields, D.L.; Liu, G.; Wei, Y. Roles of iron in plant defence and fungal virulence. Plant Signal. Behav. 2007, 2, 300–302. [Google Scholar] [CrossRef]
- Jung, W.H.; Kronstad, J.W. Iron and fungal pathogenesis: A case study with Cryptococcus neoformans. Cell. Microbiol. 2008, 10, 277–284. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Bairwa, G.; Hee Jung, W.; Kronstad, J.W. Iron acquisition in fungal pathogens of humans. Metallomics 2017, 9, 215–227. [Google Scholar] [CrossRef]
- Martinez-Pastor, M.T.; Puig, S. Adaptation to iron deficiency in human pathogenic fungi. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118797. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef]
- Pecoraro, L.; Wang, X.; Shah, D.; Song, X.; Kumar, V.; Shakoor, A.; Tripathi, K.; Ramteke, P.W.; Rani, R. Biosynthesis pathways, transport mechanisms and biotechnological applications of fungal siderophores. J. Fungi 2021, 8, 21. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Hissen, A.H.; Wan, A.N.; Warwas, M.L.; Pinto, L.J.; Moore, M.M. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 2005, 73, 5493–5503. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C. Iron uptake in fungi: A system for every source. Biochim. Biophys. Acta 2006, 1763, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Ibrahim-Granet, O.; Droin, S.; Huerre, M.; Latge, J.P.; Haas, H. The crucial role of the Aspergillus fumigatus siderophore system in interaction with alveolar macrophages. Microbes Infect. 2010, 12, 1035–1041. [Google Scholar] [CrossRef]
- Oide, S.; Krasnoff, S.B.; Gibson, D.M.; Turgeon, B.G. Intracellular siderophores are essential for ascomycete sexual development in heterothallic Cochliobolus heterostrophus and homothallic Gibberella zeae. Eukaryot. Cell 2007, 6, 1339–1353. [Google Scholar] [CrossRef]
- Condon, B.J.; Oide, S.; Gibson, D.M.; Krasnoff, S.B.; Turgeon, B.G. Reductive iron assimilation and intracellular siderophores assist extracellular siderophore-driven iron homeostasis and virulence. Mol. Plant Microbe Interact. 2014, 27, 793–808. [Google Scholar] [CrossRef][Green Version]
- Voss, B.; Kirschhofer, F.; Brenner-Weiss, G.; Fischer, R. Alternaria alternata uses two siderophore systems for iron acquisition. Sci. Rep. 2020, 10, 3587. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.H.; Chung, K.R. A nonribosomal peptide synthetase mediates siderophore production and virulence in the citrus fungal pathogen Alternaria alternata. Mol. Plant Pathol. 2013, 14, 497–505. [Google Scholar] [CrossRef]
- Chen, L.H.; Yang, S.L.; Chung, K.R. Resistance to oxidative stress via regulating siderophore-mediated iron acquisition by the citrus fungal pathogen Alternaria alternata. Microbiol.-SGM 2014, 160, 970–979. [Google Scholar] [CrossRef]
- Chung, K.R.; Wu, P.C.; Chen, Y.K.; Yago, J.I. The siderophore repressor SreA maintains growth, hydrogen peroxide resistance, and cell wall integrity in the phytopathogenic fungus Alternaria alternata. Fungal Genet. Biol. 2020, 139, 103384. [Google Scholar] [CrossRef]
- Wu, J.J.; Wu, P.C.; Yago, J.I.; Chung, K.R. The regulatory hub of siderophore biosynthesis in the phytopathogenic fungus Alternaria alternata. J. Fungi 2023, 9, 427. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Cain, T.J.; Smith, A.T. Ferric iron reductases and their contribution to unicellular ferrous iron uptake. J. Inorg. Biochem. 2021, 218, 111407. [Google Scholar] [CrossRef] [PubMed]
- Misslinger, M.; Hortschansky, P.; Brakhage, A.A.; Haas, H. Fungal iron homeostasis with a focus on Aspergillus fumigatus. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118885. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.H.; Kang, R.; Tang, D.L. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.H.; Monian, P.; Pan, Q.H.; Zhang, W.; Xiang, J.; Jiang, X.J. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- Su, T.; Li, X.Z.; Yang, M.Y.; Shao, Q.; Zhao, Y.X.; Ma, C.L.; Wang, P.P. Autophagy: An intracellular degradation pathway regulating plant survival and stress response. Front. Plant Sci. 2020, 11, 164. [Google Scholar] [CrossRef]
