Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates
Abstract
1. Introduction
2. Results
2.1. Synthetic Procedures and Chemical Characterization
2.2. Biological Characterization of the Full-Length α-MSH Drug Conjugates
2.2.1. In Vitro Antiproliferative Activity of Full-Length α-MSH Drug Conjugates
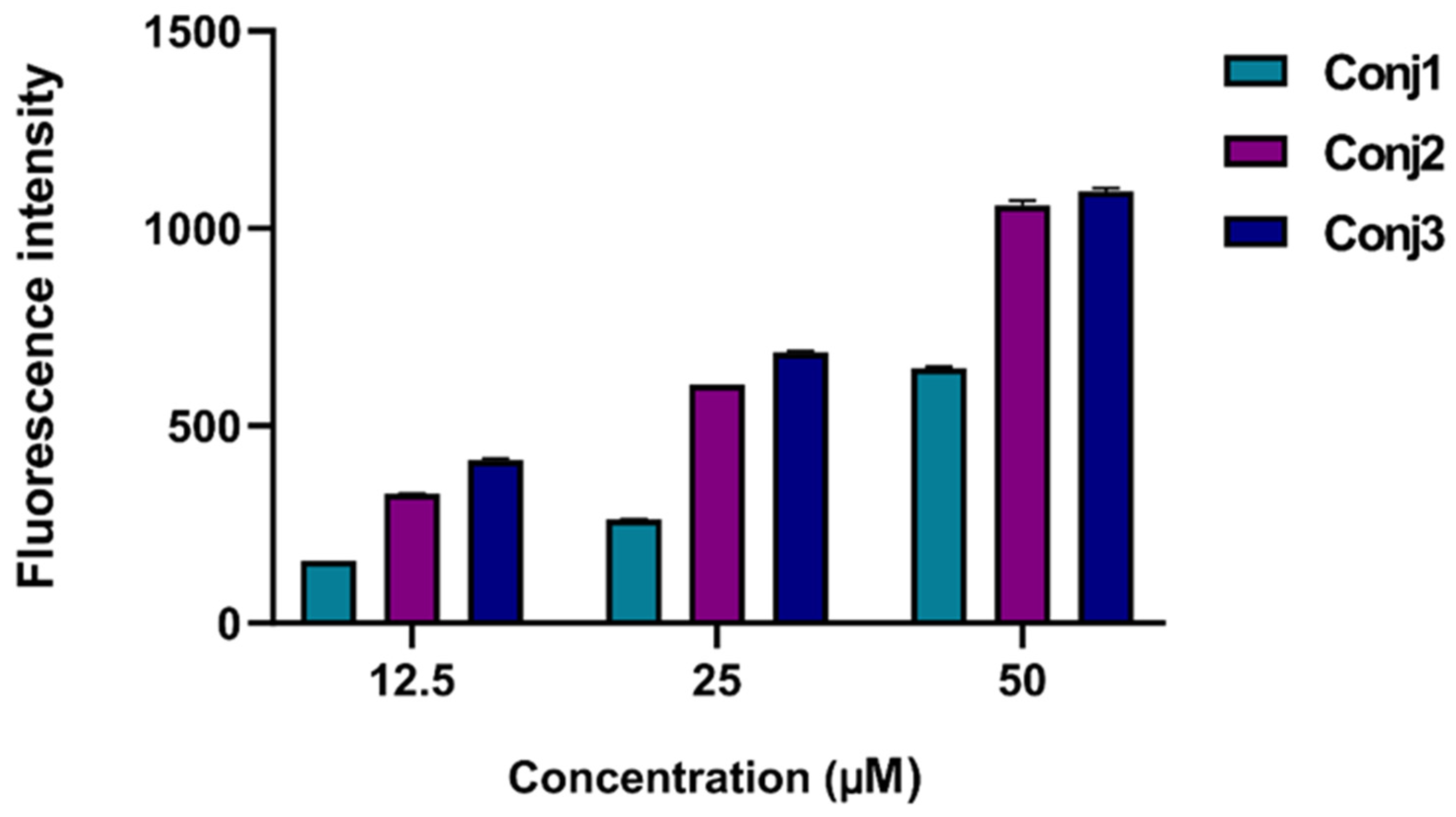
2.2.2. In Vitro Flow Cytometry Evaluation of Full-Length α-MSH Drug Conjugates
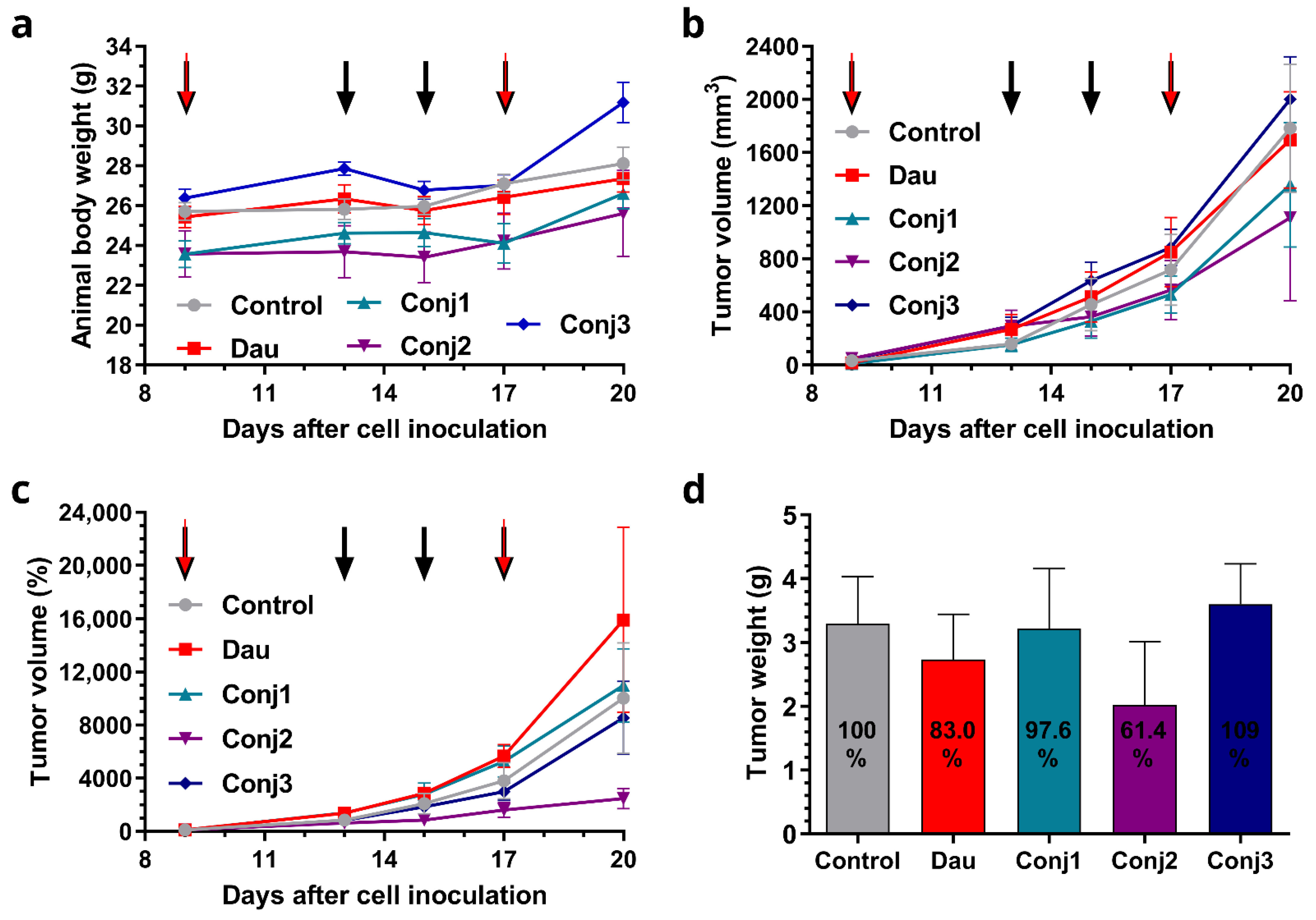
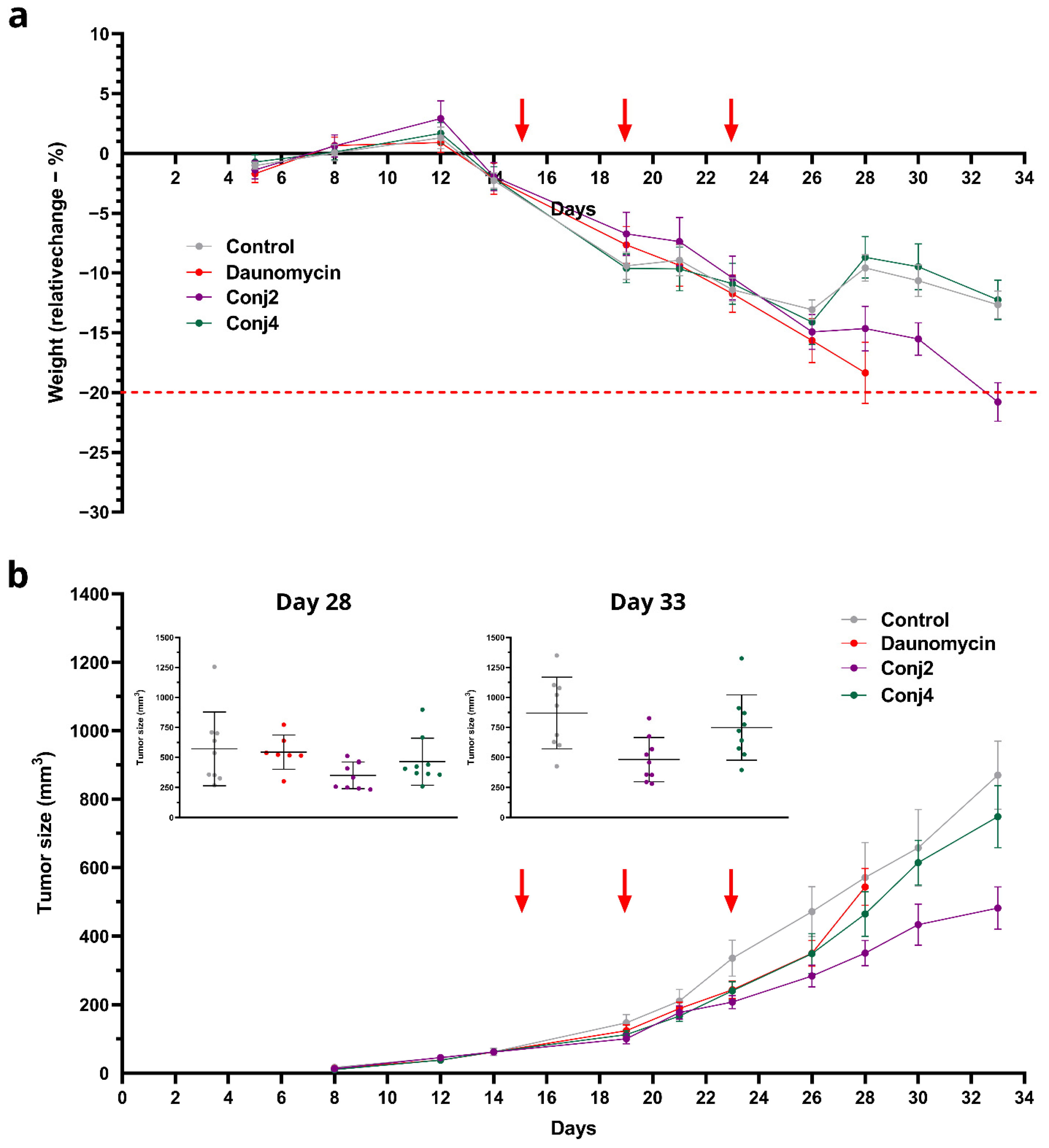
2.2.3. In Vivo Antitumor Effect of Full-Length α-MSH Drug Conjugates on B16 Melanoma Model
2.3. Biological Characterization of the Sequentially Optimized α-MSH Drug Conjugate, Conj4
2.3.1. Experimental Model Selection Based on MC1R Expression
2.3.2. In Vitro Cytostatic Effect of the Conj4 Compared to the Conj2
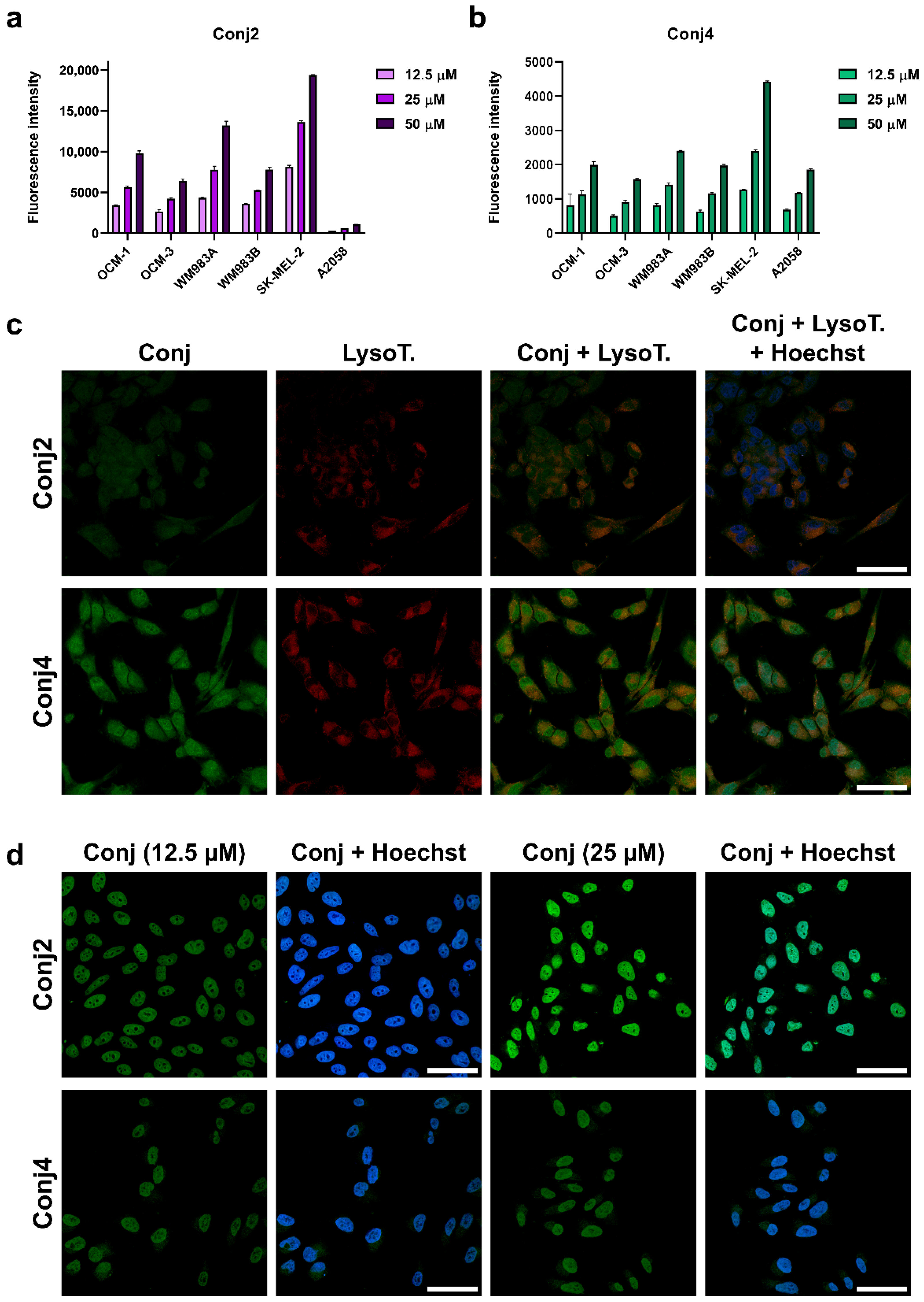
2.3.3. In Vitro Flow Cytometry and Confocal Microscopy Evaluation
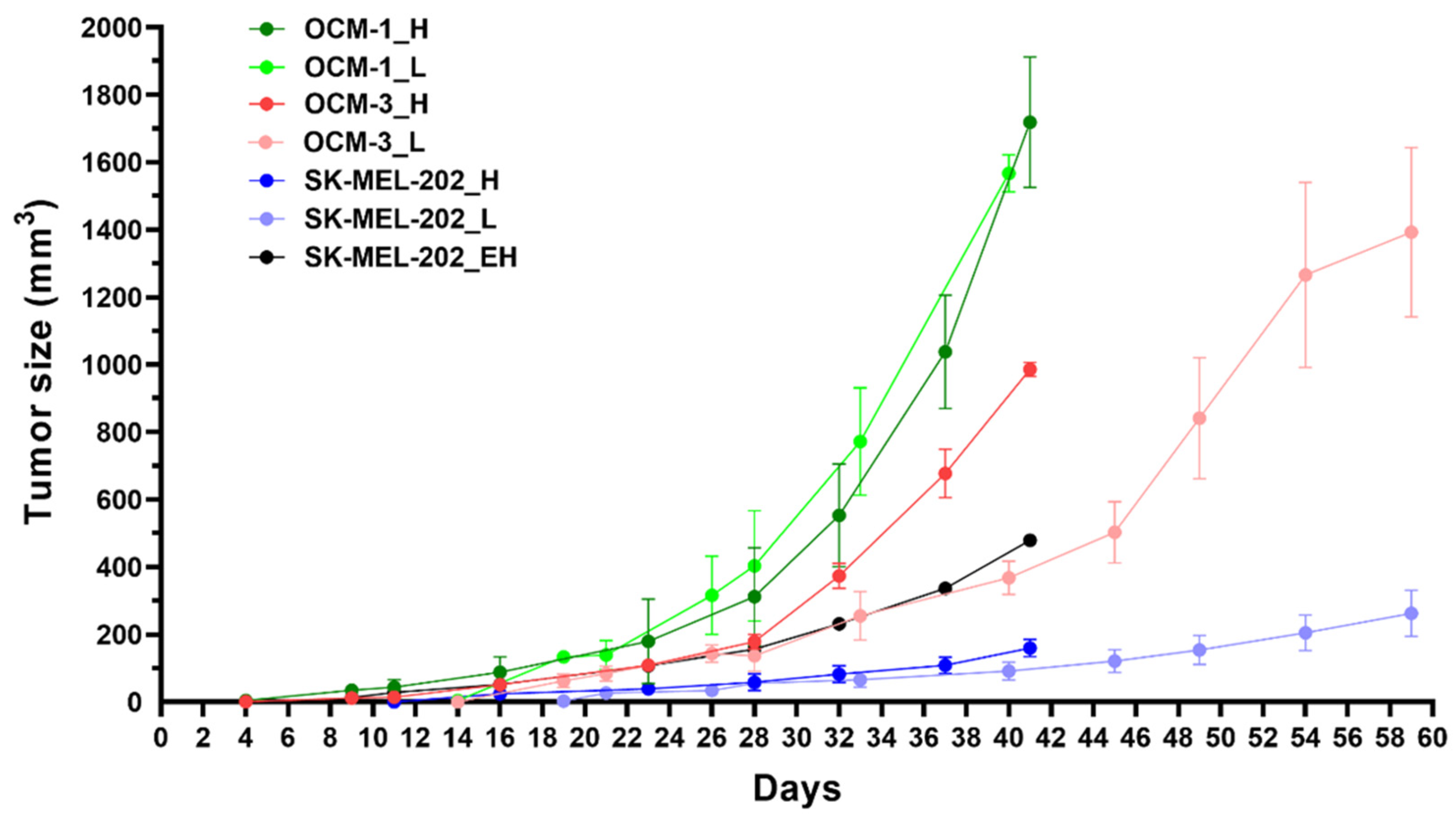
2.3.4. In Vivo Experiments
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthetic Procedures and Chemical Characterization
4.3. Reverse Phase High-Performance Liquid Chomatography (RP-HPLC)
4.4. Electrospray Ionization-High-Resolution Mass Spectromerty (ESI-HRMS)
4.5. Cell Culturing
4.6. Determination of mRNA Expression Level of Melanoma Cells by qPCR
4.7. Determination of the In Vitro Antiproliferative Activity
4.8. In Vitro Flow Cytometry Evaluation
4.9. Immunostaining and Confocal Microscopy
4.10. Experimental Animals
4.11. Acute Toxicity Study of Drug, Conj2 and Conj4
4.12. In Vivo Antitumor Effect of Drug, Conj2 and Conj4
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous Melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; From, L.; Bernardino, E.A.; Mihm, M.C. The Histogenesis and Biologic Behavior of Primary Human Malignant Melanomas of the Skin. Cancer Res. 1969, 29, 705–727. [Google Scholar]
- Hsan, K.M.; Chen, C.-C.; Shyur, L.-F. Current Research and Development of Chemotherapeutic Agents for Melanoma. Cancers 2010, 2, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.N.; Rout, B.; Bigliardi Qi, M.; Bigliardi, P.L. Synthetic Peptide Drugs for Targeting Skin Cancer: Malignant Melanoma and Melanotic Lesions. Curr. Med. Chem. 2017, 24, 1797–1826. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.N.; Siegrist, W.; Bagutti, C.; Tapia, J.C.-D.; Solca, F.; Wikberg, J.E.S.; Chhajlani, V. Receptors for Melanocyte-Stimulating Hormone on Melanoma Cells. Ann. N. Y. Acad. Sci. 1993, 680, 320–341. [Google Scholar] [CrossRef]
- Jiang, J.; Sharma, S.D.; Fink, J.L.; Hadley, M.E.; Hruby, V.J. Melanotropic Peptide Receptors: Membrane Markers of Human Melanoma Cells. Exp. Dermatol. 1996, 5, 325–333. [Google Scholar] [CrossRef]
- Salazar-Onfray, F.; López, M.; Lundqvist, A.; Aguirre, A.; Escobar, A.; Serrano, A.; Korenblit, C.; Petersson, M.; Chhajlani, V.; Larsson, O.; et al. Tissue Distribution and Differential Expression of Melanocortin 1 Receptor, a Malignant Melanoma Marker. Br. J. Cancer 2002, 87, 414–422. [Google Scholar] [CrossRef]
- Siegrist, W.; Stutz, S.; Eberle, A.N. Homologous and Heterologous Regulation of Alpha-Melanocyte-Stimulating Hormone Receptors in Human and Mouse Melanoma Cell Lines. Cancer Res. 1994, 54, 2604–2610. [Google Scholar]
- Hruby, V.J.; Sharma, S.D.; Toth, K.; Jaw, J.Y.; Al-Obeidi, F.; Sawyer, T.K.; Hadley, M.E. Design, Synthesis, and Conformation of Superpotent and Prolonged Acting Melanotropins. Ann. N. Y. Acad. Sci. 1993, 680, 51–63. [Google Scholar] [CrossRef]
- Siegrist, W.; Solca, F.; Stutz, S.; Giuffrè, L.; Carrel, S.; Girard, J.; Eberle, A.N. Characterization of Receptors for Alpha-Melanocyte-Stimulating Hormone on Human Melanoma Cells. Cancer Res. 1989, 49, 6352–6358. [Google Scholar] [PubMed]
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma Therapy via Peptide-Targeted α-Radiation. Clin. Cancer Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef] [PubMed]
- Giblin, M.F.; Wang, N.; Hoffman, T.J.; Jurisson, S.S.; Quinn, T.P. Quinn Design and Characterization of α-Melanotropin Peptide Analogs Cyclized through Rhenium and Technetium Metal Coordination. Proc. Natl. Acad. Sci. USA 1998, 95, 12814–12818. [Google Scholar] [CrossRef]