- Li, W.; He, P.C.; Huang, Y.G.; Li, Y.F.; Lu, J.H.; Li, M.; Kurihara, H.; Luo, Z.; Meng, T.; Onishi, M.; et al. Selective autophagy of intracellular organelles: Recent research advances. Theranostics 2021, 11, 222–256. [Google Scholar] [CrossRef]
- Tam, E.; Reno, C.; Nguyen, K.; Cho, S.; Sweeney, G. Importance of autophagy in mediating cellular responses to iron overload in cardiomyocytes. Rev. Cardiovasc. Med. 2022, 23, 167. [Google Scholar] [CrossRef]
- Ito, J.; Omiya, S.; Rusu, M.C.; Ueda, H.; Murakawa, T.; Tanada, Y.; Abe, H.; Nakahara, K.; Asahi, M.; Taneike, M.; et al. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. eLife 2021, 10, e62174. [Google Scholar] [CrossRef]
- Krishan, S.; Janson, P.J.; Gutierrez, E.; Lane, D.J.R.; Richardson, D.; Sahni, S. Iron metabolism and autophagy: A poorly explored relationship that has important consequences for health and disease. Nagoya J. Med. Sci. 2015, 77, 1–6. [Google Scholar] [PubMed]
- Pottier, M.; Dumont, J.; Masclaux-Daubresse, C.; Thomine, S. Autophagy is essential for optimal translocation of iron to seeds in Arabidopsis. J. Exp. Bot. 2019, 70, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Richie, D.L.; Fuller, K.K.; Fortwendel, J.; Miley, M.D.; McCarthy, J.W.; Feldmesser, M.; Rhodes, J.C.; Askew, D.S. Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Montella-Manuel, S.; Pujol-Carrion, N.; Mechoud, M.A.; de la Torre-Ruiz, M.A. Bulk autophagy induction and life extension is achieved when iron is the only limited nutrient in Saccharomyces cerevisiae. Biochem. J. 2021, 478, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Hatzipapas, P.; Kalosaka, K.; Dara, A.; Christias, C. Spore germination and appressorium formation in the entomopathogenic Alternaria alternata. Mycol. Res. 2002, 106, 1349–1359. [Google Scholar] [CrossRef]
- Ma, H.J.; Zhang, B.; Gai, Y.P.; Sun, X.P.; Chung, K.R.; Li, H.Y. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Chung, K.R. The YAP1 homolog-mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant Microbe Interact. 2009, 22, 942–952. [Google Scholar] [CrossRef]
- Yang, S.L.; Chung, K.R. Similar and distinct roles of NADPH oxidase components in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2013, 14, 543–556. [Google Scholar] [CrossRef]
- Wu, P.C.; Choo, C.Y.L.; Lu, H.Y.; Wei, X.Y.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Pexophagy is critical for fungal development, stress response, and virulence in Alternaria alternata. Mol. Plant Pathol. 2022, 23, 1538–1554. [Google Scholar] [CrossRef]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Parmley, R.T.; Spicer, S.S.; Alvarez, C.J. Ultrastructural localization of nonheme celluar iron with ferrocyanide. J. Histochem. Cytochem. 1978, 26, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.; Kijanska, M.; Kalie, E.; Siergiejuk, E.; Lee, S.S.; Semplicio, G.; Stoffel, I.; Brezovich, A.; Verma, M.; Hansmann, I.; et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012, 31, 3691–3703. [Google Scholar] [CrossRef]
- Lei, P.X.; Bai, T.; Sun, Y.L. Mechanisms of ferroptosis and relations with regulated cell death: A review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, W.K.; Bae, K.H.; Lee, S.C.; Lee, E.W. Lipid metabolism and ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L. Iron and siderophores in fungal-host interactions. Mycol. Res. 2008, 112, 170–183. [Google Scholar] [CrossRef]
- Kosman, D.J. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 2003, 47, 1185–1197. [Google Scholar] [CrossRef]
- Brandon, M.; Howard, B.; Lawrence, C.; Laubenbacher, R. Iron acquisition and oxidative stress response in Aspergillus fumigatus. BMC Syst. Biol. 2015, 9, 19. [Google Scholar] [CrossRef]
- Lopez-Berges, M.S.; Scheven, M.T.; Hortschansky, P.; Misslinger, M.; Baldin, C.; Gsaller, F.; Werner, E.R.; Kruger, T.; Kniemeyer, O.; Weber, J.; et al. The bZIP transcription factor HapX is post-translationally regulated to control iron homeostasis in Aspergillus fumigatus. Int. J. Mol. Sci. 2021, 22, 7739. [Google Scholar] [CrossRef]