- Cone, R.D.; Mountjoy, K.G.; Robbins, L.S.; Nadeau, J.H.; Johnson, K.R.; Roselli-Rehfuss, L.; Mortrud, M.T. Cloning and Functional Characterization of a Family of Receptors for the Melanotropic Peptides. Ann. N. Y. Acad. Sci. 1993, 680, 342–363. [Google Scholar] [CrossRef] [PubMed]
- Morandini, R.; Suli-Vargha, H.; Libert, A.; Loir, B.; Botyánszki, J.; Medzihradszky, K.; Ghanem, G. Receptor-Mediated Cyotoxicity of a-MSH Fragments Containing Melphalan in a Human Melanoma Cell Line. Int. J. Cancer 1994, 56, 129–133. [Google Scholar] [CrossRef]
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-Phenylalanine-Alpha-Melanocyte-Stimulating Hormone: A Highly Potent Alpha-Melanotropin with Ultralong Biological Activity. Proc. Natl. Acad. Sci. USA 1980, 77, 5754–5758. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Miao, Y. Dual Receptor-Targeting 99mTc-Labeled Arg-Gly-Asp-Conjugated Alpha-Melanocyte Stimulating Hormone Hybrid Peptides for Human Melanoma Imaging. Nucl. Med. Biol. 2015, 42, 369–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Guo, H.; Miao, Y. Technetium-99m-Labeled Arg-Gly-Asp-Conjugated Alpha-Melanocyte Stimulating Hormone Hybrid Peptides for Human Melanoma Imaging. Nucl. Med. Biol. 2010, 37, 873–883. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Gallazzi, F.; Miao, Y. Effects of the Amino Acid Linkers on the Melanoma-Targeting and Pharmacokinetic Properties of 111 In-Labeled Lactam Bridge–Cyclized α-MSH Peptides. J. Nucl. Med. 2011, 52, 608–616. [Google Scholar] [CrossRef]
- Lin, X.; Xie, J.; Niu, G.; Zhang, F.; Gao, H.; Yang, M.; Quan, Q.; Aronova, M.A.; Zhang, G.; Lee, S.; et al. Chimeric Ferritin Nanocages for Multiple Function Loading and Multimodal Imaging. Nano Lett. 2011, 11, 814–819. [Google Scholar] [CrossRef]
- Süli-Vargha, H.; Botyánszki, J.; Medzihradszky-Schweiger, H.; Medzihradszky, K. Synthesis of α-MSH Fragments Containing Phenylalanine Mustard for Receptor Studies. Int. J. Pept. Protein Res. 1990, 36, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, S.; Calame-Christe, M.; Tanner, H.; Eberle, A.N. Eberle Melanoma Targeting with DOTA-α-Melanocyte-Stimulating Hormone Analogs: Structural Parameters Affecting Tumor Uptake and Kidney Uptake. J. Nucl. Med. 2005, 46, 887–895. [Google Scholar] [PubMed]
- Uchida, M.; Flenniken, M.L.; Allen, M.; Willits, D.A.; Crowley, B.E.; Brumfield, S.; Willis, A.F.; Jackiw, L.; Jutila, M.; Young, M.J.; et al. Targeting of Cancer Cells with Ferrimagnetic Ferritin Cage Nanoparticles. J. Am. Chem. Soc. 2006, 128, 16626–16633. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Falvo, E.; Fornara, M.; Di Micco, P.; Benada, O.; Krizan, J.; Svoboda, J.; Hulikova-Capkova, K.; Morea, V.; Boffi, A.; et al. Selective Targeting of Melanoma by PEG-Masked Protein-Based Multifunctional Nanoparticles. Int. J. Nanomed. 2012, 7, 1489–1509. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Guo, H.; Padilla, R.S.; Berwick, M.; Miao, Y. Replacement of the Lys Linker with an Arg Linker Resulting in Improved Melanoma Uptake and Reduced Renal Uptake of Tc-99m-Labeled Arg-Gly-Asp-Conjugated Alpha-Melanocyte Stimulating Hormone Hybrid Peptide. Bioorg. Med. Chem. 2010, 18, 6695–6700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.; Mowlazadeh Haghighi, S.; Liu, Z.; Wang, L.; Hruby, V.J.; Cai, M. Development of Ligand-Drug Conjugates Targeting Melanoma through the Overexpressed Melanocortin 1 Receptor. ACS Pharmacol. Transl. Sci. 2020, 3, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.M.; Asato, N.; Lande, S.; Lerner, A.B. Melanotropin–Daunomycin Conjugate Shows Receptor-Mediated Cytotoxicity in Cultured Murine Melanoma Cells. Nature 1977, 267, 56–58. [Google Scholar] [CrossRef]
- Kiss, K.; Biri-Kovács, B.; Szabó, R.; Ranđelović, I.; Enyedi, K.N.; Schlosser, G.; Orosz, Á.; Kapuvári, B.; Tóvári, J.; Mező, G. Sequence Modification of Heptapeptide Selected by Phage Display as Homing Device for HT-29 Colon Cancer Cells to Improve the Anti-Tumour Activity of Drug Delivery Systems. Eur. J. Med. Chem. 2019, 176, 105–116. [Google Scholar] [CrossRef]
- Ranđelović, I.; Schuster, S.; Kapuvári, B.; Fossati, G.; Steinkühler, C.; Mező, G.; Tóvári, J. Improved In Vivo Anti-Tumor and Anti-Metastatic Effect of GnRH-III-Daunorubicin Analogs on Colorectal and Breast Carcinoma Bearing Mice. Int. J. Mol. Sci. 2019, 20, 4763. [Google Scholar] [CrossRef]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide–Drug Conjugates with Different Linkers for Cancer Therapy. J. Med. Chem. 2021, 64, 216–232. [Google Scholar] [CrossRef]