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 2003, 62, 316–330. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed cell-death by ferroptosis: Antioxidants as mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Schrettl, M.; Kragl, C.; Muller, D.; Illmer, P.; Haas, H. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Oberegger, H.; Zadra, I.; Haas, H. The siderophore system is essential for viability of Aspergillus nidulans: Functional analysis of two genes encoding L-ornithine N5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 2003, 49, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.S.; Quie, P.G.; Basford, R.E. Effect of iron on leukocyte function: Inactivation of H2O2 by iron. Infect. Immun. 1975, 12, 303–308. [Google Scholar] [CrossRef]
- Tirmenstein, M.A.; Nicholls-Grzemski, F.A.; Zhang, J.G.; Fariss, M.W. Glutathione depletion and the production of reactive oxygen species in isolated hepatocyte suspensions. Chem.-Biol. Interact. 2000, 127, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Pan, T.M. Dimerumic acid, a novel antioxidant identified from Monascus-fermented products exerts chemoprotective effects: Mini review. J. Funct. Foods 2013, 5, 2–9. [Google Scholar] [CrossRef]
- Tseng, W.T.; Hsu, Y.W.; Pan, T.M. Neuroprotective effects of dimerumic acid and deferricoprogen from Monascus purpureus NTU 568-fermented rice against 6-hydroxydopamine-induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells. Pharm. Biol. 2016, 54, 1434–1444. [Google Scholar] [CrossRef]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic. Biol. Med. 2011, 50, 1647–1658. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.M.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D.L. Autophagy-dependent ferroptosis: Machinery and regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Z.N.; Yan, X.L.; Huang, S.; Ren, J.X.; Luo, Y.; Yang, Y. Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front. Cell. Neurosci. 2020, 14, 577403. [Google Scholar] [CrossRef]
- Shen, Q.; Liang, M.L.; Yang, F.; Deng, Y.Z.; Naqvi, N.I. Ferroptosis contributes to developmental cell death in rice blast. New Phytol. 2020, 227, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- You, B.J.; Lee, M.H.; Chung, K.R. Gene-specific disruption in the filamentous fungus Cercospora nicotianae using a split-marker approach. Arch. Microbiol. 2009, 191, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Dangol, S.; Nguyen, N.K.; Singh, R.; Chen, Y.F.; Wang, J.; Lee, H.G.; Hwang, B.K.; Jwa, N.S. Mitogen-activated protein kinase OsMEK2 and OsMPK1 signaling is required for ferroptotic cell death in rice-Magnaporthe oryzae interactions. Front. Plant Sci. 2021, 12, 710794. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical-assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Wu, P.C.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Peroxisomes implicated in the biosynthesis of siderophores and biotin, cell wall integrity, autophagy, and response to hydrogen peroxide in the citrus pathogenic fungus Alternaria alternata. Front. Microbiol. 2021, 12, 645792. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-C.; Choo, Y.-L.; Wei, S.-Y.; Yago, J.I.; Chung, K.-R. Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata. Int. J. Mol. Sci. 2024, 25, 1123. https://doi.org/10.3390/ijms25021123
Wu P-C, Choo Y-L, Wei S-Y, Yago JI, Chung K-R. Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata. International Journal of Molecular Sciences. 2024; 25(2):1123. https://doi.org/10.3390/ijms25021123
Chicago/Turabian StyleWu, Pei-Ching, Yen-Ling Choo, Sian-Yong Wei, Jonar I. Yago, and Kuang-Ren Chung. 2024. "Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata" International Journal of Molecular Sciences 25, no. 2: 1123. https://doi.org/10.3390/ijms25021123
APA StyleWu, P.-C., Choo, Y.-L., Wei, S.-Y., Yago, J. I., & Chung, K.-R. (2024). Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata. International Journal of Molecular Sciences, 25(2), 1123. https://doi.org/10.3390/ijms25021123