- Pethő, L.; Oláh-Szabó, R.; Mező, G. Influence of the Drug Position on Bioactivity in Angiopep-2—Daunomycin Conjugates. Int. J. Mol. Sci. 2023, 24, 3106. [Google Scholar] [CrossRef] [PubMed]
- Gomena, J.; Vári, B.; Oláh-Szabó, R.; Biri-Kovács, B.; Bősze, S.; Borbély, A.; Soós, Á.; Ranđelović, I.; Tóvári, J.; Mező, G. Targeting the Gastrin-Releasing Peptide Receptor (GRP-R) in Cancer Therapy: Development of Bombesin-Based Peptide–Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Orbán, E.; Mező, G.; Schlage, P.; Csík, G.; Kulić, Ž.; Ansorge, P.; Fellinger, E.; Möller, H.M.; Manea, M. In Vitro Degradation and Antitumor Activity of Oxime Bond-Linked Daunorubicin–GnRH-III Bioconjugates and DNA-Binding Properties of Daunorubicin–Amino Acid Metabolites. Amino Acids 2011, 41, 469–483. [Google Scholar] [CrossRef]
- Szabó, I.; Manea, M.; Orbán, E.; Csámpai, A.; Bősze, S.; Szabó, R.; Tejeda, M.; Gaál, D.; Kapuvári, B.; Przybylski, M.; et al. Development of an Oxime Bond Containing Daunorubicin-Gonadotropin-Releasing Hormone-III Conjugate as a Potential Anticancer Drug. Bioconjugate Chem. 2009, 20, 656–665. [Google Scholar] [CrossRef]
- Baranyai, Z.; Biri-Kovács, B.; Krátký, M.; Szeder, B.; Debreczeni, M.L.; Budai, J.; Kovács, B.; Horváth, L.; Pári, E.; Németh, Z.; et al. Cellular Internalization and Inhibition Capacity of New Anti-Glioma Peptide Conjugates: Physicochemical Characterization and Evaluation on Various Monolayer- and 3D-Spheroid-Based in Vitro Platforms. J. Med. Chem. 2021, 64, 2982–3005. [Google Scholar] [CrossRef] [PubMed]
- Böhme, D.; Beck-Sickinger, A.G. Drug Delivery and Release Systems for Targeted Tumor Therapy. J. Pept. Sci. 2015, 21, 186–200. [Google Scholar] [CrossRef]
- Fodor, K.; Dobos, N.; Schally, A.; Steiber, Z.; Olah, G.; Sipos, E.; Szekvolgyi, L.; Halmos, G. The Targeted LHRH Analog AEZS-108 Alters Expression of Genes Related to Angiogenesis and Development of Metastasis in Uveal Melanoma. Oncotarget 2020, 11, 175–187. [Google Scholar] [CrossRef]
- Medzihradszky, K. Synthesis and Biological Activity of Adrenocorticotropic and Melanotropic Hormones. In Recent Developments in the Chemistry of Natural Carbon Compounds; Bogner, R., Bruckner, R., Szantay, C., Eds.; Hungarian Academy of Science: Budapest, Hungary, 1976; pp. 207–250. [Google Scholar]
- Bregman, M.D.; Sawyer, T.K.; Hadley, M.E.; Hruby, V.J. Adenosine and Divalent Cation Effects on S-91 Melanoma Adenylate Cyclase. Arch. Biochem. Biophys. 1980, 201, 1–7. [Google Scholar] [CrossRef]
- Heward, C.B.; Yang, Y.C.S.; Ormberg, J.F.; Hadley, M.E.; Hruby, V.J. Effects of Chloramine T and Lodination on the Biological Activity of Melanotropin. Hoppe Seylers Z Physiol. Chem. 1979, 360, 1851–1860. [Google Scholar] [CrossRef]
- Heward, C.B.; Yang, Y.C.S.; Sawyer, T.K.; Bregman, M.D.; Fuller, B.B.; Hruby, V.J.; Hadley, M.E. Iodination Associated Inactivation of β-Melanocyte Stimulating Hormone. Biochem. Biophys. Res. Commun. 1979, 88, 266–273. [Google Scholar] [CrossRef]
- McGill, M.R. The Past and Present of Serum Aminotransferases and the Future of Liver Injury Biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, J.M.; Burke, T.G.; Tritton, T.R. Cellular Transport of Anthracyclines by Passive Diffusion. Biochem. Pharmacol. 1985, 34, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Willingham, M.C.; Cornwell, M.M.; Cardarelli, C.O.; Gottesman, M.M.; Pastan, I. Single Cell Analysis of Daunomycin Uptake and Efflux in Multidrug-Resistant and -Sensitive KB Cells: Effects of Verapamil and Other Drugs. Cancer Res. 1986, 46, 5941–5946. [Google Scholar]
- Miao, Y.; Gallazzi, F.; Guo, H.; Quinn, T.P. 111 In-Labeled Lactam Bridge-Cyclized α-Melanocyte Stimulating Hormone Peptide Analogues for Melanoma Imaging. Bioconjugate Chem. 2008, 19, 539–547. [Google Scholar] [CrossRef][Green Version]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How Cancer Hijacks the Body’s Homeostasis through the Neuroendocrine System. Trends Neurosci 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Mező, G.; Manea, M. Receptor-Mediated Tumor Targeting Based on Peptide Hormones. Expert Opin. Drug Deliv. 2010, 7, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A. Melanocortin Receptors: Their Functions and Regulation by Physiological Agonists and Antagonists. Cell. Mol. Life Sci. 2001, 58, 434–441. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Altman, F.P. Tetrazolium Salts and Formazans. Prog. Histochem. Cytochem. 1976, 9, III-51. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.F.; Sawyer, B.; Sträuli, U. Studies on Succinate-Tetrazolium Reductase Systems. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Istivan, T.S.; Pirogova, E.; Gan, E.; Almansour, N.M.; Coloe, P.J.; Cosic, I. Biological Effects of a De Novo Designed Myxoma Virus Peptide Analogue: Evaluation of Cytotoxicity on Tumor Cells. PLoS ONE 2011, 6, e24809. [Google Scholar] [CrossRef]
- Kühn, J.; Shaffer, E.; Mena, J.; Breton, B.; Parent, J.; Rappaz, B.; Chambon, M.; Emery, Y.; Magistretti, P.; Depeursinge, C.; et al. Label-Free Cytotoxicity Screening Assay by Digital Holographic Microscopy. Assay. Drug Dev. Technol. 2013, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Ward, K.M.; Prendergast, G.C.; Ayene, I.S. Hydroxyethyl Disulfide as an Efficient Metabolic Assay for Cell Viability in Vitro. Toxicol. Vitr. 2012, 26, 603–612. [Google Scholar] [CrossRef][Green Version]
- Paulus, J.; Nachtigall, B.; Meyer, P.; Sewald, N. RGD Peptidomimetic MMAE-Conjugate Addressing Integrin AVβ3-Expressing Cells with High Targeting Index**. Chem. A Eur. J. 2023, 29, e202203476. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2000, 39, 20–21. [Google Scholar] [CrossRef]
- Teicher, B.A. Tumor Models in Cancer Research; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; ISBN 978-1-60761-967-3. [Google Scholar]


| Code | Sequence | tR (Min) 1 | Mmo (Da) 2 | ||
|---|---|---|---|---|---|
| Calc | Meas | Differences (ppm) | |||
| Conj1 | Dau=Aoa-SYSNleEHFRWGKPV-NH2 | 12.6 | 2187.0266 | 2187.0080 | 18.56 |
| Conj2 | Ac-SYSNleEHFRWGK(Dau=Aoa)PV-NH2 | 13.0 | 2229.0371 | 2229.0214 | 15.66 |
| Conj3 | Dau=Aoa-SYSNleEHFRWGK(Dau=Aoa)PV-NH2 | 12.8 | 2769.2272 | 2769.6050 | 32.76 |
| Conj4 | Ac-NleEHfRWGK(Dau=Aoa)-NH2 | 12.9 | 1694.7876 | 1694.7692 | 18.36 |
| Cell Line | IC50 1 (µM) | Relative Potency 2 | |||||
|---|---|---|---|---|---|---|---|
| Conj1 | Conj2 | Conj3 | Dau | Conj1 | Conj2 | Conj3 | |
| B16 | 2.9 ± 0.6 | 2.8 ± 0.7 | 2.0 ± 0.7 | 0.026 ± 0.008 | 0.0090 | 0.0093 | 0.0130 |
| A2058 | 9.8 ± 5.4 | 3.2 ± 0.4 | 3.0 ± 0.8 | 0.04 ± 0.007 | 0.0041 | 0.0125 | 0.0133 |
| M24 | 12.8 ± 1.6 | 11.5 ± 0.4 | 11.0 ± 0.8 | 0.119 ± 0.025 | 0.0093 | 0.0103 | 0.0108 |
| WM983B | 9.9 ± 1.5 | 7.9 ± 0.7 | 3.6 ± 0.2 | 0.050 ± 0.023 | 0.0050 | 0.0063 | 0.0138 |
| Code | IC50 (µM) 1 | |||||
|---|---|---|---|---|---|---|
| OCM-1 | OCM-3 | SK-MEL-202 | WM983A | WM983B | A2058 | |
| Free Dau | 0.73 ± 0.06 | 0.56 ± 0.05 | 0.045 ± 0.12 | 0.19 ± 0.06 | 0.33 ± 0.05 | 0.12 ± 0.07 |
| Conj2 | 2.51 ± 0.06 | 2.39 ± 0.06 | 0.13 ± 0.09 | 0.79 ± 0.05 | 1.00 ± 0.00 | 0.95 ± 0.12 |
| Conj4 | 29.68 ± 0.06 | 25.47 ± 0.08 | 2.06 ± 0.10 | 13.50 ± 0.06 | 10.18 ± 0.06 | 7.15 ± 0.07 |
| Targeting Index (TI) 2 | ||||||
| Conj2 | 2.30 | 1.85 | 2.74 | 1.90 | 2.61 | 1.00 |
| Conj4 | 1.47 | 1.31 | 1.30 | 0.84 | 1.93 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, I.; Biri-Kovács, B.; Vári, B.; Ranđelović, I.; Vári-Mező, D.; Juhász, É.; Halmos, G.; Bősze, S.; Tóvári, J.; Mező, G. Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates. Int. J. Mol. Sci. 2024, 25, 1095. https://doi.org/10.3390/ijms25021095
Szabó I, Biri-Kovács B, Vári B, Ranđelović I, Vári-Mező D, Juhász É, Halmos G, Bősze S, Tóvári J, Mező G. Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates. International Journal of Molecular Sciences. 2024; 25(2):1095. https://doi.org/10.3390/ijms25021095
Chicago/Turabian StyleSzabó, Ildikó, Beáta Biri-Kovács, Balázs Vári, Ivan Ranđelović, Diána Vári-Mező, Éva Juhász, Gábor Halmos, Szilvia Bősze, József Tóvári, and Gábor Mező. 2024. "Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates" International Journal of Molecular Sciences 25, no. 2: 1095. https://doi.org/10.3390/ijms25021095
APA StyleSzabó, I., Biri-Kovács, B., Vári, B., Ranđelović, I., Vári-Mező, D., Juhász, É., Halmos, G., Bősze, S., Tóvári, J., & Mező, G. (2024). Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates. International Journal of Molecular Sciences, 25(2), 1095. https://doi.org/10.3390/ijms25021095










